Abstract
The novel Na+/K+-ATPase inhibitor (E,Z)-3-((2-aminoethoxy)imino)androstane-6,17-dione hydrochloride (PST2744) was characterized for its inotropic and toxic properties. Inhibition potency on dog kidney Na+/K+-ATPase was comparable (0.43 μM) to that of digoxin (0.45 μM). PST2744 concentration-dependently increased force of contraction in guinea pig atria and twitch amplitude in isolated guinea pig myocytes; in the latter, aftercontractions developed significantly less than with digoxin. Intravenous infusion of 0.2 mg/kg/min PST2744 in anesthetized guinea pigs exerted an immediate and long-lasting inotropic effect (ED80 of 1.89 ± 0.37 mg/kg) without causing lethal arrhythmias up to a cumulative dose of 18 mg/kg. Conversely, an equieffective infusion of digoxin (0.016 mg/kg/min; ED80 of 0.32 mg/kg) caused lethal arrhythmias at a cumulative dose of 0.81 mg/kg. At a higher rate (0.4 mg/kg/min), PST2744 induced lethal arrhythmias, with a lethal dose/ED80 ratio significantly greater than digoxin (20.2 ± 6.3 versus 3.23 ± 0.55, p < 0.05). Decay of the inotropic effect (t1/2, min) was significantly faster for PST2744 (6.0 ± 0.39) than for digoxin (18.3 ± 4.5, p < 0.05). In anesthetized dogs, PST2744 dose-dependently increased maximum velocity of pressure rise (+dP/dtmax) in the range 32 to 500 μg/kg i.v. and was safer than digoxin. In conscious dogs with a healed myocardial infarction, PST2744 significantly increased resting values of +dP/dtmax, left ventricular pressure, and SPB, and increased +dP/dtmax throughout treadmill exercise while reverting the increase in left ventricular end diastolic pressure seen in control animals. Digoxin significantly decreased basal heart rate, while not affecting the hemodynamic response to exercise. Thus, PST2744 represents a new class of Na+/K+-ATPase inhibitors endowed with inotropic activity comparable with that of digitalis but having greater safety.
Improvement of heart failure (HF) treatment remains a major medical challenge for the coming years. Despite achievement of considerable progress during the last two decades, none of the available therapeutic agents is fully satisfactory, and HF remains a disease with a poor prognosis (Kannel, 2000). The goal of ameliorating quality of life, exercise capability, and survival is only partially met by angiotensin-converting enzyme inhibitors, diuretics, vasodilators, glycosides, and β-blocking agents, regardless of their being used in combined therapy (Consensus Recommendations for the Management of Chronic Heart Failure, 1999). Therefore, the need still remains for novel drugs able to increase workload tolerance and to reduce morbidity and mortality. Worsening of survival found with chronic administration of inotropic agents that increase intracellular cAMP (phosphodiesterase inhibitors and sympathomimetic amines) (The Xamoterol in Severe Heart Failure Study Group, 1990; Packer et al., 1991; Cohn et al., 1998) and the well known arrhythmogenic effects of cardiac glycosides have led to the general view that drug-induced inotropy per se may be associated with an increased risk of death. On the other hand, such a view is challenged by the outcome of the Digitalis Investigation Group trial (The Digitalis Investigation Group, 1997), a study specifically designed to assess the effect of long-term digoxin treatment on survival. Although improving the functional status and reducing the rate of hospitalization due to HF recurrences, digoxin did not affect overall mortality in patients receiving diuretics and angiotensin-converting enzyme inhibitors. This can be interpreted as if a beneficial increase in inotropy were balanced by a concomitant increase in the incidence of lethal arrhythmias, both effects being associated to digoxin administration. Thus, the proarrhythmic effects of inotropic agents, rather than inotropy per se, may be responsible for an increase in mortality in HF patients. This provides a strong motivation for the search of inotropic agents devoid of proarrhythmic effects. Inhibition of Na+/K+-ATPase, the “classical” mechanism of glycoside inotropy, is generally regarded with suspicion because of the view that the resulting increase in intracellular Ca2+ may be the basis for both inotropic and proarrhythmic effects. However, evidence that Na+/K+-ATPase inhibition may not be the only site of digitalis action (Sagawa et al., 2002), opens the possibility of a dissociation between inotropic and proarrhythmic effects of Na+/K+-ATPase inhibitors. The present study investigates the pharmacological properties of (E,Z)-3-((2-aminoethoxy)imino)androstane-6,17-dione hydrochloride (PST2744) (Fig. 1), a derivative of 3,6,17-androstanetrione chemically unrelated to cardiac glycosides but able to inhibit Na+/K+-ATPase and increase cardiac inotropy with similar potency. PST2744 stems from a molecular modeling study proposing a new three-dimensional model for the binding of cassaine at the digitalis receptor site (De Munari et al., 1998). Suitability of the model was corroborated by the activity of novel potent inhibitors of the Na+/K+-ATPase devoid of any of the structural characteristics peculiar to the digitalis structure (Gobbini et al., 2001). The evaluation presented herein, extending from in vitro to whole animal studies, provides concordant evidence of a substantial difference between PST2744 and digoxin, mainly consisting of a sharp dissociation between toxic and inotropic effects.
