Abstract
Objective: To gain some insight on the lesser arrhythmogenic properties of PST2744 [(E,Z)-3-((2-aminoethoxy)imino)androstane-6,17-dione hydrochloride] compared with digoxin, we compared modulation of intracellular Ca2+ dynamics by the two agents. Methods: SERCA (sarcoplasmic reticulum Ca2+-ATPase) activity and Ca2+ leak rate were measured in sarcoplasmic reticulum (SR) vesicles from guinea pig ventricles. Membrane current, intracellular Ca2+, and twitch amplitude were evaluated in guinea pig ventricular myocytes with or without blockade of the Na+/Ca2+ exchanger. Results: In SR vesicles, PST2744 (30–300 nM), but not digoxin, increased SERCA activity; digoxin only (≥0.1 nM) increased SR Ca2+ leak. In myocytes with blocked Na+/Ca2+ exchanger, Ca2+ reloading of caffeine-depleted SR was enhanced by PST2744 and slightly inhibited by digoxin. In myocytes with functioning Na+/Ca2+ exchanger, both agents increased diastolic Ca2+, SR Ca2+ content, the gain of Ca2+-induced Ca2+ release, the rate of cytosolic Ca2+ decay, twitch amplitude, and relaxation rate. Consistent with the observations in SR vesicles, the effects on SR Ca2+ content and Ca2+ decay rate were significantly larger for PST2744 than for digoxin. Conclusions: In isolated SR vesicles, PST2744 and digoxin directly affected SR function in opposite ways; this could be reproduced in myocytes during Na+/Ca2+ exchanger blockade. Under physiological conditions (functioning Na+/Ca2+ exchanger), the two agents affected Ca2+ dynamics in the same direction, as expected by their Na+/K+ pump inhibition; however, differential SR modulation was still expressed by quantitative differences. Thus, the more favorable inotropy-to-toxicity ratio previously described for PST2744 appears to be associated with direct SERCA stimulation and/or lack of enhancement of Ca2+ leak.
Due to the lack of suitable substitutes, Na+/K+ pump inhibitors remain a valuable therapeutic tool in the management of heart failure (Eichhorn and Gheorghiade, 2002); nonetheless, their use is strongly limited by their well known proarrhythmic effect. Inhibition of Na+/K+-ATPase and the resulting change in intracellular Ca2+ are commonly held to underlie both inotropy and proarrhythmia (Bers, 2002b), thus justifying the claim that these actions may be inseparable. This view is challenged by the observation that the ratio between inotropic and toxic effect (therapeutic index) varies among different Na+/K+ pump inhibitors (Wasserstrom et al., 1991), possibly due to modulation of targets other than the Na+/K+ pump. For instance, commonly used glycosides also affect sarcoplasmic reticulum (SR) function (McGarry and Williams, 1993; Sarkozi et al., 1996; Sagawa et al., 2002) with a potential impact on the relation between total intracellular Ca2+ content and its effects on cell function.
A sharp dissociation between inotropy and proarrhythmia has been recently observed with the novel nonglycoside Na+/K+ pump inhibitor (E,Z)-3-((2-aminoethoxy)imino)androstane-6,17-dione hydrochloride (PST2744) (Micheletti et al., 2002). In single-cell, tissue, and whole animal studies, the incidence of arrhythmias observed at similar inotropy (increase in unloaded cell shortening in single myocytes) was markedly lower for PST2744 than for digoxin (Micheletti et al., 2002; Rocchetti et al., 2003). Cellular electrophysiology studies showed that the changes in action potential contour and relevant membrane currents caused by equi-inotropic PST2744 and digoxin concentrations were roughly similar (Rocchetti et al., 2003). The only effect potentially accounting for the different toxicity was the inhibition of the Na+/Ca2+ exchanger current by PST2744 through a mechanism independent of Na+/K+ pump blockade (Rocchetti et al., 2003). Nonetheless, those studies could not discriminate whether such an effect resulted from PST2744 action on the exchanger itself or was secondary to changes in the dynamics of intracellular Ca2+.
The present work compares digoxin and PST2744 effects on SR function in isolated vesicle preparations and on Ca2+ dynamics in intact myocytes. The results obtained provide a basis for the differences between the two agents and a link, of more general interest, between modulation of specific SR targets and overall Ca2+ handling by ventricular myocytes.
Materials and Methods
The investigation conforms to the Guide of the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication 85-23, revised 1996) and to the guidelines for animal care endorsed by the participating institutions. Hartley guinea pigs (3–4 months old) were killed by cervical dislocation under ether (vesicle preparations) or ketamine-xylazine (myocyte preparations) anesthesia.
Studies on Isolated SR Vesicles
Vesicle Preparation. Cardiac SR vesicles were isolated according to Sitsapesan et al. (1991). Microsomes were prepared at 2°C by homogenization in a medium containing 300 mM sucrose and 20 mM imidazole (pH 7.4). Cell fragments were removed by centrifugation (6800g for 25 min) followed by filtration. The following protease inhibitors were included in all solutions: 200 μM pefabloc, 1 mM benzamidine, 100 nM aprotinin, 1 μM leupeptin, and 1 μM pepstatin A.
