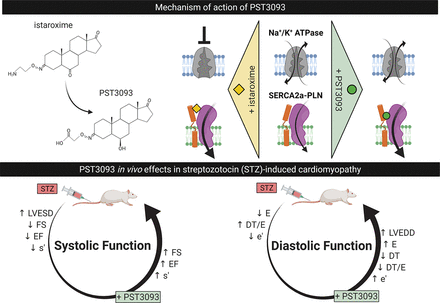
Visual Overview
Abstract
Heart failure (HF) therapeutic toolkit would strongly benefit from the availability of ino-lusitropic agents with a favorable pharmacodynamics and safety profile. Istaroxime is a promising agent, which combines Na+/K+ pump inhibition with sarcoplasmic reticulum Ca2+ ATPase (SERCA2a) stimulation; however, it has a very short half-life and extensive metabolism to a molecule named PST3093. The present work aims to investigate whether PST3093 still retains the pharmacodynamic and pharmacokinetic properties of its parent compound. We studied PST3093 for its effects on SERCA2a and Na+/K+ ATPase activities, Ca2+ dynamics in isolated myocytes, and hemodynamic effects in an in vivo rat model of diabetic [streptozotocin (STZ)-induced] cardiomyopathy. Istaroxime infusion in HF patients led to accumulation of PST3093 in the plasma; clearance was substantially slower for PST3093 than for istaroxime. In cardiac rat preparations, PST3093 did not inhibit the Na+/K+ ATPase activity but retained SERCA2a stimulatory activity. In in vivo echocardiographic assessment, PST3093 improved overall cardiac performance and reversed most STZ-induced abnormalities. PST3093 intravenous toxicity was considerably lower than that of istaroxime, and it failed to significantly interact with 50 off-targets. Overall, PST3093 is a “selective” SERCA2a activator, the prototype of a novel pharmacodynamic category with a potential in the ino-lusitropic approach to HF with prevailing diastolic dysfunction. Its pharmacodynamics are peculiar, and its pharmacokinetics are suitable to prolong the cardiac beneficial effect of istaroxime infusion.
SIGNIFICANCE STATEMENT Heart failure (HF) treatment would benefit from the availability of ino-lusitropic agents with favourable profiles. PST3093 is the main metabolite of istaroxime, a promising agent combining Na+/K+ pump inhibition and sarcoplasmic reticulum Ca2+ ATPase2a (SERCA2a) stimulation. PST3093 shows a longer half-life in human circulation compared to istaroxime, selectively activates SERCA2a, and improves cardiac performance in a model of diabetic cardiomyopathy. Overall, PST3093 as a selective SERCA2a activator can be considered the prototype of a novel pharmacodynamic category for HF treatment.
Introduction
Heart failure (HF) is characterized by abnormal Ca2+distribution among subcellular compartments, which contributes to impaired contractility and relaxation (Bers and Despa, 2006), facilitates arrhythmias (Zaza and Rocchetti, 2015), and, in the long run, contributes to myocardial remodeling (Nakayama et al., 2007). Evidence of a deficient sarcoplasmic reticulum Ca2+ ATPase2a (SERCA2a) activity in HF dates to the 1970s (Suko et al., 1970; Sulakhe and Dhalia, 1971). Since then, many studies confirmed this finding (Gwathmey et al., 1987; Arai et al., 1993; Kranias and Hajjar, 2012) showing that the impaired SERCA2a activity can often result from an over-inhibition by phospholamban (PLN) (Haghighi et al., 2001; Del Monte et al., 2002). Loss of SERCA2a function accounts for abnormal distribution of intracellular Ca2+, with numerous detrimental consequences. Interventions currently available to modulate myocyte Ca2+ handling (e.g., amines, PDE inhibitors, etc.) stimulate SERCA2a, but they do so in the context of a multitarget action, thus resulting in untoward effects. Selective SERCA2a enhancement would afford inotropic and lusitropic effects without the drawbacks of the multitarget action (Zaza and Rocchetti, 2015). Accordingly, the use of SERCA2a stimulation in HF therapy is receiving considerable attention, and many attempts to selectively stimulate SERCA2a activity with gene therapy or “small molecule” agents have been reported (Kho et al., 2011; Kaneko et al., 2017; Schaaf et al., 2020). Nonetheless, for reasons other than refutation of the principle, none of these attempts has been successfully translated into the clinic. The only exception is istaroxime, a small-molecule drug, identified as a SERCA2a enhancer by our group (Rocchetti et al., 2005) and currently under clinical development for the treatment of acute HF (Shah et al., 2009; Carubelli et al., 2020). Istaroxime has a double mechanism of action: it inhibits the Na+/K+ pump (Micheletti et al., 2002) and activates SERCA2a (Rocchetti et al., 2005). Thus, istaroxime increases overall cell Ca2+ content while promoting rapid Ca2+ sequestration into the sarcoplasmic reticulum (SR). Notably, at variance with Na+/K+ pump blockade alone, this neither facilitates spontaneous Ca2+ release from the SR (Alemanni et al., 2011) nor increases myocardial oxygen demand (Sabbah et al., 2007). Thus, istaroxime may improve systolic and diastolic performance (Shah et al., 2009) without promoting arrhythmia or ischemia (Gheorghiade et al., 2008; Carubelli et al., 2020). However, istaroxime has a plasma half-life of less than 1 hour because of extensive hepatic metabolism to a molecule named PST3093 (Gheorghiade et al., 2008; Carubelli et al., 2020); this restricts istaroxime usage to acute intravenous therapy.
The present work aims to investigate whether PST3093 may, on its own, be endowed with pharmacological activity. To this end, PST3093 has been synthesized and compared with istaroxime and digoxin (as reference compounds) in experimental setups at different levels of biologic organization and in the context of disease-induced dysfunction.
The data reported here indicate that PST3093 shows a longer half-life in human circulation compared with parent drug; it stimulates SERCA2a activity, but, at variance with istaroxime, it does not inhibit the Na+/K+ ATPase. This pharmacodynamic profile translates to positive inotropy and lusitropy in an in vivo disease model characterized by SERCA2a downregulation. Therefore, PST3093 qualifies as a “pure” SERCA2a activator, able to improve cardiac mechanical performance in vivo.
Materials and Methods
This study aims to a comprehensive characterization of PST3093 (versus istaroxime, its parent compound) as an ino-lusitropic agent to be used chronically in the treatment of HF. The evaluations performed and their scope are summarized in Table 1. Further detail on methods is given in the Supplemental Materials.