Chemical structure of PST2744.
Materials and Methods
Studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health.
In Vitro Assays
Inhibition of Na+/K+-ATPase.
Dog or guinea pig kidney outer medulla was homogenized with a Polytron in 250 mM sucrose and 30 mM histidine, at pH 7.2. The homogenate was centrifuged at 6,000g for 15 min at 4°C and the supernatant at 48,000g for 30 min at 20°C with SDS and then layered onto a discontinuous sucrose density gradient (10, 15, and 29%) and centrifuged at 60,000 rpm for 115 min at 4°C. The pellet was resuspended in 25 mM imidazole and 1 mM EDTA, pH 7.5. Protein content was measured by the method of Lowry et al. (1951). Na+/K+-ATPase activity was measured after the release of 32P from [32P]ATP. Increasing concentrations of compounds were preincubated with purified enzyme for 10 min at 37°C in 120 μl of final volume of medium containing 140 mM NaCl, 3 mM MgCl2, 50 mM HEPES-Tris, and 3 mM ATP, pH 7.5. After preincubation, 10 μl of incubation solution containing 10 mM KCl and 20 nCi of [32P]ATP (0.5–3 Ci/mmol) was added, and the reaction was carried out for 15 min at 37°C before being stopped by acidification with 30% (v/v) perchloric acid.32P was separated by centrifugation with activated charcoal and radioactivity measured by liquid scintillation counting. Inhibitory activity was expressed as percentage of control sample, carried out in the absence of standard compound. IC50 was calculated by weighed nonlinear regression curve fitting to the mass-action equilibrium.
Evaluation of Receptor Specificity.
Radioligand binding to a number of receptors was carried out by MDS Panlabs Pharmacology Services (Taipei, Taiwan) on crude membrane preparations according to published procedures, and by using appropriate reference standards. Interaction with human phosphodiesterases III was evaluated by an enzymatic assay. Interaction with Na+channels was investigated in paced rat left atria.
Ex Vivo Assays
Inotropy in Isolated Guinea Pig Ventricular Myocytes.
Guinea pigs (Charles River Italia, Calco, Italy) weighing 200 to 300 g were anesthetized with a xylazine (1.8 mM) and ketamine (22 mM) mix, killed through cervical dislocation, and exsanguinated. Ventricular myocytes were enzymatically dissociated with a procedure described previously (Zaza et al., 1998) and used within 12 h from isolation. The myocyte suspension was placed in a 30-mm Petri dish on the stage of an inverted microscope (Nikon, Tokyo, Japan). The dish was perfused at 2 ml/min with standard external Tyrode's solution, containing 154 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 5 mM HEPES-NaOH, and 5.5 mMd-glucose, adjusted to pH 7.35. The cell under study was held at the mouth of a thermostated manifold pipette, which allowed exposing the cell to different solutions, with changes completed in about 1 s. The solution temperature was monitored at the pipette tip with a fast-response digital thermometer (Physitemp Instruments, Clifton, NJ) and kept at 35 ± 0.5°C. Cells were electrically stimulated at 2 Hz through a micropipette electrode positioned in the vicinity of the cell. Extracellular stimulation was selected to avoid cytoplasm contamination and buffering, which might interfere with cell contractility and its modulation. Cell motion was monitored at a sampling rate of 200 Hz by a video edge detector (Crescent Electronics, Sandy, UT) connected to a noninterfaced camera. A voltage signal proportional to cell length was generated by the device, digitized at 1 KHz (Digidata 1200; Axon Instruments, Union City, CA), and recorded on a PC through dedicated software (pClamp 7.0; Axon Instruments). Twitch amplitude was measured as the difference between peaks and troughs of the voltage signal.
Inotropy in Isolated Guinea Pig Atria.
Male guinea pigs (350–450 g) were decapitated, the heart was rapidly excised, and the left atrium was set up under 500 mg of tension in 5-ml baths containing a solution of the following composition: 132 mM NaCl, 5.6 mM KCl, 1.8 mM CaCl2, 25 mM NaH2PO4, 11 mM glucose, and 13 mM saccarose, oxygenated with 95% O2/5% CO2, and maintained at 32°C. The preparations were stimulated to contract by means of platinum electrodes at 1 Hz, with pulses of 1-ms duration and amplitude twice the threshold. After 60-min equilibration, the compounds under study were added to the bath in cumulative concentrations until a plateau was reached or arrhythmias occurred. Results were expressed as percentage of variation of basal force. EC50 were calculated graphically.