Crude microsomes were centrifuged at 120,000g for 90 min; the pellet was resuspended in a medium containing 100 mM NaCl, 10 mM K+-PIPES, and 500 mM sucrose (pH 7.0). The suspension was layered onto a 20–45% linear sucrose gradient, spun for 16 h at 90,000g, and the lower visible protein ring was collected. Aliquots from parallel tubes were pooled and homogenized in a medium containing 400 mM KCl, 20 mM imidazole, 0.5 mM MgCl2, 0.5 mM CaCl2, and 0.5 mM EGTA. Heavy SR vesicles were then collected by centrifugation at 124,000g for 60 min, resuspended in a medium containing 300 mM sucrose and 25 mM MOPS (pH 7.0), aliquoted, and stored at –70°C until use. Protein concentration was determined by Lowry (1951) assay using bovine serum albumin as standard, and molecular purity was checked using SDS-polyacrylamide gel electrophoresis.
Measurement of SERCA Activity. ATPase activity was determined at 37°C by a coupled enzyme assay in a medium containing 100 mM KCl, 20 mM Tris-HCl, 5 mM MgCl2, 5 mM ATP, 0.42 mM phosphoenolpyruvate, 0.001 mM calcimycin (a Ca2+ ionophore), 0.2 mM NADH, 7.5 U/ml pyruvate kinase, and 18 U/ml lactate dehydrogenase (pH 7.5). Total hydrolytic activity was measured as the decrease of optical density at the NADH absorbance wavelength (340 nm) and is expressed in micromoles of inorganic phosphate per milligrams of protein per minute, abbreviated as A.U. throughout the text and figures. Total hydrolytic activity ranged from 2.53 to 2.76 A.U. SERCA-dependent ATPase activity was identified as the portion of total hydrolytic activity inhibited by thapsigargin (5 μM) (Lytton et al., 1991).
PST2744 or digoxin was added to the preparation 5 min before starting measurements of enzyme activity. The assay was performed in the presence of Ca2+ and EGTA mixtures producing estimated free Ca2+ concentrations ranging from 0.1 to 2.9 μM (Fabiato and Fabiato, 1979); the latter concentration was found to induce maximum SERCA activation.
Calcium Efflux from SR. Calcium efflux from actively loaded vesicles was determined at 37°C by monitoring time-dependent changes in free Ca2+ concentration of the extravesicular solution. The latter was determined by sampling (1 Hz) antipyrylazo-III (APIII) absorbance difference at wavelengths of 710 and 790 nM (A710–A790). The APIII signal was calibrated by successive additions of CaCl2 to vesicle samples in which Ca2+ uptake was prevented by 100 nM cyclopiazonic acid (Sarkozi et al., 1996) (see Fig. 1 in Supplemental Data). Vesicles were suspended in the cuvette up to a final protein concentration of 260 μg/ml (final volume 2 ml) in a medium containing 92.5 mM KCl, 1 mM MgCl2, 7.5 mM sodium pyrophosphate, 18.5 mM K+-MOPS, 1 mM ATP, and 0.18 mM APIII. Active loading of vesicles by CaCl2 addition (0.054 mM) resulted in an average intravesicular Ca2+ content of 240 ± 22 nmol per assay. After completion of the ATP to ADP conversion and stabilization for 1 min, Ca2+ release was initiated by addition of the compound under study (Szentesi et al., 2001); a known amount of Ca2+ was added at the end of each experiment to confirm signal calibration. Background Ca2+ efflux, i.e., that occurred over the same time period in the absence of drugs (see Fig. 2 in Supplemental Data) was evaluated for each measurement on a separate sample of vesicles from the same preparation. Data are expressed as the amount of Ca2+ released during 20 min of exposure to experimental solutions.
Studies on Intact Myocytes
Myocyte Preparation and Recording Solutions. Guinea pig ventricular myocytes were isolated by using a retrograde coronary perfusion method previously published (Zaza et al., 1998) with minor modifications. Rod-shaped, Ca2+-tolerant myocytes were used within 12 h from dissociation.
During measurements, myocytes were superfused at 2 ml/min with Tyrode's solution containing 154 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 5 mM HEPES/NaOH, and 5.5 mM d-glucose, adjusted to pH 7.35. A thermostated manifold, allowing for fast (electronically timed) solution switch, was used for cell superfusion. All measurements were performed at 35 ± 0.5°C.
Electrophysiology Techniques. Ventricular myocytes were voltage-clamped in the whole-cell configuration (Axopatch 200-A, Axon Instruments Inc., Union City, CA). The pipette solution contained 110 mM K+-aspartate, 23 mM KCl, 0.2 mM CaCl2 (calculated free-Ca2+ = 10–7 M), 3 mM MgCl2, 5 mM HEPES-KOH, 0.5 mM EGTA-KOH, 0.4 mM GTP-Na+ salt, 5 mM ATP-Na+ salt, and 5 mM creatine phosphate Na+ salt, pH 7.3. Pipette solution was modified in specific cases, as detailed in the section on Experimental Protocols (below). Membrane capacitance and series resistance were measured in every cell but left uncompensated. Current signals were filtered at 2 KHz and digitized at 5 KHz (Axon Digidata 1200). Trace acquisition and analysis was controlled by dedicated software (Axon pClamp 8.0). As preliminarily tested under the conditions used in the present experiments, peak inward current closely reflects ICaL. K+ replacement with Cs+, commonly used to isolate ICaL, was avoided because SR function may be affected by this ion (Kawai et al., 1998).