Synopsis of the studies
The animal study protocols were approved by the Institutional Review Board of Milano Bicocca (29C09.26 and 29C09.N.YRR protocol codes approved on January 2021 and June 2018, respectively) and Chang Gung (CGU107-068 protocol code approved on June 2018) Universities in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the US National Institutes of Health.
Disease Model
Streptozotocin (STZ)-induced diabetes was selected as a pathologic model because of its association with reduced SERCA2a function (Torre et al., 2022) and relevance to diastolic dysfunction (Valero-Muñoz et al., 2017), for which a lusitropic action may be more relevant. Diabetes was induced in Sprague-Dawley male rats (150–175 g) by a single STZ (50 mg·kg−1) intravenous injection (STZ group). Control (healthy group) rats received STZ vehicle (citrate buffer). Fasting glycaemia was measured after 1 week, and rats with values > 290 mg·dL−1 were considered diabetic (Torre et al., 2022). Rats were euthanized by cervical dislocation under anesthesia with ketamine-xylazine (130–7.5 mg·kg−1 i.p) 9 weeks after STZ injection.
Pharmacokinetics in Humans
Pharmacokinetics (PKs) of istaroxime and its metabolite PST3093 was assessed in 30 HF patients infused for 6 hours with istaroxime at 1.0 μg/kg per minute (secondary analysis of the HORIZON-HF study, #NCT00616161) (Gheorghiade et al., 2008). Blood samples were taken before, during, and up to 18 hours after starting the infusion. The lowest concentration resolved by the technique was 2.6 ng·mL−1 for istaroxime and 2.9 ng·mL−1 for PST3093; lower values were considered as zero. PKs parameters were estimated using the dedicated software Kinetica (version 4.4, Thermo Electron Corp., Waltham, Massachusetts). Samples were excluded from the analysis if contamination was suspected or ≥2 consecutive samples were missing. The following PKs parameters were estimated: the maximum observed concentration (Cmax), the time of Cmax (Tmax), the elimination half-life time (T0.5), and the area under the concentration curve from the start of istaroxime administration to the time of final sampling (AUClast).
SERCA and Na+/K+ ATPase Activities in Cell-Free Preparations
Total ATPase activity was assessed by measuring the rate of 32P-ATP release (μmol·min−1) at 37°C.
The inhibitory effect of compounds on Na+/K+ ATPase activities was tested, at multiple concentrations, on suspensions of the enzyme α1 isoform from dog kidney by measuring 32P-ATP hydrolysis as previously described (Ferrandi et al., 1996). Na+/K+ ATPase activity was identified as the ouabain (1 mM)-sensitive component of total one; compound efficacy was expressed as IC50.
Measurements on SERCA2a activities were performed in whole tissue homogenates (rat) or in cardiac SR–enriched microsomes (guinea pig), including SERCA2a and PLN (Torre et al., 2022). To test for PLN involvement in the effect of compounds, SERCA1 activity was also measured in PLN-free microsomes (from guinea pig skeletal muscle) before and after reconstitution with the PLN1-32 inhibitory fragment at a ratio PLN:SERCA of 300:1. The SERCA component, identified as the cyclopiazonic acid (10µM)-sensitive one, was measured at multiple Ca2+ concentrations (100–2000 nM) as 32P-ATP hydrolysis (Micheletti et al., 2007), and Ca2+ dose-response curves were fitted to estimate SERCA Vmax (μmol·min−1.mg−1 protein) and Ca2+ dissociation constant (KdCa; nM). Either an increase of Vmax or a decrease of KdCa (increased Ca2+ affinity) stand for enhancement of SERCA function.
Off-Target Actions
To predict potential off-target actions of PST3093, its interaction with a panel of 50 ligands, potentially relevant to off-target effects, was carried out by Eurofins (Taiwan) on crude membrane preparations according to Eurofins described procedures. PST3093 was tested at the concentration of 10 μM.
Na+/K+ ATPase Current and Intracellular Ca2+ Dynamics in Healthy and Diseased Myocytes
To ensure stabilization of drug effect, isolated myocytes were analyzed after incubation with PST3093 or vehicle (control) for at least 30 minutes. Difference between means was thus tested by group comparison. The experiments were performed at 35°C.
Na+/K+ ATPase current (INaK) was recorded (V-clamp) in isolated rat left ventricular (LV) myocytes as the holding current at −40 mV under conditions enhancing INaK and minimizing contamination by other conductances (Rocchetti et al., 2003; Torre et al., 2022). INaK inhibition by the compounds was expressed as percent reduction of ouabain (1 mM)-sensitive current; efficacy was expressed as the IC50 effect.
Intracellular Ca2+ dynamics were evaluated by Ca2+-dependent fluorescence (Fluo4-AM) in isolated LV myocytes and quantified by normalized units (F/F0). Properties of voltage-induced Ca2+ transients (CaT; amplitude and decay kinetics) and caffeine-induced ones (estimating total SR Ca2+ content; CaSR) were assessed in intact myocytes, field-stimulated at 2 Hz. A caffeine puff was applied 20 seconds (electronic timing) after a V-triggered loading sequence at 2 Hz. Resting Ca2+ (Carest) was measured just before caffeine application to reflect the equilibrium value of cytosolic Ca2+. In V-clamped myocytes, SR Ca2+ uptake rate was evaluated through an “SR loading” voltage protocol, specifically devised to examine the system at multiple levels of SR Ca2+ loading and to rule out Na+/Ca2+ exchanger (NCX) contribution. Current through L-type Ca2+ channel (ICaL) was simultaneously recorded, and the excitation-release (ER) “gain” was calculated as the ratio between CaT amplitude and Ca2+ influx through ICaL up to CaT peak (Rocchetti et al., 2005) (protocol in Supplemental Fig. 1).
Electrical Activity in Healthy Myocytes
Action potentials (AP) were recorded by patch clamp (I-clamp) from isolated guinea pig LV myocytes, selected because of the AP similarity to the human one (Odening et al., 2021), under Tyrode superfusion. Action potential duration (APD) at 50% and 90% repolarization (APD50 and APD90, respectively) and diastolic potential (Ediast) were measured 1) during steady-state pacing at several rates and 2) dynamically upon stepping between two rates (APD90 adaptation). During steady-state pacing, short-term APD90 variability (STV) was calculated from 20–30 subsequent APD90 values according to eq. 1 (Altomare et al., 2015):
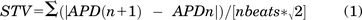
The kinetics of APD90 adaptation was quantified by the time constant (τ) of exponential data fitting.
