Abstract
(E,Z)-3-((2-Aminoethoxy)imino)androstane-6,17-dione hydrochloride (PST2744) is a novel Na+/K+ pump inhibitor with positive inotropic effects. Compared with digoxin in various experimental models, PST2744 was consistently found to be less arrhythmogenic, thus resulting in a significantly higher therapeutic index. The present work compares the electrophysiological effects of PST2744 and digoxin in guinea pig ventricular myocytes, with the aim to identify a mechanism for their different toxicity. The work showed that 1) the action potential was transiently prolonged and then similarly shortened by both agents; 2) the ratio between Na+/K+ pump inhibition and inotropy was somewhat larger for PST2744 than for digoxin; 3) both agents accelerated inactivation of high-threshold Ca2+ current (ICaL), without affecting its peak amplitude; 4) the transient inward current (ITI) induced by a Ca2+transient in the presence of complete Na+/K+pump blockade was inhibited (−43%) by PST2744 but not by digoxin; 5) the conductance of Na+/Ca2+ exchanger current (INaCa), recorded under Na+/K+ pump blockade, was only slightly inhibited by PST2744 (−14%) and unaffected by digoxin; and 6) both agents inhibited delayed rectifier currentIKs (≤−21%); delayed rectifier currentIKr was inhibited by PST2744 only, but the effect was marginal (−6%). Thus, 1) the higher therapeutic index of PST2744 may be accounted for by inhibition ofITI, a current directly involved in digitalis-induced arrhythmias. Indeed, the other differences observed concern quantitatively small effects; and 2)ITI suppression by PST2744 may be only partly accounted for by inhibition of the Na+/Ca2+ exchanger.
The focus of heart failure therapy has recently evolved from direct support of cardiac inotropy to the prevention of “maladaptive” responses underlying the evolution of myocardial damage. Nonetheless, direct inotropic support remains a primary need in the management of patients with overt heart failure (Eichhorn and Gheorghiade, 2002) and may even be a prerequisite to establish therapies (e.g., β-blockers and angiotensin converting enzyme inhibitors) potentially associated with initial hemodynamic deterioration. Inotropic interventions based on positive modulation of cAMP concentration, albeit suitable for acute treatment, cannot be used chronically because they increase the risk of sudden death (Cohn et al., 1998). This explains why, in spite of their narrow therapeutic range, cardiac glycosides remain a valuable tool in the chronic treatment of symptomatic heart failure, even when sinus rhythm is preserved (The Task Force of the Working Group on Heart Failure of the European Society of Cardiology, 1997). In particular, the use of digoxin is justified by its ability to reduce hospitalization for relapses and improve patients' functional state (The Digitalis Investigation Group, 1997). Although suggestive of hemodynamic amelioration, these effects are not associated with a decrease in mortality rate; conceivably, this reflects the proarrhythmic effect of cardiac glycosides.
Inhibition of the Na+/K+pump and the resulting increase in intracellular Ca2+ are commonly held to underlie both inotropy and proarrhythmia, thus making the two actions apparently inseparable (Schwartz and Noble, 2001; Bers, 2002). This view is challenged by the observation that the ratio between inotropic and toxic effect may vary widely among different Na+/K+ pump inhibitors (Wasserstrom et al., 1991).
A sharp dissociation between inotropy and proarrhythmia has been recently observed with a novel nonglycoside Na+/K+ pump inhibitor, (E,Z)-3-((2-aminoethoxy)imino)androstane-6,17-dione hydrochloride (PST2744). In single cell, tissue, and whole animal studies the incidence of arrhythmias, observed at similar inotropy, was markedly lower for PST2744 than for the reference compound digoxin (Micheletti et al., 2002).
The present study compares the effects of PST2744 and digoxin on the electrical activity and relevant membrane currents of guinea pig ventricular myocytes. The aim of such evaluation is to detect differences in the actions of the two agents that might account for their widely distinct therapeutic index. To this end, except when dose-response curves were obtained, drug effects were compared at concentrations of the two agents exerting similar inotropic effects (equi-inotropic concentrations) as determined by previous experiments on single guinea pig myocytes (Micheletti et al., 2002).