Measurements of Intracellular Ca2+and Cell Shortening. Single myocyte intracellular Ca2+ activity was measured fluorimetrically using the membrane permeable dye Indo1-AM (Molecular Probes, Leiden, The Netherlands). After 30 min of incubation in Indo1-AM (8 μM), myocytes were washed for 30 min at 36°C. Indo1-AM fluorescent emission was measured at two wavelengths (410 and 490 nm) (Grynkiewicz et al., 1985). The signals at the two wavelengths (F410 and F490) were separately low-pass filtered (200 Hz) and digitized at 2 KHz. Cytosolic Ca2+ activity was calculated from the F410/F490 ratio after low-pass digital filtering (fast Fourier transform, 100 Hz) and subtraction of background luminescence. F410/F490 ratio values were converted to free cytosolic Ca2+ concentration ([Ca]f) by the formula described in Table 1 using parameters determined by calibration in ionomycin permeabilized myocytes (Sipido and Callewaert, 1995).
Glossary of symbols and method of calculation
Total cytosolic Ca2+ concentration ([Ca]tot) and content (Catot), the amounts of Ca2+ moved across the sarcolemma through ICaL (CaL) or the Na+/Ca2+ exchanger (CaNCX), and the gain of Ca2+-induced Ca2+ release (CICR) were estimated as described in Table 1. In SR reloading experiments, a qualitative estimate of CICR gain was obtained simply by dividing the peak value of the Ca2+ transient by peak ICaL (Bers, 2002c); although expressed in different units, the results of this procedure were highly correlated with those obtained through the quantitative one.
Total SR Ca2+ content normalized to cell volume ([Ca]SR-tot, micromoles per liter of cytosol) was measured during caffeine pulses (protocol 1 below). [Ca]SR-tot estimate can be biased by removal of Ca2+ by the Na+/Ca2+ exchanger, which may be differently affected by the agents under the study (Rocchetti et al., 2003). To obtain an unbiased estimate of [Ca]SR-tot, CaNCX during the ascending phase of the caffeine-induced Ca2+ transient was added back to Catot measured at its peak (Table 1, Fig. 2, A and B). In control conditions, CaNCX during the ascending phase of the transient was 12 ± 1% of total SR Ca2+.
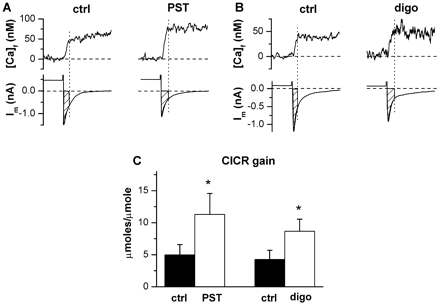
Drug effects on total SR Ca2+ content ([Ca]SR-tot). A and B, examples of free cytosolic Ca2+ concentration [Ca]f and membrane current (Im) simultaneously recorded during exposure to caffeine; traces in control and during drug superfusion (○) are shown in each panel. [Ca]f records are offset to 0 nM and time-aligned with Im traces. Dotted lines mark the time at which peak [Ca]f was read, and the hatched area on Im represents transmembrane charge movement during the rising phase of the Ca2+ transient. C, average values of [Ca]SR-tot. D, average amount of Ca2+ extruded through Na+/Ca2+ exchanger (CaNCX) during the rising phase of the caffeine-induced transient. *, p < 0.05 drug versus control; #, p < 0.05 digoxin versus PST2744; n = 8 for both digoxin (1 μM) and PST2744 (4 μM).
Intracellular caffeine concentration was monitored by measuring caffeine-induced quenching of Indo1-AM fluorescence according to the procedure described by O'Neill et al. (1990). Cell shortening was measured in intact myocytes and field-stimulated at a rate of 2 Hz by a fast acquisition camera (sampling rate 250 Hz) and a video-edge detector (Crescent Electronics, Sandy, UT).
Experimental Protocols.1. Ca2+dynamics and SR Ca2+content under physiological conditions. Transmembrane current and cytosolic Ca2+ were simultaneously measured during a train of depolarizing (200 ms) pulses to 0 mV (0.37 Hz) applied from a holding potential (EH) of –40 mV. EH was then changed to –80 mV, and a 5-s caffeine pulse (10 mM) was applied after 10 s (electronically timed).