In Vivo Hemodynamics of Diseased Hearts
Healthy and STZ rats were studied by transthoracic echocardiographic under urethane anesthesia (1.25 g·kg−1 i.p.) by means of a Mindray M9 ultrasound machine (Mindray Co, Shen Zhen, China) equipped with a P10-4s phased ultrasound transducer. LV end-diastolic (LVEDD) and end-systolic (LVESD) diameter, posterior wall thickness (PWT) and interventricular septal (IVST) thickness in systole (s) and diastole (d) were measured according to the American Society of Echocardiography guidelines (Lang et al., 2006). The Teichholz equation (7/(2.4+ D) × D3, D = LVEDD or LVESD) was used to calculate LV end-diastolic volume (EDV) and end-systolic volume (ESV). Stroke volume (SV) was calculated as the difference between EDV and ESV. LV ejection fraction (EF) was calculated as SV/EDV and expressed in percent (Tournoux et al., 2011). Fractional shortening (FS) was calculated as FS = (LVEDD-LVESD)*LVEDD−1 and expressed in percent. Transmitral flow velocity was measured (pulsed Doppler) to obtain early- and late-filling velocities (E and A waves) and E-wave deceleration time (DT). DT was also normalized to E-wave amplitude (DT/E ratio). Peak myocardial systolic (s’) and diastolic (e’ and a’) tissue velocities were measured at the mitral annulus by tissue Doppler imaging. Two-dimensional LV mass and its relative index to body weight were estimated in healthy and STZ rats.
PST3093 was intravenously infused at 0.22 mg·kg−1 (0.16 ml·min−1); echocardiographic parameters were measured before and at 15 and 30 minutes during the infusion. Istaroxime (0.22 mg·kg−1) and digoxin (0.11 mg·kg−1), both infused for 15 minutes, were used as comparators. A previous study in the same setting demonstrated that neither urethane anesthesia nor the infusion volumes per se affected echocardiographic parameters (Torre et al., 2022).
In Vivo Acute Toxicity
Acute toxicity of PST3093 was preliminarily evaluated in male albino Swiss CD-1 mice by identifying the LD50 (mg·kg−1 body weight) at 24 hours after intravenous injection. PST3093 was dissolved in DMSO and injected at 50, 100, 200, and 250 mg·kg−1 (4 animals for each group). Higher PST3093 dose levels were not tested due to the solubility limit of the compound; control animals received the vehicle only (DMSO).
Acute toxicity of istaroxime in male CD-1 mice was also evaluated for comparison by testing intravenous dose levels of 15, 22, 27, and 33 mg·kg−1 (5 animals for each group). Control animals received the vehicle only (saline).
Statistical Analysis
Individual means were compared by paired or unpaired t test; multiple means were compared by one- or two-way ANOVA for repeated measurements (RM) plus post hoc Tukey’s multiple comparisons. Drug-induced changes in overall curve steepness were defined according to significance of the “factor X group” interaction. Data are reported as mean ± S.E.M.; P < 0.05 defined statistical significance of differences in all comparisons; NS, not significant. Number of animals (N) and/or cells (n) are shown in each figure legend.
Chemicals
Istaroxime {PST2744: [E,Z]-3-[(2-aminoethoxy)imino]-androstane-6,17-dione hydrochloride} and its metabolite PST3093 {(E,Z)-[(6-beta-hydroxy-17-oxoandrostan-3-ylidene)amino]oxyacetic acid} were synthesized and produced at Prassis Research Institute and Sigma-Tau Pharmaceutical Company and then by CVie Therapeutics Limited and WindTree Therapeutics. The batch of PST3093 used for in vitro and in vivo studies was a 1:1 mixture of oxime E:Z isomers. It was synthesized according to standard procedures, characterized by NMR spectroscopy, and its purity (about 95%) was assessed by high-performance liquid chromatography (HPLC) (Supplemental Fig. 2). Digoxin was purchased from Sigma-Aldrich.
Results
Chemical Structure of PST3093
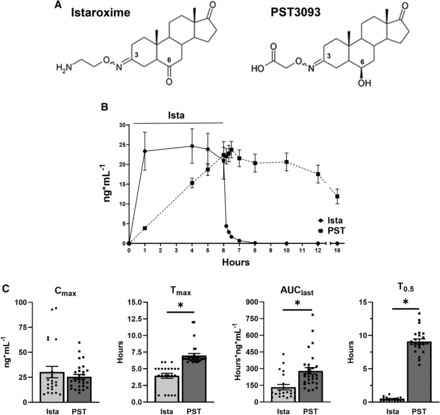
PST3093 is the final metabolite of istaroxime (Gheorghiade et al., 2008); its chemical structure is shown in Fig. 1A. Compared with istaroxime, PST3093 retains the oxime moiety at position 3 with the amino-chain oxidized into a carboxylic chain, whereas the 6-keto group of istaroxime is stereo selectively reduced to a 6β-hydroxyl group.
The chemical structure of istaroxime and its metabolite PST3093 (A) and PKs in humans (B and C). (A) The main metabolic pathways of istaroxime are reduction of the carbonyl in position 6 catalyzed by carbonyl reductases and oxidative deamination of the primary amino group catalyzed by monoamine oxidases or tissue-bound semicarbazide-sensitive amine oxidase. (B) Istaroxime (N = 22) and PST3093 (N = 29) plasma levels evaluated over 6 hours of istaroxime infusion in HF patients at 1 μg/kg per minute and up to 12 hours after discontinuation of infusion (from HORIZON-HF study, #NCT00616161). (C) Statistics of Cmax, Tmax, AUClast, and T0.5; *P < 0.05 versus istaroxime (unpaired t test). Data are the mean ± S.E.M. ista, istaroxime; PST, PST3093.
Phamacokinetics
Fig. 1B shows istaroxime and PST3093 plasma levels over a 6-hour istaroxime infusion at 1 μg·kg−1·min−1 and up to 12 hours after discontinuation of infusion in HF patients (Gheorghiade et al., 2008). All patients showed measurable istaroxime levels until 10 minutes after stopping the infusion; drug levels decreased rapidly thereafter, and just one patient had a quantifiable istaroxime level 2 hours after the end of the infusion. PST3093 plasma levels increased with a lag from the start of istaroxime infusion (as expected for a metabolite) being detectable in all patients from 1 hour after the start of infusion. Plasma PST3093 levels remained detectable long after discontinuation of the infusion, up to the last sample at 12 hours after wash out. The data suggest that, if istaroxime infusion had continued beyond 6 hours, the metabolite would have accumulated further.
In quantitative terms, PST3093 had a T0.5 of about 9 hours, i.e., substantially longer than that of istaroxime (less than 1 hour), leading to a huge enhancement of the AUClast index for PST3093; although the Cmax of PST3093 was similar to istaroxime one at this infusion rate, the Tmax was longer for PST3093 in comparison with istaroxime (Fig. 1C).
