Abstract
CRISPR was first observed in 1987 in bacteria and archaea and was later confirmed as part of bacterial adaptive immunity against the attacking phage. The CRISPR/Cas restriction system involves a restriction endonuclease enzyme guided by a hybrid strand of RNA consisting of CRISPR RNA and trans-activating RNA, which results in gene knockout or knockin followed by nonhomologous end joining and homology-directed repair. Owing to its efficiency, specificity, and reproducibility, the CRISPR/Cas restriction system was said to be a breakthrough in the field of biotechnology. Apart from its application in biotechnology, CRISPR/Cas has been explored for its therapeutic potential in several diseases including cancer, Alzheimer’s disease, sickle cell disease, Duchenne muscular dystrophy, neurologic disorders, etc., wherein CRISPR/Cas components such as Cas9/single guide RNA (sgRNA) ribonucleoprotein, sgRNA/mRNA, and plasmid were delivered. However, limitations including immunogenicity, low transfection, limited payload, instability, and off-target binding pose hurdles in its therapeutic use. Nonviral vectors (including cationic polymers, lipids, etc.), classically used as carriers for therapeutic genes, were used to deliver CRISPR/Cas components and showed interesting results. Herein, we discuss the CRISPR/Cas system and its brief history and classification, followed by its therapeutic applications using current nonviral delivery strategies.
Introduction
Genetic engineering is a modern tool used for direct editing of heritable or nonheritable genetic material to modulate the genotype or phenotype of the particular cell, tissue, or organism. This science plays with the deletion or insertion of a gene or any DNA sequence to produce revolutionary genetic changes (Collins, 2018). Gene editing has shown benefits in the management of both genetic and nongenetic conditions. Among various tools available for genome editing, CRISPR and Cas (together called a CRISPR/Cas system) have shown significant advantages in terms of simplicity and specificity, thereby generating interest in many research groups. The CRISPR/Cas gene-editing mechanism has now been well established (Doudna and Charpentier, 2014). Briefly, the CRISPR locus and array contain a cleaved protospacer from incoming bacteriophage. Due to the protospacer addition within the CRISPR locus, it becomes easy for bacteria to recognize a phage on its subsequent entry since the protospacer acts as a memory for the corresponding invading phage. Then, the bacteria synthesize their own single guide RNA (sgRNA) and Cas9 that cleave the phage DNA at the specific site complementary to the sgRNA and protect the bacteria against phage attack (Nuñez et al., 2014). The sgRNA consists of CRISPR RNA (crRNA) having a complementary sequence of phage DNA and trans-activating CRISPR RNA (tracrRNA) that join the crRNA. Following its establishment as a biotechnology tool, the CRISPR/Cas system has been explored for its therapeutic potential in several conditions including Alzheimer’s disease (Rohn et al., 2018), eye disease (Hung et al., 2016), sickle cell disease (SCD) (Park et al., 2016), Duchenne muscular dystrophy (DMD) (Nelson et al., 2016), cancer (Chen et al., 2017), and neurologic disorders (Rohn et al., 2018). Promising results of CRISPR/Cas9 editing were also seen in a metastatic lung cancer patient treated with Cas9-engineered T cells (Cyranoski, 2016).
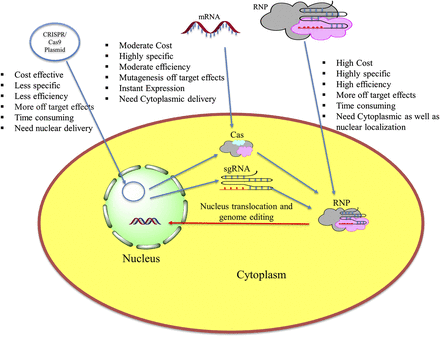
For genome editing using the CRISPR/Cas system, three approaches have been explored, i.e., delivering CRISPR plasmid, mRNA encoding for Cas protein and sgRNA, and ribonucleoprotein (RNP) complexes of sgRNA and Cas protein (Fig. 1). All these approaches have their advantages as well as disadvantages in terms of efficiency, off-target effects, specificity, and cost. CRISPR plasmid delivery is one of the simple and commonly used approaches, wherein Cas protein and sgRNA are encoded by the same vector, thus omitting the need for multiple transfections of different CRISPR/Cas components (Ran et al., 2013). However, it is more time consuming, and the plasmid need to be delivered directly into the nucleus. The second approach is to deliver sgRNA and Cas9 mRNA (mRNA encoding for the cas9 protein) separately. Cas9 mRNA translates to the cas9 protein that joins with the sgRNA in the cytoplasm to form RNP (Niu et al., 2014). This approach decreases off-target binding and needs delivery only up to the cytoplasm. In the third approach, a complex of sgRNA and Cas9 protein is delivered into the cell (Zuris et al., 2015). This approach has gained a lot of interest owing to its reduced off-target binding, less toxicity, higher efficiency, and simpler design.
Approaches for CRISPR/Cas-based editing in cells with their advantages and limitations.