2. SR reloading during Na+/Ca2+exchanger inhibition. The Na+/Ca2+ exchanger was inhibited by incubation of the cells for 30 min in aNa+- and Ca2+-free solution (replaced by equimolar Li+ and 1 mM EGTA). During measurements, the perfusate contained 0 mM Na+ and 1 mM Ca2+. The pipette solution was Na+-free (Na+ salts were replaced by K+ or Tris salts). SR was initially depleted by a brief caffeine pulse (with 154 mM Na+ to allow Ca2+ extrusion through the Na+/Ca2+ exchanger) and then progressively reloaded by a train of depolarizing pulses (0.25 Hz, pulse parameters as in protocol 1) in the presence of 1 mM Ca2+ (protocol outline in the inset of Fig. 5).
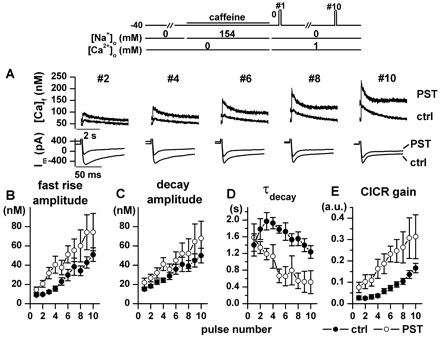
PST2744 effects during SR reloading with blocked Na+/Ca2+ exchanger. A, example of [Ca]f and membrane current (Im) during SR reload in control (ctrl) and PST2744 (PST, 4 μM); the position of the pulse in the stimulation train is shown above (#2–10). B–E, average values of Ca2+ transient parameters measured during each of the first 10 pulses (#1–10) of the stimulation train. CICR gain was estimated by the simplified method and expressed in arbitrary units (a.u.). n = 8. Inset: outline of the experimental protocol.
Substances
Stock Indo1-AM solution (1 mM in dry DMSO) was diluted in Tyrode's solution. PST2744 was dissolved in water; digoxin was dissolved in DMSO or ethanol. The final DMSO or ethanol concentrations did not exceed 0.1%. PST2744 was synthesized at Prassis Sigma-Tau (Settimo Milanese, Italy), antipyrylazo-III was purchased from MP Biomedicals (Irvine, CA), Indo1-AM from Molecular Probes, protease inhibitors from Roche Diagnostics (Mannheim, Germany) and Merck (Darmstadt, Germany), and all other chemicals from Sigma-Aldrich (St. Louis, MO).
Statistical Analysis
Least-square fitting was used to estimate the kinetics of twitch and Ca2+ decays. Means were compared by paired or unpaired t test and, wherever appropriate, by two-way analysis of variance (paired measurements). Data are expressed and plotted as the mean ± S.E. value obtained from a number of independent determinations. Statistical significance was defined as p < 0.05 (N.S. = not significant). Absolute values of the variables and sample size for each experimental condition are provided in the figures and the respective legends.
Results
Studies on Isolated SR Vesicles
Effects on SERCA Enzymatic Activity. Dependence of SERCA activity on extravesicular Ca2+ (0.1–2.3 μM) was tested in the absence (control) and in the presence of PST2744 and digoxin (Fig. 1A).
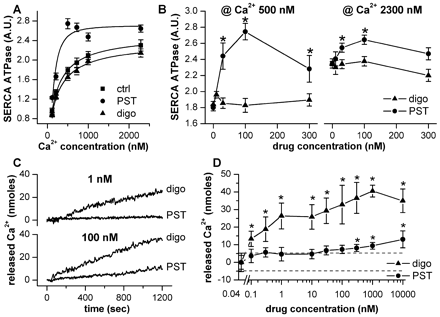
Modulation of SERCA ATPase activity and SR Ca2+ efflux in SR vesicles. A, Ca2+ dependence of ATPase activity during control (ctrl, squares), 100 nM PST2744 (PST, circles), and 100 nM digoxin (digo, triangles). Solid lines are best fits of data points with the Hill function: V = Vmax × [Ca2+]n/(Km + [Ca2+]n) whose parameters are reported in the text. Sample size: ctrl = 7; PST = 6; digo = 5. B, concentration dependence of PST2744 and digoxin effects on ATPase activity in the presence of 500 and 2300 nM Ca2+. *, p < 0.05. A.U. = activity units (see Materials and Methods); n ≥ 5 per sample point. C, time course of extravesicular Ca2+ concentration after subtraction of background Ca2+ efflux. PST2744 (PST) and digoxin (digo) were compared at concentrations of 1 and 100 nM within the same experiment. D, concentration dependence of PST2744 (circles) and digoxin (triangles) effects on Ca2+ released during 20 min of treatment; dashed lines show the confidence limits of background Ca2+ efflux. *, p < 0.05 versus control; n ≥ 5 per sample point.
Under control conditions, increments in Ca2+ enhanced SERCA activity with an asymptote (Vmax) at 2.5 ± 0.12 A.U. and Km of 209.2 ± 21.9 nM. PST2744 (100 nM) significantly reduced Km to 143.9 ± 6.3 nM (p < 0.05) and left Vmax unchanged (2.74 ± 0.05; N.S.). Conversely, 100 nM digoxin left Vmax (2.3 ± 0.11 A.U.) and Km (193.0 ± 17.1 nM) unchanged.