Effect of PST3093 on Na+/K+ ATPase
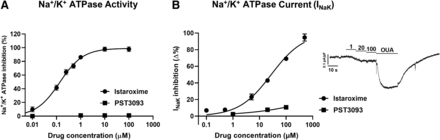
Istaroxime and PST3093 effects on Na+/K+ ATPase activity were tested in a range of concentrations from 10−9 to 10−4 M (Fig. 2A). Na+/K+ ATPase from dog kidney had a specific baseline activity of 14 μmol·min−1.mg−1 protein. The reference compound istaroxime inhibited Na+/K+ ATPase activity with IC50 of 0.14 ± 0.02 µM in dog kidney (Fig. 2A), which corresponds to a higher affinity as compared with that observed in rat renal preparations [IC50 of 55 ± 19 μM from Torre et al. (2022)]. PST3093 did not inhibit the Na+/K+ ATPase activity up to 100 µM, the maximal tested concentration (Fig. 2A).
Modulation of Na+/K+ ATPase activity. (A) Inhibition of Na+/K+ ATPase activity by istaroxime and PST3093 in dog renal preparations (N = 3). (B) Concentration-response curves for INaK inhibition by PST3093 (n = 15) and istaroxime (modified from Torre et al. (2022); this work is available under a CC-BY-NC license) in rat LV myocytes; INaK recording under increasing concentrations of PST3093 and finally to ouabain (OUA as reference) is shown on the right. Data are the mean ± S.E.M.
PST3093 effects on INaK were further evaluated in rat LV myocytes in comparison with istaroxime (Fig. 2B). The estimated IC50 for INaK inhibition by istaroxime was 32 ± 4 µM [from Torre et al. (2022)]; for PST3093, a barely detectable inhibition (9.2 ± 1.1%) was observed at the limit concentration for solubility (100 µM), an effect that can be considered insignificant.
Effect of PST3093 on SERCA ATPase Activity
Effects on SERCA2a Activity in Normal and Diseased Myocardial Preparations
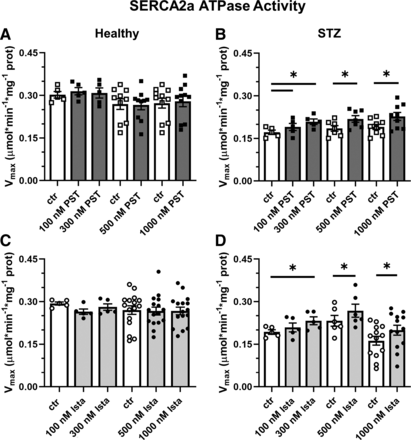
In STZ rat preparations (N = 30), baseline SERCA2a Vmax was lower (by −27%) than in healthy ones (N = 29) (0.199 ± 0.01 versus 0.272 ± 0.01 μmol*min−1*mg−1 protein, P < 0.05), with no difference in KdCa (448 ± 35 versus 393 ± 22 nM, NS), similar to what was reported recently in the same setting (Torre et al., 2022). As also reported previously (Ferrandi et al., 2013), the response of enzyme kinetics parameters to modulation was species specific: whereas in rat preparations, both PST3093 and istaroxime (Fig. 3) increased Vmax, in guinea pig ones the compounds decreased KdCa instead (Supplemental Table 1).
Modulation of SERCA2a ATPase activity in healthy and diseased (STZ) preparations. Effect of PST3093 (N = 5–11) (A and B) and istaroxime (N = 5–16) (C and D) on SERCA2a Vmax estimated from Ca2+-dose response curves in cardiac homogenates from healthy and diabetic (STZ) rats. Internal controls (ctr) are provided. Data are the mean ± S.E.M. *P < 0.05 versus ctr (RM one-way ANOVA plus post hoc Tukey’s multiple comparisons test or paired t test).
Over the whole range of concentration tested (0.1–1 µM), PST3093 and istaroxime failed to affect ATPase Ca2+ dependency in healthy rat preparations (Fig. 3, A and C) but similarly increased Vmax in STZ ones (e.g., +22% and +20%, respectively, at 300 nM) with thresholds at 100 nM and 300 nM for PST3093 and istaroxime, respectively (Fig. 3, B and D); SERCA2a KdCa in rat preparations was affected by neither istaroxime nor PST3093. Thus, PST3093 and istaroxime displayed similar potency in ameliorating disease-induced depression of SERCA2a ATPase activity.
PST3093 and istaroxime effect was also detected in preparations from normal guinea pig hearts, where they both reduced the SERCA2a KdCa value by about 20% at 100 nM (Supplemental Table 1).
To summarize, PST3093 and istaroxime equally stimulated SERCA2a ATPase activity in preparations including PLN. Regardless of the kinetic parameter affected, a stimulatory effect was present in healthy guinea pig microsomes and in rat homogenates from the STZ disease model.
Dependency of SERCA Stimulation on PLN
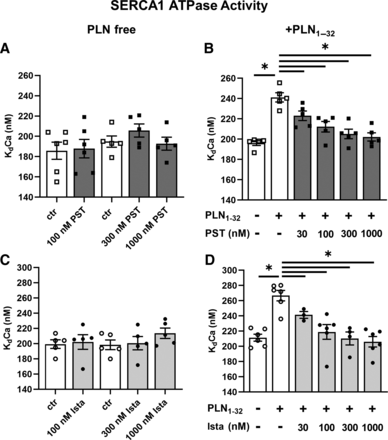
In a range of concentrations from 30 to 1000 nM, PST3093 and istaroxime failed to affect SERCA1 activity in the absence of PLN in skeletal muscle preparations (Fig. 4, A and C). Reconstitution with the PLN1-32 fragment markedly reduced SERCA1 affinity for Ca2+ (KdCa increased by 23%–26%) (Fig. 4, B and D). Under this condition, both PST3093 (Fig. 4B) and istaroxime (Fig. 4D) dose-dependently reversed PLN-induced shift in KdCa with an EC50 of 39 nM and 40 nM, respectively.
Modulation of SERCA1 ATPase activity. Concentration dependency of PST3093 and istaroxime modulation of SERCA1 KdCa in guinea pig skeletal muscle microsomes containing SERCA1 alone (A and C) (N = 5–6) and after reconstitution with the PLN1-32 fragment (B and D) (N = 5–6). Data are the mean ± S.E.M. *P < 0.05 (RM or mixed-model one-way ANOVA plus post hoc Tukey’s multiple comparisons).