CRISPR/Cas technology holds enormous potential and is very efficient; however, beyond its editing efficiency, its use as a clinically translatable therapeutic tool is limited by hurdles in its in vivo delivery. Physical methods and viral and nonviral vectors that were previously used for gene delivery applications are also adopted for delivering CRISPR/Cas components. These methods have their own pros and cons in terms of off-target effects, toxicity, mutagenesis, immunogenicity, and loading capacity. Nonviral carriers including cationic lipids, cationic polymer, micelleplexes, and inorganic nanoparticles have gained enormous interest because of the flexibility they offer in their design to overcome the limitations of other methods. However, these are also not devoid of delivery challenges and thus there is always a search for newer carrier materials with improved properties. Several reviews have discussed the use of this technology in genome editing for several diseases including cancer (Martinez-Lage et al., 2018; Yin et al., 2019; Zhan et al., 2019), SCD (Demirci et al., 2019), DMD (Lim et al., 2018), etc. A few recent reviews have also discussed the delivery aspects of CRISPR/Cas components (Li et al., 2018; Lino et al., 2018). This review particularly focuses on the progress and prospects of CRISPR/Cas technology, followed by a discussion of strategies being used for their delivery using nonviral carriers.
History and Origin of CRISPR
Gene editing has been done for a few decades through conventional homologous recombination to produce knockout/knockin mice (Smithies et al., 1985). Subsequently, two methods employing zinc-finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs) were used in gene editing, which work through a double-stranded break mechanism and can fundamentally target any sequence in the human genome. These techniques are some of the most widely used biotechnological tools for gene editing (Zhang et al., 2019). The ability to customize DNA is totally dependent on the DNA binding affinity as well as the specificity of the designed protein (zinc finger and TALEN). Despite several advantages both TALENs as well as ZFNs face challenges including difficulty in engineering (Ramirez et al., 2008), limited target site selection, off-target binding (Hockemeyer et al., 2009, 2011), and high cost. Following the discovery of the CRISPR/Cas system, interest has been diverted toward its use as a potential tool for gene editing. All of these genome-editing methods have their own adaptability and application-based uses. For example, in human pluripotent stem cells, CRISPR/Cas9 brings more advantages over the other two techniques. Furthermore, CRISPR/Cas has been found to have more feasible properties than ZFNs and TALENs, such as ease of design and versatility (Gagat et al., 2017). The CRISPR/Cas system is also cost effective and has been found to have more efficiency than other systems. Nowadays, where time is a major concern, the CRISPR/Cas system offers precise results, and editing of a genome can be achieved within weeks (Ran et al., 2013).
Looking back at the history of the CRISPR/Cas system, it was first observed as a repeatedly ordered motif of less than 50 base pairs (bp) in the genome of bacteria and archaea (Ishino et al., 1987). Earlier it was thought to be unevenly distributed in bacteria and archaea but later on was found in approximately 90% and 50% of the archaeal and bacterial genomes, respectively. These motifs were found to have common features such as noncoding and being different from each other (Lintner et al., 2011) and also interspaced (i.e., containing a foreign sequence in between), thus these were named clustered regularly interspaced short palindromic repeats (i.e., CRISPR) (Al-Attar et al., 2011). In 2007, it was suggested that when Escherichia coli was subjected to a viral attack continuously, then a DNA was introduced in the CRISPR interspacing regions derived from a phage genomic sequence and thus demonstrating CRISPR/Cas as a defense system in E. coli against phage attack (Barrangou et al., 2007). It was further put forward that bacteria have an adaptive type of immunity in the form of CRISPR (Garneau et al., 2010). Mojica et al. (2005) sequenced 4500 CRISPR sequences from 67 strains of bacteria and archaea. On comparing these sequences in GeneBank nucleotide sequence database (www.ncbi.nlm.nih.gov/BLAST/)), they surprisingly found that these sequences matched bacteriophage, invasive plasmid, and other genomic sequences (Mojica et al., 2005). Thereafter, Mojica et al. (2009) stated that CRISPR along with the spacer provided resistance against the phage attacking the bacteria. Experiments were performed to consolidate this hypothesis, wherein it was observed that the resistance of bacteria against a specific phage was reversed when the spacer sequence was removed from the bacterial genome. The integrated spacer was then termed the CRISPR-associated (Cas) gene and this whole system was named the CRISPR/Cas system (Godde and Bickerton, 2006).
Garneau et al. (2010) demonstrated that the CRISPR/Cas system is one of the defense systems of bacteria against viruses, and that Cas genes (acquired from an exochromosomal element, i.e., virus) play a vital role in cleavage of plasmid and bacteriophage DNA. Many of the ongoing studies described various silent features of the CRISPR/Cas system since it has all of the crucial characteristics required for a biotechnology editing tool and has become the subject of intensive study (Doudna and Charpentier, 2014). It has been observed that the length and sequence of the spacer may vary in the same or a different CRISPR with an ideal range of 26–72 bp spacer sequence and 21–48 bp repeats sequence (Haft et al., 2005; Labrie et al., 2010). Furthermore, the number of spacers within the CRISPR locus present in a cell’s genome or plasmid also varies from a few to hundreds (Rath et al., 2015). For example, Methanocaldococcus sp. FS406-22 and Sulfolobus tokodaii strain 7 have 18 CRISPRs with 191 spacers and five CRISPRs with 458 spacers, respectively (Rousseau et al., 2009). It has also been reported that the Cas gene is not always present along with CRISPR loci, and in this condition CRISPR depends on trans-encoded factors (Rath et al., 2015). One more important postulate about CRISPR loci is that they have a leader sequence, which is a conserved sequence located upstream of the CRISPR with respect to the direction of transcription (Pougach et al., 2010).