In Vivo Assays
Inotropic and Toxic Effect in Anesthetized Guinea Pigs.
Male guinea pigs (350–450 g) were used. Experiments were performed on normal animals or pressure-overloaded animals and their respective sham-operated controls. Pressure overload was induced in ketamine-xilazine (100–5 mg/kg i.p.)-anesthetized animals by constriction of the descending thoracic aorta; experiments were carried out 8 weeks later.
Normal animals, sham-operated, or pressure-overloaded animals were anesthetized with urethane (1.5 g/kg i.p.). Body temperature was maintained at 37°C by a homeothermic blanket system (Harvard Apparatus, South Natick, MA). A microtip pressure transducer (SPR-407; Millar Instruments, Houston, TX) was introduced into the left ventricle through the right carotid artery to measure ventricular pressure (LVP); the transducer was coupled with a transducer amplifier (model 13-4615-50; Gould, Cleveland, OH). Recordings were fed to an RS 3800 polygraph (Gould) and to an AST 486 computer and analyzed by IDAS software (Biomedica Mangoni, Pisa, Italy). A polyethylene 50 cannula was inserted into a jugular vein for drug infusion and the trachea was intubated to facilitate spontaneous respiration. ECG was telemetrically recorded by means of a TA10CA-F40 transmitter and stored on a hard disk (Dataquest software; Data Sciences, St. Paul, MN). After a stabilization period, the test substance was injected at the rate of 0.16 ml/min by means of a P22 pump (Harvard Apparatus) until the animal died, or up to a maximum of 90 min. In a second set of experiments, infusion was stopped at fixed times (40 min for digoxin and 90 min for PST2744) to evaluate the rate of recovery of basal maximum velocity of pressure rise (+dP/dtmax). The following parameters were obtained for each animal: heart rate (HR), LVP, +dP/dtmax, and maximum velocity of pressure fall (−dP/dtmax). In addition, the times elapsed until 80%, and maximum increase, of +dP/dtmaxover basal were determined to calculate the dose increasing basal +dP/dtmax by 80% (ED80) and dose inducing the maximum increase in +dP/dtmax (EDmax). The dose administered until the onset of arrhythmias and the lethal dose (dose inducing death, LD) were also calculated. The latter are given as mean ± S.E.M. when possible or as the maximum dose administered during 90 min.
Dose-Response in Anesthetized Dogs.
Beagle dogs (10–11 kg; Green Hill, Brescia, Italy) were used after being fasted for 18 h, under pentobarbital anesthesia (25 mg/kg bolus followed by 1 mg/kg/h infusion), with room air ventilation (positive pressure ventilator; Harvard Apparatus). Body temperature was maintained at 38°C by a thermostatic blanket. A 6F Millar double microtip transducer (Millar Instruments) connected to two AC preamplifiers (model 612; Biomedica Mangoni) was introduced into the left ventricle via a femoral artery for simultaneous measurement of aortic and left ventricle pressures. ECG (lead II) was recorded by subcutaneous needle electrodes (ECG recorder model 613; Biomedica Mangoni). After adequate stabilization of hemodynamic parameters, increasing doses of compounds under study were injected in a semicumulative manner by slow bolus administration. Effects were measured at their maximum or 15 min after compound administration.
Hemodynamic Effects in Dogs with Myocardial Infarction during Exercise.
Surgical procedure. Animals under pentobarbital anesthesia (25 mg/kg i.v. bolus followed by 2 mg/kg/h infusion) were implanted with one solid-state pressure transducer (PA 4.5-X6; Könisberg Instruments, Pasadena, CA) into the left ventricle and one into the aortic arch to monitor left venticular and aortic pressures. A hydraulic occluder was placed around the left circumflex coronary artery, proximal to the marginal branch to induce acute ischemia. The leads were tunneled under the skin to exit at the nape of the neck. Myocardial infarction was induced by a ligature on the second segment of the left anterior descending coronary artery. Animals were placed in an intensive care setting, and received analgesic (tramadol i.m. every 12 h) and antibiotic (ceftazidime) therapy for 2 and 5 days, respectively.
Exercise protocol.
The treadmill exercise program lasted 18 min, starting with a 3-min warm-up at 4 km/h. Thereafter, speed was increased to 9.5 km/h and then grade was increased to 4, 8, 12, and 16%. Each setting was maintained for 3 min. Animals were trained twice a week for 2 weeks before entering the study. ECG, LVP, and aortic pressures were monitored throughout the exercise test. Susceptibility to ventricular fibrillation was assessed during the last minute of exercise, when heart rate was approximately 220 bpm, by occluding the left circumflex artery. The treadmill was then abruptly stopped and the occlusion maintained for 2 min longer. The hemodynamic values reported were taken 15 min after compound administration, or at the maximum effect observed.