PST3093 Interaction with Targets other than SERCA
The targets panel (50 items) included membrane receptors, key enzymes, ion channels, and transporters relevant to potential off-target cardiac and extracardiac effects (list in Supplemental Table 2); PST3093 was tested at the concentration of 10 μM. None among the 50 items met criteria for significance of interaction. Thus, at least for the ligands shown in Supplemental Table 2, no off-target action of PST3093 is expected.
Effects of PST3093 on Intracellular Ca2± Dynamics in Cardiac Myocytes
Global Effects in Field Stimulated Myocytes
Ca2+ dynamics were analyzed in field-stimulated (2 Hz, Fig. 5) rat LV myocytes isolated from healthy or STZ rats.
Modulation of intracellular Ca2+ handling in field-stimulated myocytes from healthy and diseased (STZ) hearts. (A) Representative recordings of CaT triggered by steady-state electrical stimulation at 2 Hz in intact cells, followed after 20 seconds by caffeine-induced Ca2+ release measuring CaSR. PST3093 (1 µM) was tested in normal (healthy) and STZ myocytes. (B) Statistics for CaT amplitude (ampl), CaSR, Carest, and time for 50% CaT decay (t0.5). CTR N = 3 (n = 28 without PST3093; n = 31 with PST3093), STZ N = 4 (n = 28 without PST3093; n = 30 with PST3093). *P < 0.05 (one-way ANOVA plus post hoc Tukey’s multiple comparisons).
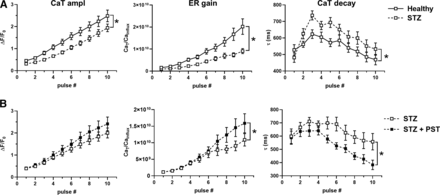
STZ myocytes had a lower CaSR and a slower CaT decay than healthy ones; however, CaT amplitude remained unchanged. These changes are compatible with reduced SERCA2a function, possibly compensated by APD prolongation, known to increase cell Ca2+ content (Torre et al., 2022). Whereas in healthy myocytes, 1 µM PST3093 failed to affect any of the Ca2+ dynamics parameters, in STZ myocytes PST3093 reduced the quiescence Ca2+ (Carest) prior to caffeine application and partially restored CaSR and CaT decay. Moreover, PST3093 restored in STZ myocytes the distribution of CaSR values peculiar of healthy ones. Comparable results have been obtained with istaroxime at a concentration marginally affecting Na+/K+ ATPase (Torre et al., 2022). Taken together, this observation suggests that PST3093 improved Ca2+ sequestration into the SR during the post-train quiescence period.
Effects on SR Ca2+ Uptake Function under NCX Inhibition
STZ-induced changes in repolarization affect Ca2+ handling in a direction masking SERCA2a downregulation (Torre et al., 2022). Thus, the “SR loading” protocol (see Methods and Supplemental Fig. 1) was performed under V-clamp and used to assess SR Ca2+ uptake under conditions emphasizing SERCA2a role (NCX inhibition). In STZ myocytes, as compared with healthy ones, SR reloading was significantly depressed (both in terms of CaT amplitude and ER gain), and CaT decay was slower at all timepoints during reloading (Fig. 6A). These changes are compatible with depressed SERCA2a function (Torre et al., 2022). PST3093 (1 µM) was tested in STZ myocytes (Fig. 6B), where it sharply accelerated CaT decay, restoring the profile observed in healthy myocytes. Albeit less evident, drug-induced changes in CaT and ER gain pointed in the same direction. Comparable results have been obtained with istaroxime at a concentration marginally affecting Na+/K+ ATPase (Torre et al., 2022).
Modulation of SR Ca2+ uptake under NCX inhibition in V-clamped myocytes from STZ hearts. (A) Disease (STZ) effect on SR Ca2+ loading in patch-clamped myocytes. SR Ca2+ loading by a train of V-clamp pulses was initiated after caffeine-induced SR depletion; NCX was blocked by Na+ substitution to identify SERCA2a-specific effects (see Methods and Supplemental Fig. 1); myocytes from healthy (N = 9; n = 32) and diseased hearts (N = 6; n = 31) are compared. (B) PST3093 effect in STZ myocytes (N = 4; without PST3093 n = 18, with PST3093 n = 19). Panels from left to right: CaT amplitude, ER gain (the ratio between CaT amplitude and Ca2+ influx through ICaL), and τ of CaT decay. *P < 0.05 for the “interaction factor” in RM two-way ANOVA, indicating a different steepness of curves.
Overall, PST3093 restored SR function in diseased myocytes, i.e., the context of the pathologic cellular environment, most likely through SERCA2a enhancement.
Effects of PST3093 on Cellular Electrical Activity
To assess the electrophysiological safety of PST3093, its effects on AP of LV myocytes were investigated. Guinea pig myocytes were used instead of rat ones because their AP is closer to the human one.
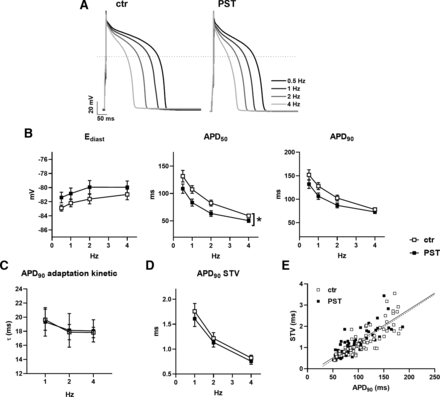
PST3093 (100 nM) marginally reduced APD50 at all pacing rates, leaving the other AP parameters unchanged (Fig. 7, A and B). Notably, also APD rate dependency at steady-state and the kinetics of APD adaptation following a step change in rate were unaffected by the agent (Fig. 7C). STV of APD90, a reporter of repolarization stability, was also unaffected by PST3093 at all pacing rates (Fig. 7, D and E). Except for the absence of APD50 reduction, similar results were obtained with PST3093 at 1 µM (Supplemental Fig. 3).
Modulation of electrical activity in guinea pig myocytes. The effect of 100 nM PST3093 was tested on AP parameters and their steady-state rate dependency in guinea pig myocytes (N = 5). (A) representative APs recorded at 0.5, 1, 2, and 4 Hz in control (left) and with 100 nM PST3093 (right). (B) Effect on the rate dependency of diastolic potential (Ediast) and AP duration (APD50, APD90) (n ≥ 24 without PST3093; n ≥ 22 with PST3093). (C) Effect on the τ of APD90 adaptation following a step change in rate (n ≥ 22 without PST3093; n ≥ 16 with PST3093). (D) Effect on the rate dependency of APD90 STV (n ≥ 24 without PST3093; n ≥ 20 with PST3093). (E) Effect on the correlation between STV of APD90 and APD90 values; data from 1, 2, and 4 Hz were pooled. *P < 0.05 for the “interaction factor” of RM two-way ANOVA. The effect of PST3093 at a higher concentration (1 µM) is reported in Supplemental Fig. 3.