Gene-Editing Mechanism of the CRISPR/Cas System
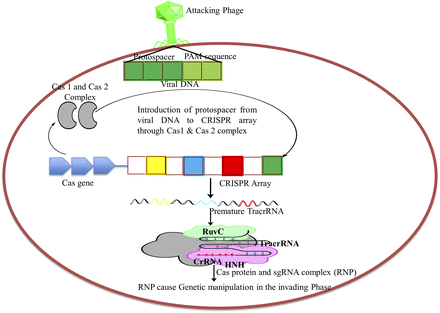
Currently, it has been fully accepted that the CRISPR/Cas system is part of the bacterial genome and plays a significant role in adaptive immunity in bacteria and archaea against an attacking phage or invading plasmid, wherein one genetic element destroys another. However, the real mechanistic role of CRISPR/Cas is still under investigation (Jiang and Doudna, 2017). It has been clearly shown that there are three distinct steps involved in CRISPR/Cas-based cleavage of plasmid or double-stranded DNA: 1) adaptation, 2) expression and maturation, and 3) interference (Fig. 2) (Amitai and Sorek, 2016). In the first stage of adaptation, a new spacer (i.e., protospacer) is incorporated in the CRISPR array by invading a mobile genetic element. For this, the Cas1-Cas2 complex (having two Cas1 dimers and one Cas2 dimer) identifies the new DNA as a target, which after identification and detection is incorporated into the CRISPR array as a new spacer along with the same adjacent sequence (Mir et al., 2018). In the second step, mature crRNA composed of RNP with Cas protein is transcribed from CRISPR. In the third stage the crRNA guides the Cas protein, both present in complex form (i.e., RNP), to locate the protospacer adjacent motif and help the Cas protein reach the target site where Cas9 acts as a double scissor to cut the DNA strand. The Cas protein has two domains (i.e., RuvC and NHN) that show a distinct function by cleaving the noncomplementary and complementary strands, respectively. Furthermore, the Cas protein makes a cut after two to three nucleotides from the protospacer adjacent motif sequence (Deveau et al., 2010). This three-step mechanism was considered as the modulator in development of viral resistance in bacteria. Furthermore, viral resistant bacteria produce different types of RNA (i.e., crRNA and tracrRNA) from two distinct regions, the first is the CRISPR spacer itself and second is outside the CRISPR repeats where Cas genes are found (Jiang and Doudna, 2017). Both crRNA and tracrRNA fragments are complementary to each other and form a double-stranded DNA that acts as a guide RNA and facilitates Cas9 along with endonuclease to blunt-ended cleavage of the invading DNA (Siksnys and Gasiunas, 2016); following this step, the repair mechanism fills the empty/cut region of the DNA with the normal sequence. This technique has been adopted in eukaryotes as a gene knockout technology with minimal cost and is an easy method compared with existing techniques for many fatal diseases (Platt et al., 2014).
Modulation in CRISPR locus (bacterial) in response to phage attack involving events of adaptive immunity in bacteria including requisition of protospacer into CRISPR array, maturation and expression of mRNA, and interference with invading phage.
Classification of the CRISPR/Cas System
The scientific literature has suggested that every Cas protein is associated with unique features and diverse nature (Makarova et al., 2011). To date, more than 13 different types of CRISPR systems have been identified (Rath et al., 2015). It is very difficult to classify a CRISPR system due to multiple CRISPR loci, fast evolution, and horizontal transfer of the CRISPR/Cas system (Fagerlund et al., 2015). The currently adopted classification regarding CRISPR/Cas systems is based on CRISPR components such as Cas gene similarities, Cas protein, the organization of genes on CRISPR/Cas loci, and variability within the CRISPR itself. This classification system has been broadly classified into three distinct categories/types (i.e., types I, II, and III) based on the Cas gene and a rare type IV, which has rudimentary CRISPR/Cas loci. These types have been further classified into various subtypes (types I A–F, II A–C, and III A and B) based on the structural differences and the gene they encode (Koonin et al., 2017). Two major classes have been defined for CRISPR/Cas systems (classes I and II), having different types as discussed subsequently (Makarova et al., 2015).
CRISPR/Cas Class I.
Class I includes types I, III (found in archaea), and IV. The effector complexes of type I and III CRISPR/Cas have a definite structure with a backbone containing paralogous repeat-associated mysterious proteins, such as Cas7 and Cas5, having the RNA recognition motif fold and additional large and small subunits. These effector complexes contain one Cas5 subunit and several Cas7 subunits. Cas3 and Cas10 are considered as the signature genes for types I and III, respectively (Shmakov et al., 2017). Type III (B) Cmr is probably rare since it has been found to cleave targeted RNAs (Majumdar and Terns, 2019).
CRISPR/Cas Class II.
In class 2, the effector system is more uniformly organized and contains simple, large, and multidomain proteins. Class 2 contains three types: II, V, and VI. Type II has endonuclease as the effector and is dominantly used as a genome-editing enzyme. On the other hand, type V contains Cpf1, a RNA guide endonuclease, as an effector that cleaves the target without needing any tracrRNA. Additionally, RuvC like endonuclease is also the main feature of types II and V. Type VI is found to target both RNA as well as DNA and contains two higher eukaryotes and prokaryotes nucleotide-binding domains. Type VI is further subdivided into two subtypes (VI-A and VI-B) containing effector proteins Cas13a and 13b, respectively (Shmakov et al., 2017).