PST2744 (300 μg/kg) or digoxin (75 μg/kg) were given by i.v. bolus 10 and 20 min before starting the exercise, respectively, and compared with vehicle. The following variables, HR, ECG, LVP, and aortic pressures, were recorded through a computerized acquisition system (IOX 1.5; Emka Technologies, Paris, France), which calculated the left ventricular rates of pressure changes. Data were analyzed from the real-time digitized recordings. Control values were obtained before compound administration.
Compounds
PST2744 was synthesized at Prassis Laboratories (U.S. patent 5914324). For in vitro studies, PST2744 was dissolved in water and digoxin in 4% DMSO. For studies in isolated myocytes, stock solutions of PST2744 (10 mM in distilled water) and digoxin (10 mM in DMSO) were diluted in Tyrode's solution. The maximum final concentration of DMSO was 0.05%.
For in vivo studies PST2744 was dissolved in physiological solution (guinea pigs) or in the vehicle of Lanoxin (Wellcome Italia, Pomezia, Italy). Digoxin (batch 75H0677; Sigma Italia, Milano, Italy) was dissolved in the vehicle of Lanoxin (quantity in 1 ml: 0.105 ml of ethanol, 0.415 g of propylene glycol, 0.75 mg of citric acid, 0.452 mg of sodium phosphate dibase, and distilled water qs to 1 ml). The vehicle was diluted with physiological solution to a final percentage of 35%. Urethane was purchased from Fluka (Buchs, Switzerland), ketamine (Inoketam) from Virbac (Carros, France), and xylazine (Rompun) from Bayer AG (Leverkusen, Germany).
Statistical Analysis
Data are presented as means ± S.E.M. Student'st tests for paired or unpaired observations and one-way analysis of variance were used for comparison of numeral variables. Dose dependence of effect was evaluated by linear regression analysis on individual data points. Differences among proportions were tested by chi square analysis (SAS 6.12 software). A p < 0.05 is used to define statistical significance.
Results
In Vitro Studies
Inhibition of Na+/K+-ATPase.
PST2744 inhibited the Na+/K+-ATPase activity from dog kidney with an IC50 value of 0.43 ± 0.15 μM. Under the same conditions, digoxin IC50 was 0.45 ± 0.07 μM. Inhibition of Na+/K+-ATPase activity in preparations from guinea pig kidney yielded potencies of 8.5 μM for PST2744 and 1.3 μM for digoxin. Thus, although PST2744 activity was 20-fold lower in this species, digoxin potency differed by less than 3-fold.
Selectivity of Receptor Interaction.
The interaction of PST2744 with several receptors was investigated at a concentration of 10 μM. The compound did not significantly interact with adenosine, α- and β-adrenergic, dopamine, estrogens, GABA, glucocorticoid, glutamate, glycine, histamine, insulin, muscarinic, opiate, progesterone, serotonin, and testosterone receptors. Similarly, no interaction could be demonstrated with calcium, potassium, or sodium channels, or with the phorbol ester or phosphodiesterases III.
Ex Vivo Studies
Inotropy and Toxicity in Isolated Guinea Pig Ventricular Myocytes.
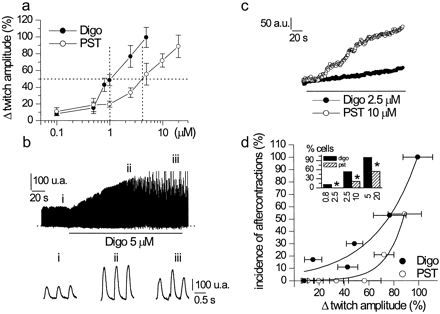
In this set of experiments, the effects of PST2744 and digoxin on the stimulated twitch were compared in single myocytes isolated from the guinea pig ventricle. Measurements were performed during steady-state electrical stimulation at 2 Hz. Twitch amplitude was expressed as cell shortening normalized (percentage) to relaxed cell length. To minimize the impact of intercellular variability on the shape of the dose-response curve, at least a low and a high concentration were tested in each cell. A total of 105 cells from 11 animals was used, with a minimum of seven cells at each drug concentration. Both PST2744 and digoxin caused a concentration-dependent increase in twitch amplitude (Fig.2a). At high concentrations, further increments of inotropic effect were often associated with the appearance of unstimulated twitches of irregular amplitude (aftercontractions; Fig. 2b), which prevented achievement of a plateau. Whenever aftercontractions appeared during development of the response, drug effect was expressed as the increment of twitch amplitude achieved just before the first aftercontraction had developed. Threshold drug concentrations were roughly similar for the two drugs, but the steep portion of PST2744 response curve was shifted to the right (Fig. 2a). The graphically estimated concentrations increasing twitch amplitude to 150% of basal were approximately 4 μM for PST2744 and 1 μM for digoxin; nonetheless, the maximum inotropic effect that could be achieved with the two agents was similar (88.9 ± 13.4 versus 99.6 ± 11.9%, N.S.). The onset and washout of PST2744 inotropic effect were considerably faster than those of digoxin, as illustrated in Fig. 2c for the onset of two equieffective concentrations. When comparing equieffective concentrations, the incidence of aftercontractions, a sign of toxicity, was significantly lower for PST2744 than for digoxin (Fig. 2d, inset). Because inotropic potency differed between the two agents, differences in toxicity can be best appreciated by plotting the proportion of cells developing aftercontractions as a function of the inotropic response that could be achieved before their onset (Fig. 2d). This analysis shows that the percentage of increase in twitch amplitude (i.e., the inotropic effect) that could be achieved in the absence of aftercontractions was up to 60% for PST2744 and below 20% for digoxin.