Materials and Methods
Myocyte Preparation
Guinea pig ventricular myocytes were isolated by using a retrograde coronary perfusion method previously published except for minor modifications (Zaza et al., 1998). The investigation conforms to the Guide of the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication 85-23, revised 1996); all experiments were carried out according to the guidelines issued by the Animal Care Committee of the University of Milan. In brief, female guinea pigs weighing 200 to 300 g were anesthetized with a xylazine (1.5 mg/kg b.wt.) and ketamine (20 mg/kg b.wt.) mix, killed through cervical dislocation, and exsanguinated. Hearts were quickly removed and perfused in sequence with 1) modified Tyrode's solution (37°C) containing 130 mM NaCl, 4.5 mM KCl, 0.75 mM CaCl2, 5 mM MgCl2, 23 mM HEPES-NaOH, 21 mM d-glucose, 1 mM NaH2PO4, 20 mM taurine, 5 mM creatine, and 5 mM pyruvate, adjusted to pH 7.3, and equilibrated with 100% O2; 2) a nominally Ca2+-free Tyrode's solution containing 3.3 μM EGTA and adjusted to pH 7.0; 3) a 0.075 mM CaCl2Tyrode's solution containing 140 U/ml collagenase (type 2; Worthington Biochemicals, Freehold, NJ), 0.17 U/ml protease (type XIV; Sigma-Aldrich, St. Louis, MO), and bovine serum albumin (1 mg/ml). The ventricles were then chopped at 37°C and triturated in nominal Ca2+-free solution adjusted to pH 7.3. The supernatant was collected every 5 min, filtered through a nylon mesh, and centrifuged at 500 rpm for 3 min. To separate myocyte and nonmyocyte cells, the pellets were resuspended (50%, v/v) in a continuous Percoll gradient containing 0.9% NaCl and centrifuged at 500 rpm for 15 min. Finally, isolated myocytes were resuspended in 1 mM CaCl2 Tyrode's solution containing gentamycin (10 μg/ml) and stored at room temperature until use. Rod-shaped, Ca2+-tolerant myocytes, obtained with this procedure, were used within 12 h from dissociation. Measurements were performed only in quiescent myocytes with clear-cut striations.
Recording Techniques
Myocytes suspension was placed in a 30-mm Petri dish, with a plastic ring to reduce total volume to ≈1 ml. The dish was perfused at 2 ml/min with standard external Tyrode's solution, containing 154 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 5 mM HEPES-NaOH, and 5.5 mMd-glucose, adjusted to pH 7.35. The cell under study was held within 300 μm from the tip (1 mm) of a thermostated multiline pipette allowing for rapid switch between solutions. The solution temperature was monitored at the pipette tip with a fast-response digital thermometer (BAT-12; Physitemp, Clifton, NJ) and kept at 35 ± 0.5°C except when otherwise specified (seeResults).
Membrane potential and current were measured in the whole cell configuration (Axopatch 200-A; Axon Instruments, Inc., Foster City, CA) by borosilicate glass pipettes with a tip resistance between 2.5 and 4 MΩ. The pipette solution contained 110 mM K+-aspartate, 23 mM KCl, 0.4 mM CaCl2 (calculated free-Ca2+= 10−7 M), 3 mM MgCl2, 5 mM HEPES-KOH, 1 mM EGTA-KOH, 0.4 mM GTP-Na+ salt, 5 mM ATP-Na+ salt, and 5 mM creatine phosphate, pH 7.3. Pipette solutions were modified in specific cases, as detailed in the relevant sections of Results. Membrane capacitance and series resistance (<5 MΩ) were measured in every cell; the latter was compensated by approx. 70% of its initial value. An average junction potential of about 5 mV, measured upon moving the electrode tip from Tyrode to “intracellular” (K+-aspartate), was left uncompensated. Potential and current signals were filtered at 2 KHz, digitized through a 12-bit A/D converter (Digidata 1200, sampling rate 5 KHz; Axon Instruments, Inc.) and simultaneously recorded by an adapted VCR system. Trace acquisition and analysis was controlled by dedicated software (pClamp 8.0; Axon Instruments, Inc.).
Experimental Protocols
Different ionic currents have been studied, each requiring specific conditions detailed here.
Na+/K+ Pump Current (INaK) Recordings.