Thus, distinct types of CRISPR/Cas systems, with numerous effectors and Cas genes, have been identified as well as classified. Types I–III are the most studied, while IV–VI are newly identified CRISPR/Cas system types (Makarova et al., 2015).
CRISPR/Cas as a Therapeutic Tool
Owing to the advantages of the CRISPR/Cas system, research is being directed toward its use as a therapeutics tool to achieve efficient genome editing through gene knockout or knockin for several fatal diseases of both genetic and nongenetic etiology. The use of the CRISPR/Cas-editing system in some of the major diseases is described subsequently.
Human Immunodeficiency Virus.
It has been more than three decades since the human immunodeficiency virus (HIV) was identified, but it is still a major health concern. Although anti-retroviral therapy effectively controls the viral load, it fails to remove the virus completely. Recently, Bella et al. (2018) demonstrated the cleavage of HIV-1 DNA from patient immune cells using lentivirus expressing CRISPR in humanized mice engrafted with patient blood. The results of the study showed the removal of virus DNA from the blood as well as other major organs including spleen, lung, and liver of the mice. Zhu et al. (2015) showed that there are 10 sites in HIV-1 that could be the potential target by the CRISPR/Cas system and also showed the effect of CRISPR/Cas-mediated removal of mutations in HIV-1–infected JLat10.6 cells (Zhu et al., 2015).
Sickle Cell Disease.
SCD is a disorder caused by a point mutation in the HBB gene that can be cured by allogenic hemopoietic stem cell transplantation; however, only a small population of patients is compatible with this treatment. Park et al. (2016) demonstrated that approximately 30% of homology-directed repair could be achieved in CD4+ cells using suitable CRISPR/Cas along with a donor templet strand. Cas9 RNP, when delivered along with the donor templet to CD34+ hematopoietic stem/progenitor cells, effectively edits the genome and increases the level of wild-type hemoglobin in a mouse model (Park et al., 2016). Reports have also stated that the Food and Drug Administration has lifted the hold on CRISPR therapeutics for SCD (Baylis and McLeod, 2017).
Duchenne Muscular Dystrophy.
DMD is another condition wherein dystrophin gene deletion causes this X-linked genetic muscle disease. The resulting product of this dystrophin gene is responsible for the development of muscles, and deletion leads to muscle weakness and muscle degeneration (Bushby et al., 2010). Mutations in exon 23 of the dystrophin gene have resulted in immature protein production and are responsible for the aforementioned consequences. The CRISPR system was delivered using adeno-associated virus (AAV) for DMD that enabled the DMD gene functions in the mouse model (Nelson et al., 2016). The results indicated that the CRISPR/Cas system deleted exon 23 from the dystrophin gene, leading to the following events: modified dystrophin gene expression, recovery of functional dystrophin protein, and enhancement of muscle force. Young et al. (2016) also reported success in the deletion of DMD exons in humans. Furthermore, a new RNA guided endonuclease (cpf1) was found to correct the mutation in DMD in human cells as well as in animal models of DMD (Zhang et al., 2017b).
Cancer.
Cancer, yet another fatal disease having multiple causes and poor treatment outcomes, has been the research agenda for genetic engineering and genome-editing techniques since they provide alternative therapeutic tools toward its cure (Table 1). The CRISPR/Cas system has gained a lot of interest in cancer treatment due to its efficient editing of the target gene directly along with adaptation for different delivery strategies. The CRISPR/Cas9 technique has been demonstrated to knockout the Ptch1 gene responsible for medulloblastoma and the Trp53, Pten, and Nf1 genes responsible for glioblastoma in mouse brain (Zuckermann et al., 2015). The editing efficiency of the CRISPR/Cas system was also evaluated in genetically engineered mouse models of colorectal cancer (Roper et al., 2017). Also, cancer suppressor genes Pten and p53 were edited by the CRISPR/Cas system in hepatocellular carcinoma. The CRISPR/Cas9 technique was used to deplete miR-210-3p in renal carcinoma cell lines (786-O, A498, and Caki2), which significantly increased tumorigenesis along with a morphologic change in A498 and Caki2 cells (Yoshino et al., 2017). The literature also provides evidence for the use of the CRISPR/Cas system in the treatment of ovarian, cervical, and acute myeloid leukemia. Long noncoding RNA is the potential target in bladder cancer. Although the CRISPR/Cas system was not widely explored for its activity in modulating their expression, Zhen et al. (2017a) recently showed that the CRISPR/Cas gene-editing tool potentially altered the expression of long noncoding RNA expression in bladder cancer. One of the major limitations in cancer treatment is the development of resistance to chemotherapy, which could be potentially avoided by knocking out the responsible gene. For example, doxorubicin efflux in MCF-7 cells was inhibited by knockout of the MDR1 gene (via a double-stranded break) using the CRSIPR/Cas system, thus providing evidence for overcoming chemoresistance via Cas9-mediated disruption of the drug resistance–related gene (Ha et al., 2016).