Concentration-response curves for the two agents were then obtained at submaximal (500 nM) and saturating (2300 nM) Ca2+ concentrations (Fig. 1B). At submaximal Ca2+, PST2744 exerted clear-cut stimulatory effects over the range 30 to 300 nM. Its concentration-response curve was bell-shaped, with maximal effect occurring at 100 nM, and decreasing thereafter. When Ca2+ was increased to the saturating level, basal activity increased and the PST2744 stimulatory effect, even if still significant, was largely hindered. At variance with PST2744, digoxin failed to stimulate SERCA activity at both Ca2+ levels.
Effects on Spontaneous Ca2+Efflux from the SR. The effect of low and high drug concentrations on spontaneous Ca2+ efflux from SR vesicles is illustrated in the sample tracings of Fig. 1C. Concentration-response curves for PST2744 and digoxin were constructed by measuring the amount of Ca2+ released by the vesicles 20 min after compound addition. Figure 1D compares curves obtained with the two agents to the confidence limits for drug-independent Ca2+ efflux. Digoxin enhanced Ca2+ efflux in a concentration-dependent way with a threshold concentration at 0.1 nM and peak effect at 1 μM (40.5 ± 0.3 nmol at 20 min, exceeding the upper confidence limit of background by approximately eight times). One hundred nanomolars of PST2744, i.e., the most effective concentration on SERCA, failed to enhance Ca2+ release; an effect appeared only at concentrations ≥300 nM and even at 10 μM, it amounted to 12.9 ± 4 nmol only (p < 0.05 versus digoxin 1 μM).
Studies on Intact Myocytes
With the aim of obtaining a functionally relevant comparison, PST2744 and digoxin were compared at equi-inotropic concentrations, as previously assessed under the same experimental setting (Micheletti et al., 2002). The concentrations used throughout the present study were 4 μM for PST2744 and 1 μM for digoxin, corresponding to the steep portion of the relation between concentration and unloaded cell shortening of isolated myocytes (Micheletti et al., 2002).
Effects on Resting Ca2+, CICR Gain, and Total SR Ca2+Content. Transmembrane current and cytosolic Ca2+ concentration were simultaneously measured under physiological ionic conditions according to experimental protocol 1 (see Materials and Methods).
Resting Ca2+ (at EH =–80 mV) was similarly increased by digoxin (from 46.5 ± 5.7 to 70.4 ± 6.1 nM; +60 ± 14%, n = 8, p < 0.05) and PST2744 (from 61.3 ± 6.4 to 92.4 ± 11.1 nM, +51 ± 12%, n = 8, p < 0.05).
Total SR Ca2+ content was estimated from the amplitude of the caffeine-induced transient after correction for the Ca2+ extruded by the Na+/Ca2+ exchanger (Fig. 2; see Materials and Methods) and normalized to cytosolic volume ([Ca]SR-tot, moles per liter of cytosol) (Shannon et al., 2000b). Digoxin increased [Ca]SR-tot by 28 ± 10% (p < 0.05, Fig. 2, B and C) and PST2744 by 47 ± 8% (p < 0.05, Fig. 2, A and C). The effect of PST2744 on [Ca]SR-tot was significantly larger than that of digoxin (p < 0.05, Fig. 2C). Both agents increased the amount of Ca2+ extruded by the Na+/Ca2+ exchanger (CaNCX) during caffeine-induced transients (digoxin +127 ± 38%, PST2744 +130 ± 46%, Fig. 2D).
As shown in Fig. 2, A and B, both digoxin and PST2744 shortened the time elapsing between the start of the (electronically timed) caffeine pulse and SR Ca2+ release. This was not associated with a matching effect on the time course of intracellular caffeine concentration (see Fig. 3 in Supplemental Data). Thus, anticipation of the Ca2+ transient may reflect drug-induced facilitation of caffeine action on RyR channels, probably a consequence of increased [Ca]SR-tot.
The gain of CICR was estimated during the last precaffeine voltage pulse as the ratio between SR-released Ca2+ and transmembrane Ca2+ influx. As previously shown (Shannon et al., 2000a), CICR gain was directly related to [Ca]SR-tot (r ≥ 0.72; p < 0.05). Digoxin and PST2744 increased CICR gain to a similar extent (+157 ± 39% versus +139 ± 24%; N.S.) (Fig. 3).
Drug effects on CICR gain. A and B, examples of free cytosolic Ca2+ concentration [Ca]f and membrane current (Im) simultaneously recorded during voltage steps. Trace alignment and symbols as in Fig. 2. C, average values of CICR gain. *, p < 0.05 drug versus control; n = 8 for both digoxin (1 μM) and PST2744 (4 μM).
Effects on the Rate of Clearance of Cytosolic Ca2+. The rate of Ca2+ clearance from the cytosol was measured by the exponential fitting of Ca2+ decays following voltage- and caffeine-induced transients, respectively (Fig. 4). The former reflects operation of all Ca2+ handling mechanisms (mainly SERCA and the Na+/Ca2+ exchanger); the latter is largely accounted for by the Na+/Ca2+ exchanger operation (Bers, 2002d).