Comparison between PST2744 and digoxin in single guinea pig ventricular myocytes. a, percentage of increase in twitch amplitude from control (predrug) value is plotted versus drug concentration. The dotted lines mark PST2744 and digoxin concentrations required to achieve 50% increase in twitch amplitude. b, example of aftercontractions induced by digoxin. Samples of the top trace at an expanded time scale are shown below. c, example illustrating the different rate of onset of inotropic effect for equieffective concentrations of PST2744 and digoxin (10 versus 2.5 μM). d, percentage of cells developing aftercontractions as a function of inotropic effect. Solid lines represent best fit of data points with an arbitrary function. The incidence of aftercontractions at different pairs of equi-inotropic drug concentrations is shown in the inset (∗,p < 0.05).
Inotropy in Isolated Guinea Pig Atria.
PST2744 concentration-dependently increased the force of contraction of guinea pig paced left atria in the range 0.3 to 30 μM. The maximum increase in force averaged 140 ± 16% of control (n = 4) with an EC50 value of 8.1 ± 1.25 μM. Digoxin was active in the range 0.03 to 1 μM, inducing a maximum inotropy of 180 ± 29% with an EC50 value of 0.38 ± 0.05 μM (n = 6) The digoxin/PST2744 potency ratio was 21.3 in this preparation.
In Vivo Assays
Inotropic and Toxic Effect in Anesthetized Guinea Pigs.
Normal animals. The main results seen during infusion of 0.2 mg/kg/min PST2744 and 0.016 mg/kg/min digoxin are shown in Figs. 3-5. Data on PST2744 refer to the whole 90-min duration of infusion, whereas those on digoxin are restricted to the first 60 min of infusion, because thereafter the occurrence of arrhythmias prevented recording of hemodynamic parameters.
PST2744 induced a progressive increase in +dP/dtmax throughout the infusion (Fig.3) that reached 80% (ED80) at the cumulative dose of 1.89 ± 0.37 mg/kg and a peak of 140 ± 3.5% at the dose (EDmax) of 4.88 ± 0.6 mg/kg (Table1). A comparable increase (127 ± 26%) was afforded by digoxin at a 6-fold lower cumulative dose of 0.76 ± 0.095 mg/kg. Digoxin ED80 was 0.32 ± 0.039 mg/kg. Both compounds increased the maximum relaxation velocity (−dP/dtmax); however, the maximum effect was substantially larger for PST2744 (72% at 3.3 mg/kg) than for digoxin (33% at 0.56 mg/kg; p < 0.05 versus PST2744).
Effect of i.v. infusion of PST2744 (squares) or digoxin (triangles) on +dP/dtmax in the anesthetized normal guinea pig.
Inotropy and toxicity due to i.v. infusion of PST 2744 or digoxin in the anesthetised intact guinea pig
Infusion of compounds resulted in a negative chronotropic effect, although with different modalities. With PST2744, HR fell from 324 ± 8 to 279 ± 5 bpm in 10 min (p < 0.01) and then progressively returned toward basal value in spite of continuing infusion (Fig. 3). With digoxin the fall in HR was slower and monotonic, going from 334 ± 15 to 298 ± 14 bpm in 25 min (p < 0.01, not significantly different from PST2744; Fig. 4).
Effect of i.v. infusion of PST2744 (squares) or digoxin (triangles) on HR in the anesthetized normal guinea pig.
Basal LVP was 62 ± 3.5 mm Hg in the PST2744 group and 71.4 ± 4.1 mm Hg in the digoxin group (N.S.). PST2744 caused a biphasic change in LVP, with a 30% increase during the early 10 min of infusion, and a decrease toward control values occurring during the remaining infusion (Fig. 5). The LVP changes caused by digoxin were monotonic and consisted in a progressive increase up to 26% of control occurring throughout the infusion period.