The paucity of PST3093 effects on the AP is consistent with the absence of hits in the analysis of PST3093 interaction (up to 10 μM) with molecular targets other than SERCA2a, among them ion channels and transporters (Supplemental Table 2).
In Vivo Acute Toxicity in Mice
In vivo toxicity after intravenous injection was investigated in mice for PST3093 and istaroxime. PST3093 was well tolerated and did not cause death up to 250 mg/kg. The LD50 was not calculated since no deaths occurred at the maximal usable dose (limited by solubility). The LD50 for istaroxime was 23.06 mg/kg. The main signs of toxicity were prostration, gasping, and convulsions. In most of the animals, death occurred within 5 minutes after istaroxime administration. Postmortem examination revealed pulmonary edema and/or hemorrhages and generalized organ congestion. No remarkable alterations were found in the surviving animals.
Therefore, when intravenously administered, PST3093 was far less toxic than istaroxime, a result ascribable to its lack of effects on the Na+/K+ ATPase.
Modulation of Cardiac Function in Vivo in Rats with Diabetic Cardiomyopathy
In vivo cardiac function was evaluated by echocardiography. In light of the uncertainty inherent to the mechanistic interpretation of drug effect on individual echo indexes, this set of experiments was designed to test whether PST3093 was able to reverse the derangements peculiar of the STZ disease model. To this end, the STZ model had to be first characterized.
Features of the Disease Model
Fasting hyperglycemia, polydipsia, polyuria, and polyphagia ensued 1 week after STZ injection; none of these symptoms was observed in healthy rats. Eight weeks after STZ, total body weight was substantially lower in STZ rats; LV mass (by echo) was reduced in absolute value but, when normalized to body weight, was not significantly different in STZ rats (Supplemental Table 3). The STZ model was largely characterized in a previous work of ours (Torre et al., 2022).
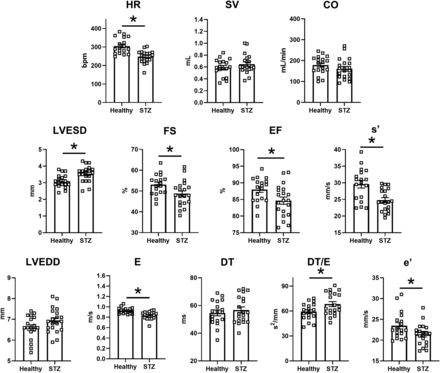
In this study, a comprehensive echocardiographic analysis of STZ rats in comparison with healthy ones was performed. Figure 8 compares some echo indexes in STZ versus healthy rats; Supplemental Table 4 lists all the measured echo parameters in the two groups. Heart rate (HR) was lower in STZ rats (−20%; P < 0.05), and SV was unchanged; nonetheless, differences in cardiac output (CO) between the two groups did not achieve significance. Systolic indexes: in STZ rats, LVESD was larger, and the EF was reduced; FS and systolic tissue velocity (s’) were depressed. Diastolic indexes: in STZ rats, LVEDD tended to be larger; peak E-wave velocity (E) was slightly smaller, and A-wave velocity (A) was unchanged (E/A unchanged); E-wave DT was unchanged in absolute, but the DT/E ratio was increased. Changes in early (e’) and late (a’) diastolic tissue velocities paralleled those in E and A waves; therefore, the e’/a’ and E/e’ ratios did not differ between STZ and healthy rats.
Disease (STZ) effects on in vivo echocardiographic parameters. Echocardiographic parameters are compared between healthy rats (N = 18) and 8 weeks after STZ treatment (N = 20). Top row, global function parameters; mid row, systolic function parameters; bottom row, diastolic function parameters: *P < 0.05 (unpaired t test).
To summarize, in STZ rat, echocardiographic abnormalities were rather subtle; nonetheless, 11 out of 21 indexes were significantly affected, indicating derangements in both systolic and diastolic function.
Drug Effects in the Disease Model
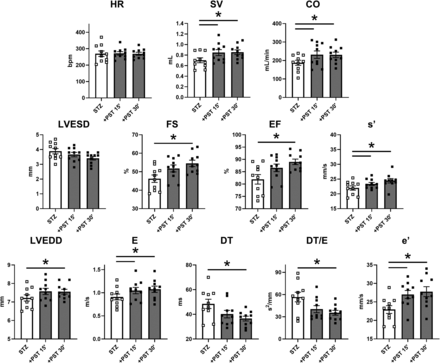
The in vivo acute effect of PST3093 (0.22 mg·kg−1.min−1) on echo indexes of STZ rats was investigated at 15 and 30 minutes of infusion (Fig. 9). Data at 15 minutes were also obtained with istaroxime (0.22 mg·kg−1.min−1) and digoxin (0.11 mg·kg−1.min−1) (Table 2). Overall, PST3093 increased SV volume and CO, without changing HR (Fig. 9). Systolic indexes: PST3093 tended to decrease LVESD and increased FS, EF, and s’. Diastolic indexes: PST3093 increased LVEDD, E, A (at 30 minutes), and e’ (E/A and E/e’ unchanged, Table 2); DT and DT·/E were reduced. PST3093 effect was almost complete at 15 minutes of infusion; only minor increments were observed at 30 minutes (Fig. 9). Collectively, PST3093 improved overall cardiac function, both systolic and diastolic, beyond simple recovery of STZ-induced derangements. As shown in Fig. 9, PST3093 “reversed” STZ-induced changes in 7 out of 11 indexes; moreover, five additional indexes, unaffected by STZ, were changed by PST3093 in a direction compatible with positive inotropy/lusitropy (Table 2).
Effects of PST3093 (0.22 mg.kg−1.min−1), istaroxime (0.22 mg.kg−1.min−1), and digoxin (0.11 mg.kg−1.min−1) on echo indexes of STZ rats at 15 minutes of infusion Data are the mean ± S.E.M.
PST3093 effects on in vivo echocardiographic parameters in diseased (STZ) rats. PST3093 was intravenously infused (0.22 mg.kg−1.min−1) in rats 8 weeks after STZ treatment. Echocardiographic parameters were measured before and at 15 and 30 minutes during drug infusion. Rows and symbols as in Fig. 8; N = 10 rats. Data are the mean ± S.E.M. *P < 0.05 (RM one-way ANOVA plus post hoc Tukey’s multiple comparisons). Effects of PST3093 on all echocardiographic parameters at 15 minutes of infusion in STZ rats in comparison with istaroxime and digoxin are reported in Table 2.