Drug effects on the rate of clearance of cytosolic Ca2+. A and B, examples of normalized [Ca]f records during the decay from voltage- and caffeine-induced transients, respectively; traces in control and during drug superfusion (○) are shown in each panel. C and D, average values of the time constant of Ca2+ decay (τdecay) under all experimental conditions. Closed bars refer to voltage-induced transients and open ones to caffeine-induced transients; *, p < 0.05 drug versus control; #, p < 0.05 voltage versus caffeine pulses; n = 6 for both digoxin (1 μM) and PST2744 (4 μM).
As expected, under control conditions Ca2+ decay time constant (τdecay) was smaller during voltage- than during caffeine-induced transients (851 ± 104 versus 1415 ± 280 ms; p < 0.05). Digoxin similarly reduced τdecay during voltage-(–30.1 ± 8.4%, p < 0.05) and caffeine-induced transients (–36.5 ± 7.4%, p < 0.05) (Fig. 4, B and D). PST2744 reduced τdecay in voltage-induced transients (–41.3 ± 7.0%, p < 0.05) significantly more than in caffeine-induced transients (–20.0 ± 3.9%, p < 0.05) (Fig. 4, A and C). For both compounds, higher resting Ca2+ levels were associated with a faster decay of the caffeine-induced transient (r ≥ 0.74, p < 0.05).
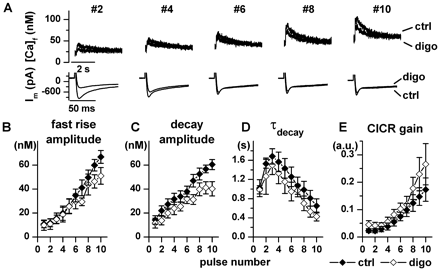
Effects on SR Reloading during Na+/Ca2+Exchanger Inhibition. To evaluate drug effects on SR function in isolation, the Na+/Ca2+ exchanger was blocked by removing Na+ from both sides of the sarcolemma (see protocol 2 under Materials and Methods). After the SR was depleted, all the parameters of Ca2+ transients progressively recovered during subsequent depolarizations. A progressive accumulation of resting Ca2+ occurred due to the absence of Na+/Ca2+ exchanger-mediated Ca2+ extrusion (Fig. 5A). During SR reloading, PST2744 markedly accelerated the recovery of Ca2+ transient amplitude (Fig. 5B), slightly increased decay amplitude (Fig. 5C), and sharply accelerated Ca2+ decay (smaller τdecay values, Fig. 5D). Since ICaL decreased significantly during both digoxin and PST2744 superfusion, data were also analyzed in terms of CICR gain (Fig. 5E) by using a simplified method (see Materials and Methods). Under control conditions, CICR gain started to increase only after several cycles of the loading train, thus suggesting that a “threshold” Ca2+ concentration within the SR is required for efficient Ca2+ release (Shannon et al., 2000a). PST2744 markedly increased CICR gain and eliminated the initial increase of τdecay (Fig. 5D) and the delay in gain changes (Fig. 5E). Effects on SR reloading qualitatively similar to those of PST2744, but of larger magnitude, were observed with the β1-adrenergic agonist isoproterenol (0.1 μM, see Fig. 4 in Supplemental Data); at variance with PST2744, isoproterenol increased ICaL.
Digoxin did not modify any of the Ca2+ transient parameters during early SR reloading cycles and tended to depress Ca2+ reuptake in the late ones (Fig. 6C). Thus, digoxin displayed a behavior distinctly different from PST2744 (compare Fig. 6 to Fig. 5).
Digoxin effects during SR reloading with blocked Na+/Ca2+ exchanger. Protocol and captions as in Fig. 5. n = 6, digoxin 1 μM.
Since SERCA function is up-regulated by high cytosolic Ca2+, the acceleration of Ca2+ decays induced by PST2744 might simply reflect drug-induced increase in cytosolic Ca2+. If so, PST2744 should not affect the relation between τdecay and the Ca2+ concentration present at the onset of decay (initial Ca2+) (Bers and Berlin, 1995). Contrary to this hypothesis, the relation was modified by PST2744, which reduced τdecay at each at each initial Ca2+ level (Fig. 7A). This effect prevailed at low initial Ca2+ levels (leftmost part of the relation in Fig. 7A) and was not shared by digoxin (Fig. 7B).
Dependence of Ca2+ decay rate on initial Ca2+ level. Ca2+ decay time constants (τdecay) measured from the experiments of Figs. 5 and 6 plotted against Ca2+ concentration at the beginning of the decay phase (initial Ca2+). Initial Ca2+ values were binned, and the median of each bin is reported on the abscissa. A, control (ctrl, solid symbols) versus PST2744 (PST, open symbols). B, control versus digoxin (digo, open symbols).