Effect of i.v. infusion of PST2744 (squares) or digoxin (triangles) on LVP in the anesthetized normal guinea pig.
The effect of PST2744 on systolic and diastolic pressure was biphasic and paralleled that on LVP; the effect of digoxin was also parallel to that of LVP, i.e., was maintained throughout the infusion period (data not shown).
Although nonlethal arrhythmias occurred in 30% (3 of 10) of animals treated with PST2744 at a cumulative dose of 7.4 ± 2.2 mg/kg, a cumulative dose of 0.81 ± 0.12 mg/kg digoxin induced lethal arrhythmias in 100% of the animals (10 of 10; p < 0.05 versus PST2744). Accordingly, none of the animals receiving PST2744 died up to a maximum cumulative dose of 18 mg/kg. In contrast, 100% mortality was observed in the digoxin group at the mean cumulative dose of 1.07 ± 0.10 mg/kg, (i.e., an 18-fold lower dose). Thus, at equieffective infusion rates, PST2744 was consistently safer than digoxin.
To better assess the safety ratio of PST2744, experiments were repeated, doubling the infusion rate of compound. At 0.4 mg/kg/min, PST2744 yielded ED80 and EDmax values substantially unchanged (Table 1) and a nonsignificantly greater increase in +dP/dtmax (p = 0.06). The main effect of doubling the concentration of PST2744 was that seven of eight animals displayed arrhythmias and subsequently died. However, when the safety of the two compounds was compared, it appeared that the LD/ED80 ratio was significantly better for PST2744 than for digoxin (p < 0.05; Table 1).
Animals with cardiac failure.
Total heart weight was 2742 ± 56 mg in pressure-overloaded animals compared with 2164 ± 50 mg in the respective sham group (p < 0.001). The left ventricle weight normalized to body weight increased by 28% (p < 0.01) and atrial weight by 70% (p < 0.01), showing the presence of significant left ventricle hypertrophy and atrial enlargement. Lung congestion was also observed, with lung weight-to-body weight index (milligrams per gram) increasing from 4.47 ± 0.07 to 7.94 ± 0.33 (p < 0.001). Thus, 8 weeks after constriction of the descending aorta left ventricle hypertrophy and failure could be demonstrated. These morphometric parameters were equivalent in the animals randomly assigned to treatment with 0.2 mg/kg/min PST 2744 or 0.018 mg/kg/min digoxin.
As detailed in Table 2, potency and toxicity of either compound were unaffected by the presence of HF. In total, 44% of HF animals receiving PST 2744 died at a cumulative dose 8 times higher than the ED80(16.9 versus 2.05 mg/kg), whereas the remaining 56% survived to a maximum dose of 18 mg/kg. In contrast, digoxin caused death in 100% of the animals at a cumulative dose (1.16 ± 0.07 mg/kg) only 3 times higher than the ED80 (0.39 mg/kg).
Inotropy and toxicity due to i.v. infusion of PST 2744 or digoxin in the anesthetized guinea pigs with (HF) and without (sham) aortic constriction
Reversal of Inotropy.
In some experiments (n = 6 each), infusion was discontinued to evaluate the time course of washout of inotropic effects. At the interruption of infusions producing comparable inotropic effects of the two drugs, the time required for a 50% reduction of +dP/dtmax was 6.0 ± 0.39 min for PST2744 and 18.3 ± 4.5 min for digoxin (p < 0.05).
Dose-Response in Anesthetized Dog.
Basal HR and +dP/dtmax values were similar in PST2744 (n = 4) and digoxin (n = 3) treatment groups [HR (bpm): 135 ± 13 versus 140 ± 7; +dP/dtmax (mm Hg/s): 2658 ± 96 versus 3122 ± 202]. Both agents dose-dependently increased +dP/dtmax (p < 0.001; Fig.6) with a threshold at 32 μg/kg for PST2744 and at 60 μg/kg for digoxin; at the latter dose digoxin produced an effect comparable with the one of PST2744 at a similar dose. Although HR was dose-dependently decreased by digoxin (p < 0.05), to a minimum of 97 bpm (n= 2 for this dose), an increase to a maximum of 187 ± 4 bpm was observed with PST2744 (p < 0.05), mostly due to the effect of the highest dose.
Changes in +dP/dtmax (Δ over basal) induced by i.v. bolus administration of increasing doses of PST2744 (squares) or digoxin (triangles) in the anesthetized dog.
With PST2744, rhythm disturbances (slow junctional rhythm) were observed at 250 μg/kg in two of four dogs; junctional tachycardia occurred at 500 μg/kg in one of three dogs. In all instances, dogs recovered a normal rhythm within 30 min after the bolus injection.