PST3093 effects were only partially shared by istaroxime at the same infusion rate (Table 2). Istaroxime failed to increase SV and systolic indexes (SV, FS, EF, s’); it increased CO, but, at variance with PST3093, this was because HR increased. Similar to PST3093, istaroxime shortened DT and DT/E and increased e’, a’, and A; however, it did not change E, thus reducing E/e’. Digoxin (Table 2), as expected from its inotropic effect, increased systolic indexes (FS, EF, and s’); however, at variance with PST3093, it did not affect SV or CO. Notably, digoxin also improved diastolic function to some extent: E/A, DT, and DT/E were reduced, and e’ was increased.
Discussion
In the present study, we have investigated the effects of the istaroxime metabolite PST3093 at molecular, cellular, and in vivo levels. The interest in this molecule is motivated by 1) the possibility that it may actually contribute to (i.e., be endowed with) the unique mechanism of action and interesting therapeutic profile of istaroxime (inotropy and lusitropy at low proarrhythmic risk, confirmed in phase 2 clinical trials) (Sabbah et al., 2007; Gheorghiade et al., 2008; Shah et al., 2009; Carubelli et al., 2020) and 2) the possibility that it would afford to test the clinical benefit associated specifically with the rescue of SERCA2a depression, which is widely recognized as the basis for many among HF abnormalities.
PST3093 effect has been tested in three experimental settings with incremental levels of biologic organization, including in vivo measurements from diseased hearts. The consistency of effects across these three sets of experiments confers robustness to the findings.
The results of molecular studies indicate that PST3093 differs from istaroxime because it is devoid of any inhibitory activity on the Na+/K+ ATPase while retaining SERCA2a stimulatory action. This identifies PST3093 as a “selective” SERCA2a activator. The results also indicate that, similar to istaroxime (Ferrandi et al., 2013), PST3093 may act by weakening SERCA-PLN interaction. In rat preparations, PST3093 (and istaroxime) increased SERCA2a Vmax. Although this apparently conflicts with the notion that interference with PLN should decrease the KdCa instead (Brittsan et al., 2003), the same pattern coexisted with evidence, by several independent approaches, of istaroxime antagonism of SERCA2a-PLN interaction (Ferrandi et al., 2013). The reason for this apparent discrepancy is unclear; the observation that PST3093 effect on SR Ca2+ uptake was present in rat cardiac myocytes (i.e., at physiologic Ca2+ concentrations) suggests that it may reside in specificities of the microsomal preparation.
Investigations in intact ventricular myocytes confirm a negligible effect of PST3093 on Na+/K+ pump function. Furthermore, PST3093 abolished STZ-induced intracellular Ca2+ abnormalities likely dependent on SERCA2a downregulation. Although PST3093 clearly affected several Ca2+ cycling parameters under V-clamp conditions (Fig. 6), its effects in field-stimulated cells were apparently small (Fig. 5). As we have previously shown in this experimental model (Torre et al., 2022), evaluation of Ca2+ handling without controlling membrane potential (field stimulation) and without disabling competing mechanisms (NCX) may be unsuitable to detect SERCA2a activation. This is why we also performed experiments under V-clamp and with disabled NCX function, shown in Fig. 6. Field-stimulation experiments are nonetheless informative because they better represent drug effects at the cell level under “physiological” conditions. In particular, the reduction in Carest indicates that PST3093 allows the other Ca2+ cycling parameters to be preserved (or even showing a trend to improve) at a lower cytosolic Ca2+ level. Considering that all Ca2+ homeostatic mechanisms are in place in this setting, this is precisely what should be expected from pure SERCA2a activation (Alemanni et al., 2011; Zaza and Rocchetti, 2015), i.e., improved subcellular Ca2+ compartmentalization. That this apparently small change in myocyte physiology has an impact on in vivo cardiac performance is shown by the in vivo echo measurements (Fig. 9).
The present in vivo studies were conducted by echocardiography in a disease model characterized by impairment of SERCA2a function (Choi et al., 2002; Torre et al., 2022). In this model, PST3093 infusion improved overall cardiac performance (SV and CO); both systolic and diastolic indexes were positively affected by the agent. Mechanistic interpretation of echocardiographic indexes is often ambiguous. For instance, both DT prolongation and shortening have been associated with deterioration of diastolic function (Eren et al., 2004; Sabbah et al., 2007). These puzzling observations can be interpreted by considering the contribution to DT of opposing factors, each prevailing in a specific condition (Mossahebi et al., 2015). At any rate, whenever HF was associated with DT shortening, istaroxime (having PST3093 as a metabolite) prolonged it (Sabbah et al., 2007; Shah et al., 2009). A further difficulty may arise from the expectation that ino-lusitropy may increase atrial contraction (A amplitude) and ventricular relaxation (E amplitude) at the same time, thus conceivably making their ratio (E/A) unable to detect drug effects on diastolic function. A similar consideration applies to the E/e’ index. An approach to the interpretation of drug effects, independent of mechanistic models, is to check whether the drug counters disease-induced abnormalities. In the case of PST3093, this was true for the majority of indexes (7 out of 11), the most notable exception being a small further increase in LVEDD. Although increments in LVEDD are usually associated with deterioration of systolic function, PST3093 tended to decrease LVESD instead. The LVEDD increment was indeed associated with increased SV and EF, to which it likely contributed.
With the exception of guinea pigs, PST3093 efficacy on SERCA2a function in the diseased condition consistently contrasted with the lack of effect in healthy preparations (Figs. 3 and 5). This suggests that SERCA2a function, although not strictly limiting in health, may become so whenever its “reserve” is diminished. This view may not clash with the clear-cut effect of PLN knockout in healthy murine myocytes; indeed, SERCA2a modulation by PST3093 may be, albeit functionally significant, subtler than complete PLN ablation.
Although failing to increase the amplitude of CaT, PST3093 improved echo indexes of systolic function. Mechanisms at two levels may account for this observation: at the intracellular level, increased compartmentation of Ca2+ within the SR may improve the energetic efficiency of Ca2+ cycling (Shannon et al., 2001); at the organ level, improved relaxation may increase preload, with its well known impact on systolic force (Shiels and White, 2008). Indeed, normalization of diastolic function in HF patients with preserved EF may restore CO irrespective of changes in the latter (Tobushi et al., 2017). On the other hand, digoxin, whose mechanism of action is purely inotropic, accelerated early relaxation (DT shortening and e’ increase). This is consistent with systo-diastolic coupling, i.e., the contribution to early relaxation of elastic restitution (recoil) of systolic force (Burns et al., 2009).