Effects on Twitch Decay Rate. Twitch decay rate was measured by fitting an exponential through the decay phase and defined by its time constant (τrelax). τrelax was decreased by digoxin (–9.4 ± 1.4%, n = 5, p < 0.05) and, to a larger extent, by PST2744 (–28.6 ± 6.8%, n = 5, p < 0.05 versus digoxin; Fig. 8). Notably, the present results are fully consistent with those in vivo showing that PST2744 increased maximum relaxation velocity substantially more than digoxin (Micheletti et al., 2002).
Drug effects on twitch relaxation rate. A and B, sample records of cell shortening during field stimulation. In each panel, raw traces are shown on the left and normalized ones on the right. ^, traces during drug superfusion. C, average values of the time constant of twitch relaxation (τrelax). *, p < 0.05 versus control; #, p < 0.05 digoxin versus PST2744. n = 5 for both digoxin (1 μM) and PST2744 (4 μM).
Discussion
Studies in Isolated Vesicles. The results obtained in isolated vesicles demonstrate that PST2744 and digoxin modulate SR function in opposite ways. PST2744, but not digoxin, stimulated the ATPase activity of SERCA. Conversely, digoxin enhanced spontaneous Ca2+ efflux from the SR, starting at concentrations 1000-fold lower than PST2744.
Cytosolic Ca2+ has been shown to affect SERCA activity both by being its rate-limiting substrate (Bers and Berlin, 1995; Shannon et al., 2000b) and by allosteric modulation through phospholamban (Kranias, 1985). PST2744 markedly increased SERCA Ca2+ affinity (lower Km), thus suggesting that PST2744 may modulate SERCA mainly by affecting its interaction with cytosolic Ca2+. The finding that, in the presence of saturating Ca2+ concentrations, PST2744 effect was minimal is consistent with this hypothesis. In isolated SR vesicles, the number of targets potentially accounting for such an action is limited to SERCA itself and to those of its regulatory proteins, which remain associated with the SR membrane. Among them, phospholamban may be contained in variable amounts in SR vesicles prepared according to the present technique (R. Micheletti, unpublished data), thus constituting a potential target for PST2744 action.
Ca2+ efflux from SR vesicles was sharply enhanced by digoxin. Several cardiac glycosides, including digoxin, were previously shown to increase the open probability of RyR channels in cardiac (digoxin EC50 = 0.20–0.65 nM) (McGarry and Williams, 1993; Sagawa et al., 2002) and skeletal myocytes (Sarkozi et al., 1996). Hence, digoxin effects reported here were not unexpected, and although other mechanisms cannot be ruled out, they may be accounted for by its action on RyR channels. On the other hand, at least at the concentrations acting on SERCA (see above), PST2744 did not affect spontaneous Ca2+ efflux significantly.
Studies in Intact Myocytes. Although SERCA stimulation may increase SR Ca2+ content, an opposite effect is expected from enhancement of Ca2+ leak. In myocytes, such qualitative differences between the two agents were observed when Na+/Ca2+ exchanger was blocked, thus precluding effects secondary to Na+/K+ pump inhibition and unveiling direct modulation of SR targets.
Under such conditions, PST2744 effects were all compatible with a sharp enhancement of Ca2+ uptake by the SR, as expected from stimulation of SERCA activity. Indeed, PST2744 increased the magnitude and rate of Ca2+ reuptake (Fig. 5, C and D), thus stimulating the SR reloading process (Fig. 5B). The marked increase in CICR gain caused by PST2744 (Fig. 5E) may be secondary to larger increments in SR Ca2+ content during the reloading protocol (Shannon et al., 2000a). Consistent with Ca2+ dependence of SERCA stimulation in SR vesicles, acceleration of cytosolic Ca2+ decay by PST2744 prevailed at low Ca2+ levels (Fig. 7A).
Similarity between PST2744 and isoproterenol effects might suggest a receptor- or protein kinase A-mediated mechanism (Tsien et al., 1986). However, PST2744 did not enhance ICaL (Rocchetti et al., 2003), and binding studies showed that this agent is not a ligand of β-adrenergic receptors (Micheletti et al., 2002).
During Na+/Ca2+ exchanger blockade, digoxin effects were compatible with a slight inhibition of the SR reloading process. In particular, the amount of Ca2+ cleared at each cycle was reduced by this agent in the late cycles of the reloading protocol (Fig. 6C). This effect might reflect drug-induced enhancement of Ca2+ leak, which would become more significant as SR Ca2+ content increases (Sagawa et al., 2002). In the light of this, the tendency of digoxin to increase CICR gain (Fig. 6E) implies the presence of a compensatory mechanism. This could be represented by the facilitation of the RyR channel opening, which may favor CICR (McGarry and Williams, 1993). In other words, in the absence of Na+/Ca2+ exchanger, digoxin modulation of CICR gain might be the net result of decreased SR Ca2+ content and RyR channel sensitization to cytosolic Ca2+. However, the latter mechanism may lose its relevance in the presence of normal Na+/Ca2+ exchanger function (Trafford et al., 2000).