Death after sinoatrial block and ventricular fibrillation was observed in one case at 60 μg/kg digoxin. The two remaining animals that received 80 μg/kg displayed junctional ectopic beats followed by junctional tachycardia. Thus, the increase in dP/dtmax induced by the highest nontoxic dose was 115% for PST2744 and 42% for digoxin.
Hemodynamic Effects in Dogs with Myocardial Infarction during Exercise.
The resting hemodynamic parameters for the three experimental groups obtained before and after compound administration are shown in Table 3. Pretreatment values did not differ. PST2744 significantly increased resting values of LVP (p < 0.001), SBP (p < 0.05), and +dP/dtmax (p < 0.01). Digoxin significantly decreased HR (p < 0.05), without modifying the other parameters.
Resting hemodynamic parameters in conscious dogs before and after compound administration
As expected, exercise significantly increased HR, systolic LVP, systemic pressures, and +dP/dtmax; the increase in HR and +dP/dtmax reached a maximum at the same workload (Figs. 7 and8). A tendency to exercise-induced increase in LVEDP occurred that roughly paralleled the increase in HR (Fig. 9). Neither compound affected the response of HR and systolic LVP to exercise (data not shown). A clear-cut increase in +dP/dtmax was induced by PST2744 at all exercise levels, whereas digoxin failed to do so (Fig.8). The exercise-induced increase in LVEDP seemed blunted by digoxin and reversed by PST2744 (Fig. 9). Reported in Table4 are comparisons made at the submaximal exercise level, 9.5 km/h with an 8% grade.
Changes in HR induced by treadmill exercise after bolus i.v. administration of PST2744 (squares) and digoxin (triangles) in conscious dog with myocardial infarction in comparison with vehicle-treated animals (asterisks).
Increase in +dP/dtmax (Δ from basal) induced by treadmill exercise after bolus i.v. administration of PST2744 (squares) and digoxin (triangles) in conscious dog with myocardial infarction in comparison with vehicle-treated animals (asterisks).
Changes in LVEDP induced by treadmill exercise after bolus i.v. administration of PST2744 (squares) and digoxin (triangles) in conscious dog with myocardial infarction in comparison with vehicle-treated animals (asterisks).
Effect of treatment vs. vehicle on hemodynamic parameters at submaximal exercise level (9.5 Km/h, 8% grade)
None of the control animals displayed arrhythmias during coronary occlusion, or in the recovery period subsequent to reperfusion. PST2744 did not modify this behavior and neither did digoxin.
Discussion
Results from these studies carried out in two species, guinea pig and dog, show that PST2744 is a potent inotropic agent both under anesthesia and in the conscious state, and signally during exercise-induced increase in workload. Importantly, under all experimental conditions PST2744 seems endowed with substantially greater safety than the reference inotropic drug digoxin. Safety in vivo is related to a lower incidence of arrhythmias and arrhythmia-sustained death. Experiments in isolated myocytes indicate that this correlates with a lower incidence of aftercontractions.
The conclusion that PST2744 possesses a significantly higher safety ratio than digoxin is supported by the following findings. In isolated guinea pig myocytes no aftercontractions were elicited by a PST2744 concentration able to increase cell shortening up to 60%, whereas with digoxin, aftercontractions ensued already at a 20% increase in cell shortening. In anesthetized guinea pigs, inotropic effects that could be obtained with PST2744 in the absence of death were achieved by a dose of digoxin causing 100% mortality. Even when PST2744 infusion rate was increased to induce death, its LD/ED80ratio was over 6-fold larger than that of digoxin. PST2744 maintained a greater safety than digoxin also in the presence of heart failure induced by pressure overload. In anesthetized dogs digoxin induced arrhythmias and death at the threshold inotropic dose; conversely, nonlethal arrhythmias with PST2744 were first observed at a dose increasing +dP/dtmax by over 4000 mm Hg/s and 7.8 times higher than the threshold inotropic one.
Some important qualitative differences also exist between PST2744 and digoxin inotropic effects. First, the rate of onset and decay of inotropic effect was significantly faster for PST2744 both in isolated myocytes and in anesthetized guinea pigs. Second, during sustained drug exposure, although the inotropic effect of PST2744 easily reached a plateau, the one of digoxin slowly drifted until the appearance of aftercontractions (myocytes) or arrhythmias (anesthetized guinea pigs). Finally, in dogs with a myocardial infarction, exercise was associated with an increase in LVEDP, possibly reflecting latent diastolic dysfunction. Noticeably, PST2744 reversed such a pattern. This effect of PST2744 may conceivably be related to the positive lusitropic properties clearly seen in the anesthetized guinea pig.
We did not aim herein at clarifying the mechanism underlying the greater safety of PST2744. The differences disclosed in this study between the two Na+/K+-ATPase inhibitors PST27444 and digoxin might underlie differences in their mechanism of action. We might speculate that because aftercontractions due to digitalis glycosides have recently been shown to depend on the reversal of the Na+-Ca2+ exchanger transport mode (Satoh et al., 2000; Sagawa et al., 2002), PST2744 interaction with this transporter may differ from that of digoxin. Characterization of PST2744 effect on the Na+-Ca2+ exchanger needs to be performed in view of the reported up-regulation of this transporter in human failing hearts (Studer et al., 1994; Flesch et al., 1996;Reinecke et al., 1996) and of the reciprocal regulation between cardiac Na+/K+-ATPase and Na+-Ca2+ exchanger (Magyar et al., 1995). Although we demonstrated herein a selective interaction of PST2744 for the Na+/K+-ATPase receptor, findings such as the positive lusitropy not shared by digoxin are strongly suggestive of peculiarities in Ca2+handling mechanisms. Likewise, it may not be excluded that PST2744 interacts with a discrete isoform of the Na+/K+-ATPase enzyme, because it has been suggested that different isoforms may be responsible for the inotropic and arrhythmogenic effects (Zahler et al., 1992, 1996; James et al., 1999). In this respect, the wide disparity in potencies toward guinea pig and canine Na+/K+-ATPase kidney receptor displayed by PST2744 at variance with digoxin might reflect interaction with different isoforms, although species specificity is probably more likely.
Further peculiarities of PST2744 in comparison with digoxin were found in whole animal experiments. In anesthetized guinea pigs, a transient decrease in HR occurred during the early infusion period with PST2744, followed by a return toward control values in spite of continuing infusion. This pattern of HR changes was paralleled by a transient increase in SBP having the same time course. In contrast, a slow and steady negative chronotropic effect was observed with digoxin. Thus, the HR reduction induced by PST 2744 in the guinea pig seems to be secondary to baroreceptor response to blood pressure increase and may thus differ from that of digoxin, commonly attributed to vagal activation. The negative chronotropic effect of digoxin was also found in both anesthetized and conscious dog. Conversely, in the former, although at the highest doses, the effect of PST2744 on HR was opposite to that of digoxin. Thus, it may be concluded that, unlike digoxin, PST2744 does not stimulate the vagal tone.
The experimental exercise model used herein deserves comment because it mimics a functionally relevant condition in humans, i.e., the increase in workload induced by physical activity in a condition of mild ischemia. Importantly, PST2744-treated dogs show a favorable hemodynamic profile at a stage of submaximal well tolerated stress (Table 4). This infarction model does not easily evolve into heart failure. The healed infarcted myocardium has, however, been reported as a site of enhanced susceptibility to glycosides toxicity (Iesaka et al., 1983). Importantly, when an acute ischemic episode was added to the background ischemia by occluding the circumflex artery, PST2744 did not exhibit proarrhythmogenic properties. To this regard it should be mentioned that the doses used in this experiment for either compounds were 20 to 25% above those found to be arrhythmogenic in the anesthetized dog, because preliminary experiments showed that anesthesia increases the susceptibility to arrhythmias.
Whether the peculiar features of PST2744 described above reflect different pharmacokinetic properties, interaction with specific Na+/K+-ATPase isoforms, or a different regulation of the Na+-Ca2+ exchanger is not known. We demonstrated a selective interaction of PST2744 with the Na+/K+-ATPase receptor, excluding all known inotropic mechanisms; however, interaction with yet unknown mechanisms or different postreceptor mechanisms may not be ruled out.
In conclusion, a low propensity of PST2744 to induce rhythm disturbance was seen in all experimental settings used, and differentiates the compound from digoxin, the most commonly prescribed cardiac glycoside used as reference drug. Results in isolated myocytes suggest that the underlying cause for this difference resides mainly at the cell level. Finally, if the faster rate of onset and dissipation of effects seen with PST2744 could be extrapolated to the clinical setting, PST2744 might be anticipated to have better therapeutic handling and a larger safety margin against fluctuation of drug plasma levels than digoxin.
Footnotes
-
DOI: 10.1124/jpet.102.038331
- Abbreviations:
- HF
- heart failure
- LVP
- left ventricular pressure
- +dP/dtmax
- maximum velocity of pressure rise
- HR
- heart rate
- −dP/dtmax
- maximum velocity of pressure fall
- ED80
- dose increasing +dP/dtmax by 80%
- EDmax
- dose inducing the maximum increase in +dP/dtmax
- LD
- lethal dose
- DMSO
- dimethyl sulfoxide
- bpm
- beats per minute
- LVEDP
- left ventricular end diastolic pressure
- SBP
- systolic blood pressure
- Received May 9, 2002.
- Accepted July 3, 2002.
- The American Society for Pharmacology and Experimental Therapeutics