Besides affording inotropy and lusitropy, SERCA2a stimulation may improve intracellular Ca2+ compartmentalization, with potential long-term effects on energetic efficiency and biology of cardiac myocytes (Zaza and Rocchetti, 2015).
PST3093 is remarkably less toxic than istaroxime, which, in turn, has a lower proarrhythmic risk as compared with digoxin (Micheletti et al., 2002). We speculate that the low PST3093 toxicity, relative to istaroxime, may be due to its failure to inhibit the Na+/K+ pump. The absence of interaction with 50 cardiac and noncardiac targets commonly involved in drug toxicity provides at least a first-level evidence of PST3093 suitability as a therapeutic agent.
Limitations
Whereas in the in vivo experiments, PST3093 effects generally achieved a maximum at 30 minutes of infusion, the istaroxime infusion period was limited to 15 minutes. Our previous study (Torre et al., 2022) indicates that a 15-minute infusion is sufficient for modulation of diastolic parameters by istaroxime. This timepoint was selected in the present study to minimize metabolism to PST3093, thus allowing it to differentiate istaroxime’s own effect from that of its metabolite. Nonetheless, istaroxime effects reported here might differ from the steady-state ones described in previous studies (Sabbah et al., 2007; Shah et al., 2009; Carubelli et al., 2020), to which PST3093 (the metabolite) might actually contribute.
Echo parameters are sensitive to changes in blood pressure, which was not directly measured. Nonetheless, previous in vivo studies ruled out the effect of infused isatroxime and, implicitly, of PST3093 on pulmonary and peripheral resistances (Gheorghiade et al., 2008).
Translation of the present in vivo results to human therapy has to consider differences between clinical HF and the STZ rat model, which has specific hemodynamic features (Mihm et al., 2001). However, consistency of the istaroxime effect reported here with that described in HF patients (Carubelli et al., 2020) supports this translation.
Therapeutic Relevance and Perspective
The results of this study identify PST3093 as a prototype “selective” (i.e., devoid of Na+/K+ pump inhibition) SERCA2a activator. This may entail significant differences from the already characterized pharmacodynamic profile of istaroxime.
In the case of istaroxime, lack of the proarrhythmic effect [expected from Na+/K+ ATPase inhibition (Rocchetti et al., 2005)] is likely due to SERCA2a stimulation. Indeed, the latter may reduce the occurrence of “Ca2+ waves” and the resulting “triggered activity” (Bai et al., 2013; Zaza and Rocchetti, 2015; Fernandez-Tenorio and Niggli, 2018). It is logical to predict that a pure SERCA2a activator may exert substantial antiarrhythmic effects, at least under the common conditions characterized by SR instability (e.g., HF). On the other hand, Na+/K+ pump inhibition may contribute to inotropy; thus, at least theoretically, PST3093 should increase systolic force less than istaroxime. The present results argue for a PST3093 effect on global cardiac function, including positive inotropy. Moreover, compared with istaroxime, PST3093 has a much longer half-life that, per se, may also prolong the beneficial hemodynamic effect of istaroxime infusion.
Overall, PST3093 acting as “selective” SERCA2a activator can be considered the prototype of a novel pharmacodynamic class for the ino-lusitropic approach of HF. After more than 50 years from the suggestion of the involvement of a reduced SERCA2a function as a cause of the depressed cardiac function and the increased arrhythmias in HF, we may have the possibility to prove this hypothesis and provide a “causal” and selective therapy for HF patients.
Authorship Contributions
Participated in research design: Peri, Ferrari, Bianchi, Rocchetti, Zaza.
Conducted experiments: Arici, Ferrandi, Barassi, Hsu, Chang.
Performed data analysis: Arici, Ferrandi, Hsu, Torre, Luraghi, Ronchi.
Wrote or contributed to the writing of the manuscript: Rocchetti, Zaza.
Footnotes
- Received May 30, 2022.
- Accepted August 1, 2022.
This research was supported by CVie Therapeutics Limited (Taipei, Taiwan), WindTree Therapeutics (Warrington, PA), and University of Milano Bicocca. This work received no external funding.
M.F. and P.B. are Windtree employees, P.F. and G.B. are Windtree consultants, and S.-C.H. is an employee of CVie Therapeutics Limited. All the other authors declare no conflicts of interest.
↵1 M.A. and M.F. contributed equally to this work as first authors.
↵2 M.R. and A.Z. contributed equally to this work as senior authors.
↵
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
Abbreviations
- a′
- peak late diastolic tissue velocity
- A
- late diastolic transmitral flow velocity
- AP
- action potential
- APD
- action potential duration
- APD50
- AP duration at 50% repolarization
- APD90
- AP duration at 90% repolarization
- AUClast
- the area under the curve from the start of drug administration to the time of final sampling
- Carest
- resting Ca2+ at 20-second pause end
- CaSR
- SR Ca2+ content
- CaT
- Ca2+ transient
- CO
- cardiac output
- DT
- deceleration time
- e′
- peak early diastolic tissue velocity
- E
- early diastolic transmitral flow velocity
- Ediast
- diastolic potential
- EDV
- end-diastolic volume
- EF
- ejection fraction
- ER
- excitation-release
- ESV
- end-systolic volume
- FS
- fractional shortening
- HF
- heart failure
- HR
- heart rate
- ICaL
- L-type Ca2+ current
- INaK
- Na+/K+ ATPase current
- IVSTd
- interventricular septal thickness in diastole
- IVSTs
- interventricular septal thickness in systole
- KdCa
- Ca2+ dissociation constant
- LV
- left ventricle
- LVEDD
- LV end-diastolic diameter
- LVESD
- LV end-systolic diameter
- N
- number of animals
- n
- number of cells
- NCX
- Na+/Ca2+ exchanger
- PK
- pharmacokinetic
- PLN
- phospholamban
- PWTd
- posterior wall thickness in diastole
- PWTs
- posterior wall thickness in systole
- RM
- repeated measurement
- SERCA
- sarcoplasmic reticulum Ca2+ ATPase
- SR
- sarcoplasmic reticulum
- STV
- short-term variability
- STZ
- streptozotocin
- SV
- stroke volume
- s′
- peak systolic tissue velocity
- t0.5
- time for 50% Ca2+ transient decay
- T0.5
- drug elimination half-life time
- Tmax
- the time of maximum observed concentration
- τ
- time constant
- Copyright © 2022 by The Author(s)
This is an open access article distributed under the CC BY-NC Attribution 4.0 International license.