When drug effects on Ca2+ dynamics were evaluated in the presence of a normal Na+/Ca2+ exchanger activity, digoxin and PST2744 still differed, but in quantitative rather than in qualitative terms. PST2744 modulation of SERCA was manifested by 1) a larger increment in SR Ca2+ content (Fig. 2C), and 2) preferential acceleration of the Ca2+ decay following voltage-compared with caffeine-induced transients (Fig. 4C). On the other hand, the observation that digoxin and PST2744 effects were qualitatively similar (i.e., in the same direction) implies the contribution of a mechanism based on Na+/Ca2+ exchanger and shared by the two agents. Such requirements are satisfied by Na+/K+ pump blockade, which increases cytosolic Ca2+ through a change in the Na+/Ca2+ exchanger equilibrium potential. Indeed, an increment in cytosolic Ca2+ may, on its own, account for increased [Ca]SR-tot (Bers and Berlin, 1995) and for the resulting increment in CICR gain (Shannon et al., 2000a). Higher Ca2+ may also explain acceleration of Ca2+ decay and twitch relaxation through allosteric Na+/Ca2+ exchanger activation (Weber et al., 2001). This view is reinforced by the observation that τdecay of caffeine-induced transients was inversely related to resting Ca2+ levels.
A finding which might appear at odds with the observation that PST2744 and digoxin differently affect Ca2+ confinement in the SR is the similar increase in resting Ca2+ levels induced by the two agents. However, it should be stressed that diastolic Ca2+ levels closely depend on the Na+/Ca2+ exchanger equilibrium potential (Bers and Bridge, 1989) and, in turn, on Na+/K+ pump function, which was equally affected by the two drugs at the concentrations used (Rocchetti et al., 2003). Thus, a similar elevation of diastolic Ca2+ with the two agents was expected under physiological conditions.
In the presence of Na+/Ca2+ exchanger blockade, SR reloading was associated with a progressive increment in resting cytosolic Ca2+, which is likely to reflect the increase in free SR Ca2+ concentration (with the maximum Ca2+ gradient across SERCA remaining constant) (Shannon et al., 2000b). The observation that PST2744 enhanced resting Ca2+ accumulation (Fig. 5) is compatible with faster SR Ca2+ loading through SERCA stimulation. Indeed, recruitment of more SERCA molecules (e.g., by increasing their affinity for cytosolic Ca2+) may increase Ca2+ flow rate without necessarily affecting the maximum Ca2+ gradient (as batteries connected in parallel supply more current with unchanged voltage).
Conclusions and Practical Implications. Although under physiological conditions Na+/K+ pump inhibition contributed to both inotropy and lusitropy, concomitant SERCA stimulation was associated with quantitative differences in these effects and, most notably, with a widely different therapeutic index (Micheletti et al., 2002; Rocchetti et al., 2003). This suggests that SERCA stimulation may be a key mechanism in tempering the proarrhythmic effect of an increased Ca2+ load, such as that caused by many among inotropic interventions. In light of this, SERCA down-regulation in the failing myocardium (Beuckelmann et al., 1992; Movsesian et al., 1994; Mishra et al., 2002, 2003) might sensitize to the detrimental effect of inotropes. Whether PST2744 may be able to revert this condition is unknown and deserves further investigation.
Limitations of the Study. Although the Ca2+ handling mechanisms considered in this work are largely prevalent (Bers, 2002a), Ca2+ extrusion by the sarcolemmal Ca2+ ATPase and mitochondrial Ca2+ uptake may also contribute to determine cytosolic Ca2+ levels. Based on the present results, it is impossible to rule out interference with such mechanisms as a further cause for the differences between PST2744 and digoxin.
Cytosolic Ca2+ buffering parameters (Bmax and Kd) and the cell surface/volume ratio used in the estimation of cytosolic Ca2+ levels were not available for guinea pig myocytes, and the values reported for rabbit ventricular myocytes have been used instead (Table 1). This might make absolute quantitative estimates imprecise, but it would not affect the comparison between drug effects.
Acknowledgments
We are grateful to Prof. Giuseppe Bianchi for providing stimulating discussion and thoughtful criticism throughout the execution of the study and compilation of the manuscript.
Footnotes
-
This work was funded by Istituto di Ricerche Prassis Sigma-Tau and from Grant Ministero Università e Ricerca Scientifica e Tecnologica (MURST) 2000 (to A.Z.). Collaboration with Debrecen University was supported by an Italy/Hungary cooperation program of the Ministry for Foreign Affairs.
-
doi:10.1124/jpet.104.077933.
-
ABBREVIATIONS: SR, sarcoplasmic reticulum; PST2744, (E,Z)-3-((2-aminoethoxy)imino)androstane-6,17-dione hydrochloride; PIPES, 1,4-piperazinediethanesulfonic acid; SERCA, sarcoplasmic reticulum Ca2+-ATPase; MOPS, 4-morpholinepropanesulfonic acid; A.U., activity units (micromoles of Pi per milligrams of protein per minute; APIII, antipyrylazo-III; CICR, Ca2+-induced Ca2+ release; DMSO, dimethyl sulfoxide.
-
↵
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material. - Received September 15, 2004.
- Accepted November 30, 2004.
- The American Society for Pharmacology and Experimental Therapeutics