INaK was measured as the holding current recorded at −40 mV in the presence of Ni2+ (5 mM), nifedipine (5 μM), and Ba2+ (1 mM) to minimize contamination by changes in Na+/Ca2+ exchanger, Ca2+ and K+ currents, respectively. Tetraethylammonium-Cl (20 mM) and EGTA (5 mM) were added to the pipette solution and intracellular K+ was replaced by Cs+. To optimize the recording conditions, INaK was enhanced by increasing intracellular Na+ (to 10 mM) and extracellular K+ (to 5.4 mM). In each cell, the current was recorded at steady state during exposure to increasing concentrations of the drug under test and, finally, to a saturating concentration of ouabain (20 μM) (Fig. 2a). BecauseINaK inhibition was expressed as percentage of ouabain effect, the latter was used as the asymptote for the estimation of EC50 values. The rate of onset of INaK inhibition was measured by linear interpolation of the early phase of the change in current.
Drug effects onINaK. a) membrane current recorded at −40 mV from an individual cell during exposure to increasing concentrations (micromolar) of PST2744 (PST) followed by 20 μM ouabain (ouab). The effect of a single digoxin (digo) concentration (micromolar) applied after ouabain washout is shown for comparison. B, dose dependence ofINaK inhibition by PST2744 (open circles,n = 9, 14, 17, and 6) and digoxin (filled circles,n = 7, 9, 13, and 7). c, myocyte twitch amplitude is plotted as a function of INaK inhibition at various PST2744 (0.5, 2.5, 10, and 20 μM) and digoxin (0.5, 0.8, 2.5, and 5 μM) concentrations. Data of twitch amplitude are fromMicheletti et al. (2002).
High-Threshold Ca2+ Current (ICaL) Recordings.
ICaL was recorded during depolarizing pulses to 0 mV from a holding potential of −40 mV as the difference between currents recorded in the absence and presence of 10 μM nifedipine, respectively (nifedipine-sensitive current,Inife). To prevent contamination by K+ currents, intracellular K+ was replaced by equimolar Cs+, the latter was also added to the superfusing solution at a concentration of 10 mM. A low EGTA concentration (0.5 mM) was used to control basal Ca2+ levels without suppressing Ca2+ transients.
Transient Inward Current (ITI) Recordings.
ITI was elicited by repolarization during a Ca2+ transient. Because the aim was to test the effects of PST2744 and digoxin independently of the inhibition of the Na+/K+ pump, the latter was blocked during all measurements by exposure to a K+-free solution containing 20 μM ouabain. To minimize contamination by components independent of the Ca2+ transient,ITI was obtained as the subtraction of traces recorded, respectively, under loading (Fig. 4, inset 1) and depletion (Fig. 4, inset 2) of the sarcoplasmic reticulum. Under such conditions, ITI should be mostly carried by the Na+/Ca2+exchanger (NaCaX) (Fedida et al., 1987; Zygmunt et al., 2000), whose driving force is transiently increased upon repolarization.ITI was measured at room temperature under ruptured-patch conditions with low intracellular EGTA concentration (1 mM). To prevent contamination by K+ currents, intracellular K+ was replaced by equimolar Cs+ and the extracellular solution contained Ba2+ (1 mM) and Cs+ (2 mM). Because the contour of ITI was also affected by digoxin, effects were more accurately quantified by measuring “average ITI” as the ratio of ITI time integral over the integration interval (avg ITI).
Drug effects on ITI in the presence of Na+/K+ pump blockade. a and b,INaCa measured asITI elicited by repolarization during a Ca2+ transient in the presence of 20 μM ouabain. The voltage protocols (inset) included a conditioning train (1 Hz) followed by a test pulse, after which ITI was measured (shown by the box). The duty cycle of the conditioning train was such as to favor cell Ca2+ loading in protocol 1 and Ca2+ depletion in protocol 2. Protocols 1 and 2 were applied in each cell, and ITI was obtained by subtraction between the respective current traces. c and d, effects of PST2744 (PST 4 μM, n = 14) and digoxin (digo 1 μM, n = 12) on avgITI. ∗, P < 0.05.
Na+/Ca2+ Exchanger Current (INaCa) Recordings.