CRISPR/Cas9 technology in the treatment of different cancers
Challenges in the Delivery of CRISPR/Cas Components
Nowadays, CRISPR/Cas9 has been under intensive research as a genetic engineering tool and is also providing satisfactory results in preclinical practices. Three major approaches, which differ in their properties and nature, have been used for attaining CRISPR/Cas9 expression in target cells. These are delivering 1) plasmid DNA (pDNA) expressing Cas9 protein and sgRNA, which is a very simple and cost-effective method; 2) mRNA (encoding the Cas protein) that shows instant gene expression and reduces the risk of mutagenesis; and 3) RNP (i.e., complexes of Cas9 protein and sgRNA), which has the advantage of reduced off-target cleavage. The efficacy of gene cleavage not only depends on the selectivity of the CRISPR/Cas nature (pDNA, mRNA, or Cas protein) but is also affected by the methodology of transportation of the CRISPR gene to the target cells or tissue. Selection of a carrier for delivering the payload into the target cells has been seen as a bottleneck in achieving efficient editing. Both viral and nonviral vectors have been reported to deliver CRISPR/Cas components; however, various hurdles have limited their therapeutic use, as outlined in the following sections.
Packing.
CRISPR/Cas editing can be achieved either by pDNA, mRNA or RNP complexes; however, all of these delivery methods face packing issues in the carrier owing to their macromolecular size. For example, the size of the spCas9 gene is ∼4.3 kilobase pairs, while negatively charged spCas9 protein has a size of 160 kDa with hydrodynamic diameter of ∼7.5 nm and sgRNA has a size of ∼31 kDa and hydrodynamic diameter of 5.5 nm (Mout et al., 2017). Since there is a limited capacity of various delivery vectors, the packing of CRISPR/Cas components is a major concern (Wu et al., 2010).
Targeted Delivery.
Although viral vectors provide targeted delivery through tissue tropism, they have several disadvantages including immunogenicity, payload limitation, and delivery challenges (Zincarelli et al., 2008). On the other hand, delivery of CRISPR/Cas components via nonviral vectors requires an antibody or peptide-mediated targeting strategy to avoid off-site distribution (Peer et al., 2007). Designing actively targeted carriers with required packaging capabilities is much more difficult.
Efficiency, Off-Target Binding, and Mutagenesis.
Although this technology has been demonstrated to be much more specific and efficient, diseases such as cancer require much more editing efficiency in order to achieve the therapeutic outcome. Off-target effects for the CRISPR/Cas system is also of major concern (Choi and Meyerson, 2014). In particular, Cas9/sgRNA shows expression for an extended period of time and can interact with other genes, leading to off-target effects. Editing of a gene other than the potential site could also lead to mutations and hence complicate the condition.
Immunogenicity.
The components of the CRISPR/Cas system are derived from bacteria, which potentially could induce immune responses. It has been reported that major histocompatibility complex class one was elicited by the Cas gene and Cas protein. Literature evidence has shown that in vivo delivery of CRISPR can elicit immune responses not against the vector but against the Cas protein itself (Chew et al., 2016).
Delivery Strategies for CRISPR/Cas Components
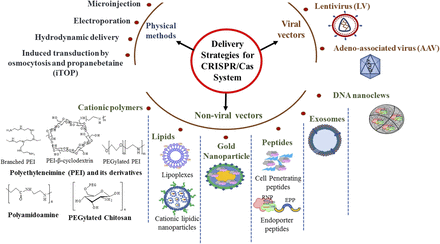
Genome editing using CRISPR/Cas9 could be achieved either by gene-based (plasmids or viral vectors expressing Cas9 and sgRNA), RNA-based (Cas9 mRNA and sgRNA), or protein-based (Cas9 protein and sgRNA) strategies (Mout et al., 2017). The science of gene delivery is one of the diverse fields in biomedical science that has been under investigation for a long time, wherein physical, viral, and nonviral methods have been employed (Fig. 3). Conventional physical methods such as microinjection and electroporation have limited in vivo applications due to their disadvantages, including off-target binding, requirement of manual operations, and damage to cells. Some newer techniques include induced transduction by osmocytosis and propanebetaine, hydrodynamic injection, and mechanical cell deformation. CRISPR components in a plasmid format along with single-stranded DNA were delivered by tail-vein hydrodynamic injection into a mouse model of tyrosinemia that resulted in correction of the Fah mutation in hepatocytes (Yin et al., 2014). Furthermore, this technique inhibited hepatitis B virus replication and expression in mice (Zhen et al., 2015). However, certain limitations constrained their use in gene therapy. For instance, cardiovascular dysfunction is a common consequence in the case of hydrodynamic injection and induced transduction by osmocytosis and propanebetaine.
Strategies used for delivering CRISPR/cas components.
Viral vectors, mainly lentivirus and AAV, have been reported for delivering gene-editing components with high efficiency. However, mutagenesis, immunogenicity, and limited loading capacity pose challenges in their use as carriers for therapeutic genes. CRISPR/Cas9 adenoviruses disrupted the Pcsk9 gene with ∼50% of insertion and deletion mutation (indel) in adult mouse liver after retroorbital injection. This further resulted in a decrease of 35%−40% blood cholesterol in mice (Ding et al., 2014). Although adenoviruses do not get incorporated into the host genome, they can produce an immune reaction in the host (Wang et al., 2004). AAVs have been used to deliver CRISPR components to rectify the mutated dystrophin gene in DMD disease (Long et al., 2016). In another study by Kim et al. (2017a), AAVs were used to deliver CjCas9 (derived from Campylobacter jejuni) that induced targeted mutations with high frequencies in mouse muscle cells or retinal pigment epithelium cells.