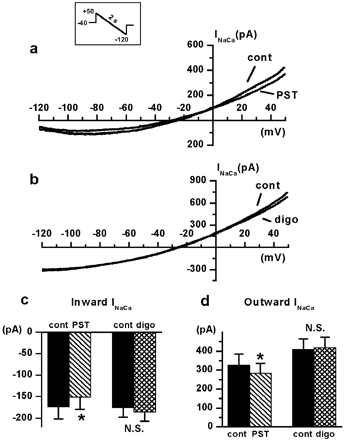
INaCa was measured as the current sensitive to 5 mM Ni2+ (Kimura et al., 1987) by the voltage protocol shown in the inset of Fig. 5. As forITI, measurements were performed in the presence of Na+/K+ pump blockade (see described above), and Na+concentration in the pipette solution was increased to 20 mM to improve measurement of outward INaCa. Contamination by ICaL and cytosolic Ca2+ oscillations were minimized by adding 0.2 μM nisoldipine and 5 mM EGTA to the extra- and intracellular solutions, respectively. Current/voltage relations ofINaCa were obtained by applying repolarizing voltage ramps (dV/dt = −85 mV/s), which allowed measurement of INaCa reversal potential (Erev) (Hobai et al., 1997). Drug effects on forward and backward NaCaX operation modes were separately quantified by measuringINaCa at potentials symmetrical toErev (±60 mV fromErev). This type of measurement provides an estimate of inward and outward NaCaX “conductances”.
Drug effects on INaCa in the presence of Na+/K+ pump blockade. a,INaCa measured as the current sensitive to 5 mM Ni2+ (see text for details). Current/voltage curves were obtained by applying slow hyperpolarizing V ramps (−0.085 V/s, inset); inward and outward INaCa were measured at ±60 mV from the current reversal potential, respectively. a and b, examples of the effects of PST2744 (PST 4 μM) and digoxin (digo 1 μM) on INaCa current/voltage curves. Drug effects on inward and outward INaCa are summarized in c and d, respectively (n = 10 for PST and n = 11 for digo). ∗, P < 0.05.
Delayed Rectifier Current (IKr andIKs) Recordings.
Delayed rectifier currents were measured as the amplitude of the “tail” elicited upon returning to the holding potential after an activating pulse (protocols at the top of Figs. 6 and 7). To isolateIKs,IKr was blocked by 5 μM E-4031, and tail contamination by drug-induced changes inICaL andINaCa were prevented by ouabain (20 μM), Ni2+ (5 mM), and nisoldipine (0.2 μM). Under such conditions, the time-dependent outward current was completely suppressed by 10 μM chromanol 293B, thus confirming its identity with IKs.IKr was isolated by subtracting currents recorded in the absence and presence of 5 μM E-4031 (Sanguinetti and Jurkiewicz, 1990).
Drug effects on IKs. The voltage protocol used to measure IKs is shown at the top (see text for details). Examples of PST2744 (PST 4 μM) and digoxin (digo 1 μM) effects are shown in a and b, respectively. Drug-induced changes in the amplitude ofIKs tail current density are summarized in c (n = 5 for PST and n = 4 for digo). ∗, P < 0.05.
Drug effects on IKr. The voltage protocol used to measure IKr(current sensitive to 5 μM E-4031, IE-4031) is shown at the top (see text for details). Examples of PST2744 (PST 4 μM) and digoxin (digo 1 μM) effect are shown in a and b, respectively; because IKr flowing during the activating step was very small, only IKr tail currents are displayed at high magnification. Drug-induced changes in the amplitude of IKr tail current amplitude are summarized in c (n = 6 for PST andn = 8 for digo). ∗, P < 0.05.
Substances
Nifedipine (Sigma-Aldrich) and nisoldipine, E-4031, and chromanol 293B stock solutions were prepared by dissolving the substances in ethanol, water, and dimethyl sulfoxide, respectively. PST2744 and ouabain (Sigma-Aldrich) were dissolved in water, and digoxin (Sigma-Aldrich) was dissolved in dimethyl sulfoxide; E-4031, chromanol 293B, and nisoldipine were generous gifts from Sanofi Recherche (Montpellier, France), Roche Diagnostics, and Bayer Pharmaceuticals (Milano, Italy), respectively.
Statistical Analysis
Means were compared by Student's t test or analysis of variance for paired or unpaired observations as appropriate. A probability level P < 0.05 was used to define significance throughout the study (N.S., not significant). In the text and figures, values are presented as mean ± standard error.
Results
Effect on Membrane Potential.
Myocytes were studied in the ruptured patch mode, during stimulation by 2-ms current pulses delivered through the patch pipette at a cycle length of 0.5 s (Fig. 1, a–c).
Drug effects on the action potential. a, APD90 measured in an individual cell during stimulation at 2 Hz is plotted as a function of time. Exposure to PST2744 (PST) and digoxin (digo) is marked by the bars. The insets show action potentials recorded at the times marked on the APD graph by the labels a–f. b and c, summary of Ediast and APD90changes observed during exposure to the specified equi-inotropic drug concentration pairs (n = 5, 7, and 9 for both agents). ∗, P < 0.05 versus control; #,P < 0.05 PST versus digo.
Both PST2744 and digoxin depolarized diastolic membrane potential slightly and only at concentrations belonging to the rightmost portion of the inotropy dose-response curve (Micheletti et al., 2002). The effect of equi-inotropic concentrations of PST2744 and digoxin on diastolic membrane potential was similar (Fig. 1b).
In the majority of cells, action potential duration (APD) was modulated by PST2744 in a biphasic manner, i.e., a transient prolongation was followed by a larger shortening (Fig. 1a). Mirror-like effects were observed upon washout, i.e., return to control values was preceded by transient APD shortening beyond the level achieved during steady-state drug perfusion (Fig. 1a). A biphasic response was less consistently observed with digoxin. Transient APD prolongation at washin occurred in three of seven cells with 2.5 μM PST2744 (10.2 ± 3.5%) and in zero of seven cells with the equi-inotropic digoxin concentration of 0.8 μM. At higher concentrations, transient prolongation occurred in seven of nine cells with PST2744 (10 μM, 27.8 ± 3.7%) and in two of nine cells with digoxin (2.5 μM, 6.9%). As previously observed for inotropic effects (Micheletti et al., 2002), the onset and decay of APD changes were faster with PST2744 than with digoxin. The extent of APD shortening induced by equi-inotropic PST2744 and digoxin concentrations was similar (Fig. 1c). APD shortening occurred mainly through depression of the plateau phase (Fig. 1a, insets). Diastolic oscillations of membrane potential (DADs) were seldom observed and only at the highest concentration tested; their incidence was too small to allow a meaningful comparison between the two drugs.
Effect on INaK.
This set of experiments was designed to compare directly the concentration dependence of Na+/K+ pump inhibition by PST2744 and digoxin (Fig.2, a–c). The effect of each drug concentration on INaK was expressed as percentage of the ouabain-induced change (see Materials and Methods). As occurred for inotropic effects (Micheletti et al., 2002), digoxin (EC50 = 2.66 μM) was more potent than PST2744 (EC50 = 6.75 μM) (Fig. 2b) in inhibiting INaK. However, the extent of INaK inhibition associated to a given inotropic effect was slightly greater for PST2744 than for digoxin (Fig. 2c). Thus, digoxin may require less Na+/K+ pump inhibition than PST2744 to induce the same inotropic response. When measured at the same concentrations, the onset of INaKinhibition was faster for PST2744 than for digoxin (e.g., at 2.5 μM, 4.9 ± 0.9 versus 2.1 ± 0.9 pA/s; P < 0.05).
Effect on ICaL.
The purpose of this set of experiments (Fig. 3, a–d) was to test whether inhibition of ICaL might contribute to drug-induced changes in the action potential contour. Equi-inotropic drug concentrations corresponding to the mid-portion of the inotropy dose-response curve (Micheletti et al., 2002) were used. Neither PST2744 (4 μM; Fig. 3a) nor digoxin (1 μM; Fig. 3b) significantly affected the peak amplitude ofICaL (PST2744, −0.52 ± 5.5%, N.S.; digoxin, −6.23 ± 4.2%, N.S.). The current decay was best fitted by a biexponential function. The faster exponential component, reflecting Ca2+-dependentICaL inactivation, was significantly accelerated by both PST2744 (τfast, −17.05 ± 3.2%, P < 0.05) and digoxin (τfast, −13.53 ± 2.8%,P < 0.05) (Fig. 3d). The slow component was unchanged by PST2744 and slightly slowed by digoxin (τslow, 5.4 ± 1.5%, P < 0.05).
Drug effects on ICaL. a and b, nifedipine-sensitive currents (10 μM,Inife) in control and during drugs superfusion at equi-inotropic concentrations (PST2744 4 μM,n = 4 and digoxin 1 μM, n = 6). Current traces were recorded during steps to 0 mV from a holding potential of −40 mV every 2 s; to emphasize changes inICaL inactivation, each trace was normalized to the peak amplitude. c and d, average results on peakInife and τfast ofICaL inactivation. ∗,P < 0.05.
Effect on ITI.
Representative examples of the effects of PST2744 and digoxin onITI are shown in Fig.4, a and b, respectively. PST2744 (4 μM) consistently reduced avg ITI(−42.8 ± 7.9%, P < 0.05; Fig. 4c). Although digoxin (1 μM) also caused a small decrease inITI in part of the cells, its effect was inconsistent and failed to achieve significance (−8.8 ± 11.5%, N.S.; Fig. 4d).
Effect on INaCa.
Representative examples of the effects of PST2744 and digoxin onINaCa are shown in Fig.5, a and b, respectively. BaselineINaCa current and itsErev were similar between PST2744 and digoxin groups. PST2744 (4 μM; Fig. 5a) decreased inward (Fig. 5c) and outward (Fig. 5d) INaCaconductance similarly (inward, −14.9 ± 4.8%, P< 0.05; outward, −13.5 ± 4.1%, P < 0.05) and slightly shifted Erev in the negative direction (−26.3 ± 3 versus −23.6 ± 3.2 mV,P < 0.05). Digoxin (1 μM; Fig. 5b) affected neitherINaCa norErev (−18.5 ± 2.1 versus −17.1 ± 1.9 mV; N.S.)
In summary, PST2744, but not digoxin, markedly inhibitedITI (i.e.,INaCa triggered by a Ca2+ transient), but only slightly reducedINaCa conductance measured under conditions minimizing cytosolic Ca2+fluctuations. Because such effects were measured in the presence of ouabain, they were not secondary to Na+/K+ pump inhibition.
Effect on Delayed Rectifier Currents.
Evaluation of PST2744 and digoxin effects on delayed rectifier currents was prompted by the arrhythmogenic potential of their inhibition and by the observation of a difference in transient APD changes upon drug washin and washout (see above). PST2744 (4 μM) and digoxin (1 μM) were applied during separate measurement of IKs (Fig.6, a–c) andIKr (Fig. 7, a–c) (Sanguinetti and Jurkiewicz, 1990) (see Materials and Methods). IKr was marginally reduced by PST2744 (−5.99 ± 1.5%, P < 0.05) and unaffected by digoxin (2.99 ± 5.3%; N.S.).IKs was slightly but significantly reduced by both PST2744 and digoxin to a similar extent (−21.5 ± 4.0 versus −14.6 ± 2.6%; N.S.). Thus, concentrations of PST2744 and digoxin exerting similar inotropic effect slightly reducedIKs. PST2744 also inhibitedIKr but to a very small extent.
Discussion
Effects on Membrane Potential.
The aim of this set of experiments was to look for peculiarities in the modulation of action potential contour that might suggest a mechanism for the different proarrhythmic effects of PST2744 and digoxin.
Slight depolarization of diastolic membrane potential was similarly induced by PST2744 and digoxin and was expected as a result of Na+/K+ pump inhibition. Transient APD prolongation followed by shortening is commonly observed with cardiac glycosides and is respectively interpreted as primary and secondary effects of Na+/K+pump inhibition (Levi et al., 1994). Indeed, although removal ofINaK would primarily prolong APD, the concomitant increase in intracellular Na+ would move the NaCaX electrochemical balance to shiftINaCa in the outward (i.e., repolarizing) direction, thus causing APD shortening (Levi, 1993).
Transient APD changes (wash-in prolongation and wash-out overshoot shortening) were more prominent for PST2744 than for digoxin. This can be hardly attributed to modulation of delayed rectifier currents; indeed, the two agents differed only slightly in their effects on IK, which were small even in absolute terms. An alternative and more plausible explanation was provided by the faster rate of onset and dissipation of PST2744 effects, probably attributable to a difference between PST2744 and digoxin in the kinetics of Na+/K+ pump inhibition. This might reduce the overlap betweenINaK inhibition and intracellular Na+ accumulation, thereby emphasizing the transient changes in APD.
DADs, expected in view of the previously described after- contractions (Micheletti et al., 2002), did not occur in the present setting. This might reflect partial buffering of drug-induced Ca2+ loading by cell dialysis through the recording pipette.
Effects on Na+/K+ Pump Current.
According to the classical interpretation of glycosides action, inotropy should be directly related to Na+/K+ pump blockade. However, other mechanisms may contribute to their inotropic effect (Tian et al., 2001; Sagawa et al., 2002) and affect the relationship between Na+/K+ pump inhibition and inotropy (Wasserstrom et al., 1991; Wasserstrom, 2002). This set of experiments was designed to assess whether differences might exist in such a relationship between PST2744 and digoxin. Data from a previous study were used for the concentration dependence of the inotropic effect (Micheletti et al., 2002). Comparison of such data with the present results shows that the extent of Na+/K+ pump inhibition required to produce a given inotropic effect was slightly smaller for digoxin than for PST2744. The ratio between Na+/K+ pump inhibition and inotropy for various digitalis compounds may correlate with the ability to facilitate opening of Ca2+ release channels of the sarcoplasmic reticulum (Wasserstrom et al., 1991). Such an action has been demonstrated for digoxin (Sarkozi et al., 1996; Sagawa et al., 2002) and, if not shared by PST2744, might justify the difference observed between the two agents. The onset of Na+/K+ pump inhibition was faster for PST2744 than for digoxin. This is consistent with the previous observation that association and dissociation rate constants of digitalis compounds to the Na+/K+ pump are decreased by the glycosidic group (aglycones bind faster than the respective glycoside) (Yoda, 1974), which is not present in PST2744 structure.
It is fair to stress that precise quantitation of Na+/K+ pump inhibition byINaK measurements could be obtained only if subsarcolemmal Na+ activity remained strictly constant (Levi et al., 1994). This would require instantaneous equilibration of subsarcolemmal space with pipette solution, a condition only grossly approximated under real experimental conditions. Thus, the curves shown in Fig. 2c are meant to compare the two agents, rather than to establish the relation between Na+/K+ pump inhibition and inotropy in absolute terms.
Effects on ITI and Na+/Ca2+ Exchanger Current.
This group of experiments was aimed at testing the primary effects (i.e., independent of Na+/K+ pump inhibition) of PST2744 and digoxin on ITI and its charge carrier NaCaX.
At variance with digoxin, PST2744 significantly inhibitedITI. This current is directly responsible for digitalis-induced DADs; therefore inhibition ofITI can be viewed as an antiarrhythmic effect and might account for the different toxicity of the two agents. Two mechanisms may account for ITIreduction by PST2744: 1) a direct inhibition of NaCaX; or 2) a reduction in the subsarcolemmal Ca2+concentration transiently available after repolarization to drive the exchanger (e.g., a decrease in the amplitude or duration of the Ca2+ transient induced by the depolarizing step). Although the former mechanism would take place at sarcolemmal level, the latter would imply a change in the function of sarcoplasmic reticulum components. Clues to discriminate between such mechanisms are provided by the second set of experiments, in whichINaCa was dissected by pharmacological means under conditions unlikely to induce significant fluctuations of subsarcolemmal Ca2+. In this case, NaCaX conductance was still consistently reduced by PST2744, but the effect was small compared with that on ITI. Thus, although PST2744 may directly inhibit NaCaX to a small extent, other mechanisms (e.g., changes in the kinetics of intracellular Ca2+ transients) are likely to contribute to the observed inhibition of ITI. PST2744 also slightly shifted Erev in the negative direction. This effect cannot be explained by an increase in intracellular Na+. First, because the Na+/K+ pump was fully blocked, and second, because although an increase in intracellular Na+ should cause largerINaCa conductance (LabHeart version 4.8, model; D. M. Bers and J. L Puglisi), the latter was decreased by PST2744. Thus, PST2744-induced change in NaCaX conductance is likely to result from a direct action on the exchanger, rather than from a change in transarcolemmal ion distribution.
Effects on Delayed Rectifier and Ca2+ Currents.
Due to its proarrhythmic potential, inhibition of delayed rectifier currents has recently become a major concern in drug safety; this motivated an analysis of PST and digoxin effects onIKr andIKs. While both agents exerted some effects, their functional relevance is questionable for the following reasons. Although major inhibition of delayed rectifier currents is generally required to modulate repolarization (Bosch et al., 1998), the effect on IKs were relatively small and those on IKr almost negligible. Second, IKs inhibition is generally associated with a relatively low proarrhythmic risk, because of the lack of reverse use dependence of its effect on APD (Bosch et al., 1998). Finally, although induction of early after-depolarizations and arrhythmias related to IK inhibition may be secondary to APD prolongation (Brachmann et al., 1983; January et al., 1988; Antzelevitch and Sicouri, 1994), the net steady-state effect of PST2744 and digoxin was a shortening of APD (see above). Nonetheless, it is fair to stress that some APD prolongation might theoretically occur in other cardiac tissues expressing drug-sensitive currents in a different proportion (e.g., Purkinje fibers and M cells).
PST2744 and digoxin did not affectICaL peak amplitude; as expected (Hancox and Levi, 1996), both drugs acceleratedICaL inactivation. On the other hand, high concentrations of cytosolic Cs+ may obstruct Ca2+ fluxes through the SR membrane (Kawai et al., 1998); thus, drug-induced increase in Ca2+-dependent inactivation ofICaL might have been underestimated. Thus, PST2744 and digoxin effects on the action potential, as well as the different toxicity of two agents, cannot be attributed toICaL modulation.
Relevance to Arrhythmogenic Effects.
The cell targets of drug action evaluated in this study encompass those most likely to be involved in arrhythmogenic effects based on electrophysiological alterations. Among the actions observed, the one not shared by digoxin and most likely to account for the lower arrhythmogenic effect of PST2744 is inhibition of ITI. Indeed,ITI is directly involved in the genesis of DADs, the main mechanism of digitalis-induced arrhythmias (Bers, 2002). The extent of ITIreduction was large compared with direct inhibition ofINaCa, thus suggesting that PST2744 might modulate the amplitude and/or kinetics of intracellular Ca2+ transients, a hypothesis that requires further investigation. Although in guinea pig and human ventricular myocytes ITI is mostly carried by NaCaX (Fedida et al., 1987; Koster et al., 1999), other Ca2+-sensitive conductances may contribute to it in other species (Zygmunt et al., 1998). On the other hand, Ca2+-sensitive conductances would still be affected by drug-induced changes in cytosolic Ca2+ dynamics. Although other differences have been disclosed between the two agents, they concern quantitatively small effects, which, as discussed above, are unlikely to contribute to the arrhythmogenic profile.
Digitalis-induced triggered activity may more frequently originate from Purkinje fibers or M cells (Antzelevitch and Sicouri, 1994), probably because a longer plateau favors Ca2+ overload. Nonetheless, the conductances involved in the genesis of DADs are the same in all cell types. Thus, except for a potential difference in modulation of APD (see above), the findings of the present study may be relevant to all ventricular cell types.
Androgen steroids have been shown to counteract the tendency of estrogens to inhibit repolarizing currents (Pham and Rosen, 2002). In the present studies, performed on myocytes freshly isolated from female hearts, PST2744 slightly inhibited delayed rectifier currents and only minimally affected ICaL. Thus, it seems unlikely that the antiarrhythmic effect of PST2744 may be related to the “repolarizing” effect of androgen steroids. Moreover, in spite of its chemical structure, PST2744 has no affinity for steroid receptors (Micheletti et al., 2002).
Acknowledgments
We thank Dr. Elena Colombo for assisting in part of the experiments.
Footnotes
-
This work was partly funded by a grant from Prassis Research Institute Sigma Tau and from Grant Ministero dell'Università e della Ricerca Scientifica e Tecnologica 2000 (to A.Z.).
-
DOI: 10.1124/jpet.102.047696
- Abbreviations:
- INaK
- Na+/K+ pump current
- ICaL
- high-threshold Ca2+current
- Inife
- nifedipine-sensitive current
- ITI
- transient inward current
- INaCa
- current generated by the Na+/Ca2+ exchanger
- Erev
- current reversal potential
- IKr
- rapid component of the delayed rectifier K+ current
- IKs
- slow component of the delayed rectifier K+ current
- NaCaX
- Na+/Ca2+ exchanger
- APD
- action potential duration
- DAD
- delayed after depolarization
- E-4031
- N-[4-[[1-[2-(6-methyl-2-pyridinyl)-ethyl]-4-piperidinyl]carbonyl]phenyl]methanesulfonamide dihydrochloride
- Received December 4, 2002.
- Accepted February 14, 2003.
- The American Society for Pharmacology and Experimental Therapeutics