Most trending gene delivery systems in recent times rely on nonviral methods such as cationic polymers, lipid nanoparticles, cell-penetrating peptides (CPPs), DNA nanoclews, and gold nanoparticles (Wong et al., 2017). These carriers have shown efficient transfection with ample opportunity in the design owing to their synthetic or semisynthetic nature. Furthermore, hybrid systems have been proposed to confer biomimetic properties to these carriers. Although nonviral vectors are cost effective and have better safety profiles, they also share some limitations including low transfection efficiency, irregular cellular uptake, and poor delivery to target cell/tissue (Nayerossadat et al., 2012; Ramamoorth and Narvekar, 2015). Selection of a nonviral carrier will depend on the type of payload to be delivered. Since there are different approaches (e.g., pDNA, mRNA or RNP) used in CRISPR/Cas-based editing, delivery carriers are designed accordingly. When either DNA or RNA is to be delivered, most of the nanoviral gene delivery approaches could be adopted for transfection. On the other hand, direct delivery of Cas protein has an advantage over the delivery of conventional pDNA expressing Cas protein because of the shorter exposure time at the cellular level, resulting in reduced toxicity and off-target actions (Ramakrishna et al., 2014). Recently, several methods were developed for delivering Cas protein to overcome existing delivery limitations, such as instability in serum, poor uptake and endosomal escape, and limited in vivo efficiency (Zuris et al., 2015).
Cationic Polymers.
A wide range of polymers, both natural and synthetic, with the desired characteristics are available for designing gene delivery vehicles. Cationic polymers from the past decade are one of the most explored carriers for various peptides and gene silencing oligonucleotides (such as short interfering RNA and microRNA) and are available with a dispersive range of derivatives (Samal et al., 2012). Among various cationic polymers, polyethyleneimine (PEI) has been widely used for gene delivery application owing to its advantages, such as efficient complexation and proton sponge effect. PEI has a branched structure with multiple amine functionality and is easy to assemble, easily available, and has been fully explored for its gene delivery efficacy (Ahn et al., 2008). It has been demonstrated that high molecular weight PEI will exert better transfection effects compared with low molecular weight PEI. The major problem associated with PEI is its toxicity, which to some extent has been circumvented by the use of branched PEI or modifications of PEI with polyethylene glycol (PEG) (He et al., 2013). Recently, Zhang et al. studied polyethyleneimine-β-cyclodextrin for delivering large pDNA encoding Cas9 and guide RNA for in vitro genome editing (Zhang et al., 2019). A nitrogen to phosphate (N/P) ratio of 20 or above resulted in condensation of all free pDNA molecules of different sizes ranging from 3487 to 8506 bp. Furthermore, as the N/P ratio increased, the size of polyethyleneimine-β-cyclodextrin/pDNA complexes decreased and all of the pDNAs showed an average particle size below 200 nm at an N/P ratio of 60. These complexes were also efficiently internalized by HeLa cells with negligible cytotoxicity. The genome-editing efficiency was confirmed by using plasmids expressing Cas9 and sgRNA targeting the hemoglobin subunit beta (19.1%) and RHBDF1 (7.0%) locus (Zhang et al., 2019). Chitosan is another natural polymer that is nontoxic and biodegradable and has been investigated for delivering CRISPR components. One recent study showed that polyethylene glycol monomethyl ether conjugated chitosan for nonviral aerosol and mucosal delivery of the CRISPR/Cas system. Low and medium molecular weight chitosan was PEGylated with a high polyethylene glycol monomethyl ether degree of substitution and complexed with pSpCas9-2A-GFP at different N/P ratios (5, 10, 20, and 30). The positively charged amines of chitosan interact with the negatively charged nucleic acid and significantly promote delivery. It was observed that low molecular weight PEGylated chitosan showed optimal transfection at an N/P ratio of 20, while PEGylated medium molecular weight chitosan showed optimal transfection at an N/P ratio of 5 (Zhang et al., 2018). In another study, CRISPR/pCas9 was delivered intravenously using PEG-b-poly-(lactic acid-co-glycolic acid)–based cationic lipid-assisted polymeric nanoparticles that efficiently disrupted the chronic myeloid leukemia–related BCR-ABL fusion gene and increased the survival of a chronic myeloid leukemia mouse model (Liu et al., 2018). Kretzmann et al. (2017) showed the capability of a poly amidoamine dendrimer to efficiently load and deliver the CRISPR/Cas system. A library of the dendritic copolymer was prepared by click chemistry and studied to improve the delivery of the target pDNA. Literature evidence has suggested that lipofectamine 2000 could be used to efficiently deliver small pDNA (∼5 kilobase); however, to deliver large pDNA (∼10.3 kilobase), the modified poly amidoamine polymer could be better in terms of transfection effciency (Kretzmann et al., 2017).
Cationic Lipids.
Lipoplexes (containing cationic lipids) are one of the most efficient nonviral vector systems for the delivery of genetic material. Cationic lipid forms a stable nanocomplex via electrostatic interaction with negatively charged Cas9/sgRNA. Zhen et al. (2017b) delivered CRISPR/Cas9 for the treatment of prostate cancer by using cationic liposome containing PEG-grafted 1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine. In cancer therapy, lipid nanoparticles showed significant delivery of the CRISPR/Cas system. Cas9/sgRNA plasmid targeting PLK-1 was encapsulated in a PEG phospholipid-modified cationic lipid nanoparticle to form a core-shell structure that showed an in vitro transfection of 47.4% in A375 cells. An in vivo study of these phospholipid-modified cationic lipid nanoparticles in melanoma tumor–bearing mice showed significant downregulation of PLK-1 protein and suppression of tumor growth (Zhang et al., 2017a).
Cas9 endonuclease proteins have a net positive charge, and hence cannot be complexed directly with cationic lipids. Zuris et al. (2015) demonstrated that these proteins can be fused with anionic supercharged proteins or anionic nucleic acids. They efficiently delivered Cre recombinase, transcription activator-like effector–based and Cas9-based transcriptional activators, and Cas9:sgRNA nuclease complexes into cultured human cells. Furthermore, up to 80% genome modification was observed with Cas9:sgRNA complexes compared with DNA transfection (Zuris et al., 2015). In a recent study, Cho et al. (2019) used nanoliposomes prepared using lecithin to deliver cas9-sgRNA RNPs directed against the DPP-4 gene to modulate the function of glucagon-like peptide 1. In vivo delivery of these complexes in type 2 diabetes mellitus db/db mice disrupted DPP-4 gene expression, leading to a decline in DPP-4 enzyme activity that resulted in normalized blood glucose levels, a declined in insulin resistance, and negligible impact on liver and kidney function (Cho et al., 2019). In another study, Kim et al. complexed cas9 RNPs with lipofectamine 2000 delivered subretinally for the treatment of wet age-related macular degeneration (Kim et al., 2017b). The authors designed sgRNAs targeting the VEGF A gene that encodes VEGF receptors in mouse NIH3T3 cells and human ARPE-19 cells. VEGF A sgRNA/Cas9 RNPs were delivered using lipofectamine 2000, resulting in indels at the target site with frequencies of 82% and 57% in NIH3T3 and ARPE-19 cells, respectively. These RNPs were further delivered subretinally into the adult mouse eye, wherein it was observed that RNPs could induce indels in the injected area. In the mouse model of wet age-related macular degeneration, these RNPs induced indels at a frequency of 22% and effectively reduced the concentration of the VEGF A protein in the choroidal neovascularization area, demonstrating that subretinal injection of VEGF A/Cas9 RNP could lead to local treatment in the eye (Kim et al., 2017b).
Bioreducible lipids have recently been used as a nanocarrier for the delivery of the CRISPR/Cas system. These lipids contain disulfide linkage in the hydrophobic tail of the lipid that leads to the degradation of lipid in the reductive intracellular (glutathione-rich) environment, promote the release of loaded cargo into the cytoplasm without endosomal degradation (Wang et al., 2016), and enhance the efficiency of gene delivery. Wang et al. (2016) demonstrated the synthesis of cationic lipids containing a disulfide bond created by the Michael addition of primary and secondary amines along with acrylate and a long chain of carbon. The head group modification leads to the synthesis of derivatives with distinct activities and acts as an effective system for the delivery of Cas protein/sgRNA for editing of the allele. It was further demonstrated that the RNP complex, with a supernegative charge, is more efficiently delivered by using bioreducible lipids compared with commercial lipids. The results of the study showed more than 70% gene knockout efficiency of Cas9/sgRNA in cultured human cells (Wang et al., 2016). In another study, cationic lipids were used to deliver sgRNA/Cas (RNP) in MCF-7 cells to knockout the MDR1 gene, which is responsible for efflux of doxorubicin. The results showed a 4-fold increase in drug uptake relative to the untreated cells by decreasing MDR1 gene–mediated resistance (Ha et al., 2016).
Cell-Penetrating Peptide.
CPP has been used as a means to attain effective Cas9 protein delivery because of its inherent ability to translocate through plasma membranes (Gagat et al., 2017). The conjugation of CPP with various cargos can be achieved through electrostatic interaction or covalent bonding. Suresh et al. showed endogenous gene disruption in human cell lines mediated via CPP-conjugated recombinant Cas9 protein (Suresh B et al., 2017). Another report also showed enhancement in the Cas9 delivery to a nucleus by utilizing CPP. A novel CPP, TAT-calmodulin, was explored to effectively deliver the cargo into the nucleus (Axford et al., 2017). Ramakrishna et al. (2014) showed that CPP mediated delivery of Cas protein as well as guide RNA with lesser off-target effects (Ramakrishna et al., 2014).
Endo-Porter Peptides.
Another strategy utilizing Endo-Porter peptides has been reported for delivering Cas protein and sgRNA. These are α-helical and amphipathic peptides with weak basic amino acids, leucine and histidine, as their major components and can deliver nonionic substances into the cell (Summerton, 2005). It has been reported that the Endo-Porter peptide enters the cell through endocytosis and escapes the endosome through a proton sponge effect (Bartz et al., 2011). A recent study by Shen et al. (2018) showed efficient delivery of Cas protein and sgRNA with reduced off-target effects by complexing it with Endo-Porter peptides via electrostatic interaction. Another study reported the results of using PEGylated nanoparticles along with a membrane disruptive and endosomolytic helical polypeptide, in which more than 71% suppression in the growth of cancer cells was observed (Wang et al., 2018).
Gold Nanoparticles.
Multiple surfaces functionality makes gold nanoparticles a unique and versatile delivery system for various cargos (Yeh et al., 2012). The literature has described the role of gold nanoparticles in distinct applications, such as sensing, imaging, delivery, etc. One study showed that direct cytosolic delivery of ribonucleoprotein complexed with gold nanoparticles provided effective (∼30%) gene-editing efficiency (Mout et al., 2017). Lee et al. (2017) also demonstrated the delivery of Cas9/sgRNA RNP using 15 nm gold nanoparticles conjugated with thiolated short DNA oligos and conjugated further with donor single-stranded DNA, coated with a polymer, Poly{N-[N-(2-aminoethyl)-2-aminoethyl] aspartamide} (PAsp-DET), that disrupted the endosome in mice suffering from DMD. The outcomes of the study showed that the complex was more effective and helpful in correcting 5.4% of the mutated gene in DMD (Lee et al., 2017). Furthermore, it has been recently observed that intracranial injection of CRISPR/gold nanoparticles containing Cas9 CNP edit the gene within the mouse brain through the mGluR5 gene (Lee et al., 2018).
Exosomes.
Exosomes as an advanced delivery system have emerged as a potential area of research owing to their small size and ability to transit molecules such as lipids, protein, and mRNA and to cross the blood-brain barrier as well as the placental barrier (Shi et al., 2017). These are stable nanosized vesicles (having a diameter of 30–100 nm) that are secreted by almost every type of cell (Ibrahim and Marbán, 2016). It has also been reported that exosomes express surface proteins like tetraspanin, thereby exhibiting cell targeting (Hoshino et al., 2015). Kim et al. derived natural exosomes from the cancer cell itself and used them as a carrier to deliver CRISPR/Cas plasmid to treat cancer (Kim et al., 2017c). This strategy provides a natural carrier with less risk of immunogenicity and effectively delivers cargo to treat ovarian cancer in SKOV3 xenograft mice (Kim et al., 2017c). The main limitation with exosomes for delivering macromolecules such as proteins is its limited payload capacity. To overcome this, hybrid exosomes (incorporating exosomes and liposomes) were prepared for delivering the CRISPR/Cas component in mesenchymal stem cells (Lin et al., 2018). Another biologically inspired carrier, DNA nanoclews, has been reported to deliver CRISPR/Cas9 both in vitro and in vivo (Sun et al., 2015). DNA nanoclews are made up of a single strand of DNA having a yarn-like structure prepared by a rolling circle amplification method with palindromic sequences encoded to drive the self-assembly of the nanoparticles. Cas9/sgRNA is loaded in them, which is further coated with PEI to enable cellular internalization and endosomal escape.
Conclusions and Future Prospectives
The CRISPR/Cas system is an adaptive (acquired) immune system in bacteria and archaea with immune memory that is stored in the form of spacer sequences derived from foreign genomes and inserted into CRISPR arrays. CRISPR/Cas has been explored as a potential biotechnology tool for genome editing in order to decipher complex components of gene expression and has been preferred over ZFNs and TALENs in terms of easiness, simplicity, and specificity. CRISPR-associated DNA endonuclease (Cas) provides a novel opportunity for therapeutic genome editing in diseased cells and tissue. The CRISPR/Cas tool can be used by either delivering a Cas expression plasmid or Cas mRNA or RNP complex. Although gene delivery using viral vectors is the most popular choice for gene therapies, this type of in vivo application is disadvantageous for a number of reasons, including possible integration into genomic DNA, immune responses due to persistent expression of bacterial Cas9, and off-target effects. There is still a large gap in translating these tools to the clinic. To exploit the full therapeutic potential of this technology, it has to be merged with the advancements taking place in nanotechnology, particularly in the area of delivery strategies. Nonviral vectors, including cationic polymers and lipids, used for gene delivery have been adopted for delivering CRISPR/Cas components owing to their advantages over viral constructs. However, several issues that needed to be addressed by pharmaceutical and medical scientists are ensuring efficient packing of CRISPR components into the carrier, targeting delivery to the diseased site, avoiding off-target binding, improving in vivo efficiency, avoiding mutagenesis, and eliminating immunogenicity. Translating this tool to therapeutic purposes requires thorough investigation of carriers with spatiotemporal control over in vivo delivery in order to achieve therapeutic concentrations with minimal side effects.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Sahel, Mittal, Chitkara.
Note Added in Proof—The original Table 1 provided in the proof was deleted due to copyright issues. Table 2 has now been renamed Table 1.
Footnotes
- Received February 11, 2019.
- Accepted May 20, 2019.
The authors declare no conflict of interest.
The work was supported by the Department of Biotechnology, Government of India [Research Grant BT/PR26897/NNT/28/1489/2017].
Abbreviations
- AAV
- adeno-associated virus
- bp
- base pair
- CPP
- cell-penetrating peptide
- crRNA
- CRISPR RNA
- DMD
- Duchenne muscular dystrophy
- HIV
- human immunodeficiency virus
- N/P
- Nitrogen to phosphate ratio
- pDNA
- plasmid DNA
- PEG
- polyethylene glycol
- PEI
- polyethyleneimine
- RNP
- ribonucleoprotein
- SCD
- sickle cell disease
- sgRNA
- single guide RNA
- TALEN
- transcription activator-like effector nuclease
- tracrRNA
- trans-activating CRISPR RNA
- ZFN
- zinc-finger nuclease
- Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics