Visual Overview
Abstract
Pasty polymers offer a platform for injectable implants for drug delivery. A library of biodegradable pasty polymers was synthesized by bulk ring-opening polymerization of lactide, glycolide, trimethylene carbonate, or caprolactone using castor oil or 12-hydroxy stearic acid as hydroxyl initiators and stannous octoate as the catalyst. Some of the polymers behaved as Newtonian liquids. Pasty polymers of poly(caprolactone) and poly(trimethylene carbonate) were stable under physiologic conditions for over 1 month in vitro, whereas polymers of poly(lactic-co-glycolic acid) degraded within 10 days. These pasty polymers offer a platform for pasty injectable biodegradable carriers for drugs and fillers.
SIGNIFICANCE STATEMENT New injectable pasty, in situ forming drug delivery systems are described and are advantageous due to their ease of administration, tunable viscosity, and biodegradability. Polyesters based on lactide, glycolide, trimethylene carbonate, and caprolactone, which are commonly used as absorbable implants and drug carriers, were conjugated onto natural hydroxyl fatty acids. These polymers have potential use as wrinkle fillers and drug carriers.
Introduction
Biomaterials have made an enormous beneficial impact on human healthcare as tissue engineering scaffolds and drug delivery systems (Prestwich and Luo, 2001; Ulery et al., 2011; Ramot et al., 2016). The design of biomaterials for specific biomedical applications has rapidly expanded during recent years. (Basu et al., 2016; Ning et al., 2018; Lei et al., 2019; Recek, 2019). Drug delivery platforms in particular have been developed as ubiquitous and integral contributors to modern medicine. Therefore, their continued development is of paramount importance.
Drug delivery systems often comprise biodegradable polymers that may be employed as drug carriers in the human body (Doppalapudi et al., 2014). Poly(caprolactone) (PCL), poly(trimethylene carbonate) (PTMC), and poly(lactic-co-glycolic acid) (PLGA) are examples of safe polymers with varying degrees of hydrophilicity and hydrophobicity that each display unique mechanical properties and patterns of hydrolytic or enzymatic degradation based on the chemical linkages along the polymer backbone (Chen et al., 2018; Duval et al., 2018; Spearman et al., 2018; Zhang et al., 2018), allowing for the fine-tuning of polymers for a given delivery system.
A significant in vivo drawback of hydrolytically degradable polymers stems from the high acidity of the degradation products (Fu et al., 2000). The acidic microenvironment may lead to the degradation of encapsulated therapeutic material, especially in the delivery of proteins (Estey et al., 2006). Thus, the development of hydrophobic polymers with less acidic degradation products, released over a longer time, is expected to minimally reduce the local pH during degradation.
Drug-eluting injectable polymer implants offer an effective route of localized drug administration (Exner and Saidel, 2008). Drugs may be incorporated into the implant and injected to affect highly localized delivery with minimal invasion and highly predictable rates of drug release. Highly viscous, or pasty, injectable polymers offer the distinct advantage of immobility postinjection (Krasko et al., 2007), releasing a drug at a defined rate at the desired site of delivery. Hence, the development of pasty, biodegradable, and biocompatible polymers is of utmost importance.
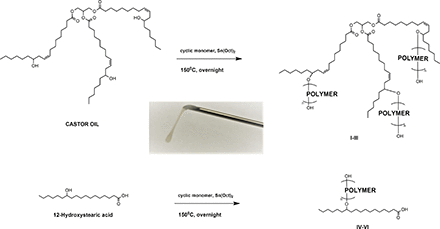
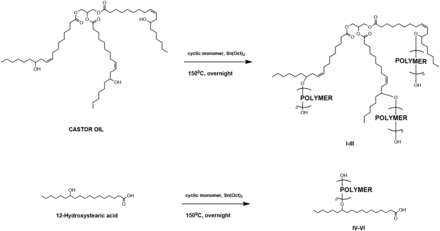
We present here a library of pasty, biocompatible, and hydrolytically degradable injectable polymers based on castor oil (CO) and 12-hydroxystearic acid (HS) for potential use as drug-eluting implants (Fig. 1).
Biodegradable pasty polymers may be injected through a syringe. These injectable polymers have the potential to be incorporated with drugs and used as site-specific drug-eluting implants.
Materials and Methods
Materials.
Castor oil was obtained from Tamar Pharma (Jerusalem, Israel). 12-Hydroxystearic acid was purchased from Holland Moran (Yehud, Israel). Trimethylene carbonate (TMC) was purchased from Richman Chemical (Lower Gwynedd, PA). D,L-Lactide (L) and glycolide (G) were purchased from Purasorb, Purac (Gorinchem, Netherlands). Ɛ-Caprolactone was purchased from Tzamal D-Chem (Petach Tikva, Israel). Stannous octoate was purchased from Sigma-Aldrich (Jerusalem, Israel). All solvents were of analytical grade from Biolab (Jerusalem, Israel).
General Methods.
Chemical reactions were performed in oven-dried glassware under N2 gas. 1H and 13C NMR spectra were obtained on a Varian 300 MHz spectrometer with CDCl3 as the solvent and tetramethylsilane as the shift reference. The molecular weights of the polymers were estimated using a gel permeation chromatography system consisting of a Waters 1515 Isocratic HPLC pump with a Waters 2410 Refractive Index Detector and a Rheodyne (Cotati, CA) injection valve with a 20 μl loop (Waters, Milford, MA). Samples were eluted with CHCl3 through a linear Styragel HR4E column (7.8 × 300 mm i.d.; Waters) at a flow rate of 1 ml/min. The molecular weights were determined relative to polystyrene standards (Polyscience, Warrington, PA) using a Breeze computer program. Mass spectrometry electrospray ionization was recorded on a ThermoQuest Finnigan LCQ-Duo instrument in positive ionization mode. Fourier-transform infrared spectroscopy analysis was performed using a Smart iTR ATR sampling accessory for a Nicolet iS10 spectrometer with a diamond crystal.
Synthesis.
Pasty polymers were prepared by ring-opening polymerization (ROP) of Ɛ-caprolactone, TMC, or D,L-lactide and glycolide by CO or HS in the melt and in the presence of a stannous octoate catalyst. A sample synthesis was as follows: 50 μl of a 0.33 g ml−1 solution of stannous octoate in dichloromethane was added to a melt of CO (0.65 g, 0.70 mmol) and TMC (2.1 g, 21 mmol) purged with N2. Solvent was allowed to evaporate and the vial was purged again with N2. The mixture was stirred at 150°C overnight to afford CO:PTMC (entry 9).
Six polymer series were synthesized by choosing one fatty acid initiator (CO or HS) and one monomer set (caprolactone, TMC, or L + G) and performing ROP in the melt. Individual polymers within the series were synthesized by modifying the relative amounts of initiator and monomer in the melt. L and G were added in a 6:1 molar ratio (Steinman et al., 2019).
Rheological Studies.
Polymer viscosity was measured on a Physica MCR 101 rheometer. Samples were measured at shear rates from 0.01 to 100 second−1 at 25°C.
Degradation Studies.
The polymers (50 mg) were loaded into an ampule and covered with 5 ml of 0.1 M PBS solution (pH 7.4). The ampule was shaken at 37°C for 2 months. Media were exchanged for fresh PBS at regular intervals (1, 5, 10, 23, 29, and 57 days) to avoid solution saturation and checked for small molecules by mass spectrometry electrospray ionization. When medium was exchanged, polymers were weighed after drying to determine weight loss.
Results
Synthesis of Pasty Polymers
Viscosity and degradation of polymeric substances are influenced by both molecular makeup and polymer molecular weight (Colby et al., 1987; Park, 1994). To obtain a significant variety of polymer viscosities and degradation profiles, six series of hydrolytically degradable pasty polymers were synthesized via ROP with either CO or HS as the fatty acid initiator (Figure 1). We chose these fatty acids due to their biocompatibility and prior use in drug delivery (Teomim et al., 1999; Shelke et al., 2007), and also due to the presence of hydroxyl groups available for ROP initiation. Polymers of PCL, PTMC, or PLGA were prepared at the reactive hydroxyl positions of each fatty acid, affording a total of six polymer series. These polymers were selected based on their established use as biodegradable polymers for drug delivery, known degradation profiles, and varying mechanical properties in order to afford a variety of delivery systems with varying viscosities, degradation rates, and flow behaviors. Six unique polymer series were prepared by varying the cyclic monomers and hydroxyl fatty acids employed in the reaction mixture (Table 1). Reaction yields were all quantitative, since homogeneous polymers were obtained without need for purification steps after ROP was performed in the melt in the presence of stannous octoate catalyst (Fig. 2).
Six series of polymers were synthesized, each with its own fatty acid: polymer pair
Preparation of polymers from secondary alcohols on castor oil or 12-hydroxystearic acid. The cyclic monomers used were Ɛ-caprolactone, trimethylene carbonate, or a 6:1 molar ratio of D,L-lactide and glycolide.
Characterization of Pasty Polymers
Each polymer series (I–VI) was prepared in various molecular weights by modifying the fatty acid initiator:monomer feed ratios. The weight average molecular weight values were estimated by gel permeation chromatography, and fatty acid:polymer ratios as well as true number average molecular weight values were calculated by comparing relative 1H NMR peaks of fatty acids and polymers (Table 2).
Molecular weight and fatty acid:polymer ratios of all polymers within series I–VI (Table 1)
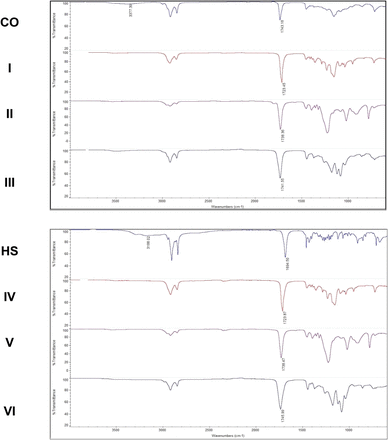
Formation of pasty polymers was confirmed spectroscopically by 1H NMR and infrared (IR) spectroscopy. Ester and carbonate linkages were visualized along the polymer backbone by characteristic strong C=O bends ranging between 1746 and 1723 cm−1. The next section gives an example of the spectroscopic characterization of the highest viscosity polymer of each series by IR spectroscopy. 1H NMR spectra are available as supplementary information (Supplemental Figs. 1–19).
IR.
IR spectroscopy showed the decreasing intensity of the OH bands in fatty acid starting materials (3377 cm−1 in CO; 3188 cm−1 in HS) and the simultaneous appearance of characteristic strong C=O bands in pasty polymers. CO is a natural triglyceride with a characteristic strong ester C=O band at 1743 cm−1. CO-based polymers (entries 1–11) displayed new C=O bands with the polymeric carbonyls overlapping the ester bands from the starting material. HS-based polymers (entries 12–19) also displayed the appearance of characteristic strong C=O bands between 1746 and 1724 cm−1. This was consistent with the terminal OH groups of fatty acids (CO or HS) having been converted to ester/carbonate bonds in pasty polymers (Fig. 3).
Fourier-transform infrared spectroscopy spectra of pasty polymers confirmed polymerization. Characteristic C=O bands between 1746 and 1723 cm−1 were observed for polymers, as well as the disappearance of the characteristic hydroxyl bands in CO and HS.
Viscosity Studies
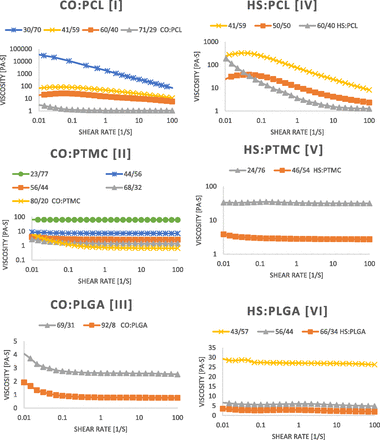
Polymer viscosity was measured under increasing shear rates. As the molecular weight increased within a polymer series, the viscosity increased dramatically (Table 2; Figure 4). Any further increase in molecular weight than what is reported here resulted in solid polymers, unsuitable for injectable drug delivery implants. All polymers were injectable through a 23G syringe.
Viscosity was measured at varying shear rates. Polymers with the highest molecular weight were the most viscous. Any further increase in molecular weight resulted in solid polymers.
Degradation Studies
Hydrolytic degradation under physiologic conditions of the pasty polymers was determined. A slow rate of degradation may allow for controlled release of drugs over a significant time period, while faster degradation profiles may allow release of high levels of drug concentration over a shorter time period (Hatefi and Amsden, 2002). Therefore, a significant variety of polymer degradation profiles is desirable, allowing fine-tuning for the desired application.
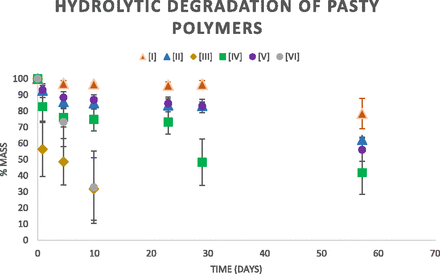
The highest molecular weight (and hence most viscous) pasty polymer of each series was loaded into an ampule and covered with PBS (pH 7.4) at 37°C. PLGA polymers (series III and VI) displayed rapid degradation, with only 30% weight retention after 10 days. PTMC polymers (series II and V) degraded at similar slow rates, with over 80% weight retention after 29 days and over 55% weight retention after 57 days. PCL polymers (series I and IV) also degraded slowly, although CO:PCL (series I) degraded at the slowest rate (over 96% weight retention through 29 days; 78% weight retention through 57 days), while HS:PCL (series IV) displayed 73% weight retention through 23 days before rapid weight loss (Fig. 5).
Hydrolytic degradation of pasty polymer in each series I–VI was determined in phosphate buffer (pH 7.4) at 37°C. Weight loss was determined by drying the sample at each time point to determine the weight of the polymer.
Discussion
Biocompatible and biodegradable drug-eluting implants are essential for drug delivery (Exner and Saidel, 2008; Wolinsky et al., 2012; Water et al., 2015; Sharma et al., 2018). Pasty polymers derived from natural fatty acids and biocompatible polymers offer the potential of acting as injectable, depot-forming implants with incorporated drugs for localized drug delivery (Krasko and Domb, 2007; Krasko et al., 2007). Ideally, an implant should be injectable, viscous enough such that it maintains its localization and integrity throughout the therapeutic time frame, biocompatible to prevent unwanted side effects, biodegradable such that no postinjection implant removal would be necessary, and permeable to allow for controlled release of therapeutic material over an extended time frame. Furthermore, polymer degradation should be tuned for the desired drug release profile (Ickowicz et al., 2016).
We present here six series of pasty polymers comprised of natural fatty acids (CO and HS) and biocompatible, hydrolytically degradable polymers (PCL, PTMC, and PLGA). Each series was synthesized in a variety of initiator:monomer ratios to afford a range of polymer molecular weights. As the polymer content was increased relative to the fatty acid initiator, the polymers displayed dramatic increases in viscosity (Fig. 4).
Viscosity and Degradation Studies.
Castor oil is a low viscosity fluid that permits intramuscular injections (Behre et al., 1999). The biodegradable and biocompatible polymers PCL, PTMC, and PLGA are all solids at physiologic temperatures; therefore, they may only be injected in particle dispersions, preventing their use as templates for injectable drug delivery depots (Weiniger et al., 2012). Consequently, we used CO as a polymerization initiator of PCL, PTMC, and PLGA to afford a platform of pasty polymers that may be applied as injectable delivery systems. We prepared similar polymers with HS, a solid hydroxyl fatty acid, as the initiator for lactone polymerization. Polymerization initiated by HS yielded pasty materials with low molecular weight, and thus may be referred to as pasty oligomers.
The CO:PLGA polymer (series III, entry 11) displayed the lowest viscosity as well as the most rapid degradation profile, consistent with low molecular weight PLGA, which is known to degrade quickly in aqueous media (Shive and Anderson, 1997). Higher molecular weight PLGA polymers with slower degradation rates were solid, and thus were unsuitable as injectable drug delivery implants. The HS:PLGA oligomer (series VI, entry 19) displayed higher viscosity and slower degradation than CO:PLGA; therefore, it may be considered as a platform for drug release over several days. CO:PTMC (series II, entry 9) and HS:PTMC (series V, entry 16) each displayed great potential for application as in situ drug-eluting implants due to their high viscosities and slow rates of degradation. These polymers were slow to degrade due to the increased hydrolytic stability of the carbonate bond along the PTMC chain (Yang et al., 2010), and their injectability, high viscosity, and pastiness provide excellent platforms for drug-eluting implants. The HS:PCL oligomer (series IV, entry 14) displayed high viscosity and hydrolytic stability for up to 23 days. However, the oligomer did not maintain pasty integrity, as it formed oily globules in aqueous media.
The polymer with the highest potential for in vivo application as a polymeric drug-eluting implant was CO:PCL (series I, entry 4). It exhibited a viscosity of 1.1 × 104 Pa·s and presented as a thick paste that also maintained injectability even at molecular weight of 8.2 kDa. Furthermore, the polymer displayed hydrolytic stability for over 1 month while maintaining its pastiness. Due to polymer composition (>20% CO or HS) and its slow degradation, the local pH during degradation should be higher than unmodified polymer degradation.
Flow Behavior.
In addition to biocompatibility and biodegradability, injectable polymeric drug delivery depots should possess low viscosity at the time of injection and high viscosity in situ such that the polymer may remain affixed at the injection site and slowly release the loaded drug. It is thus highly desirable for a polymer implant to display non-Newtonian pseudoplastic behavior in order to undergo shear thinning, aiding in the ease of injectability while maintaining its high viscosity in situ (Guvendiren et al., 2012). Therefore, the high performance of CO:PCL (entry 4) in this regard is advantageous. At low shear rate (0.1 second−1), the polymer exhibits high viscosity. However, under high shear rate (68 second−1) this viscosity dropped by three orders of magnitude (Fig. 4, series I). This dramatic shear thinning, in addition to the slow hydrolytic degradation of the polymer, offers an encouraging platform for a pasty polymer in localized drug delivery.
Conclusions
We report on the synthesis of six series of pasty polymers based on hydroxyl fatty acids (CO and HS) and common biocompatible and biodegradable polymers (PCL, PTMC, and PLGA). Several polymer molecular weights of each series were synthesized by modifying the fatty acid initiator:monomer feed ratios, and polymer viscosity was shown to increase with increasing molecular weight. The highest viscosity polymer of each series formed pasty, injectable, and hydrolytically degradable depots in aqueous media. Some polymers displayed non-Newtonian pseudoplastic flow behavior, adding a crucial element of ease of injectability while forming hard semisolids in situ. This new class of polymers offers potential use as carriers for hydrophobic drugs as localized, injectable polymer implants. The polymer carrier is biodegradable and should eliminate from the body after the drug has been depleted. The fatty acid components of the polymers should reduce the severity of the acidic microenvironment produced upon polymer degradation, since fewer acidic units are released per polymer chain degraded. This feature should support the delivery of acid-sensitive therapeutic materials, particularly proteins.
Acknowledgments
We thank Dr. Ofra Moshel for assistance in performing the viscosity measurements.
Authorship Contributions
Participated in research design: Steinman, Domb.
Conducted experiments: Steinman.
Performed data analysis: Steinman, Domb.
Wrote or contributed to the writing of the manuscript: Steinman, Domb.
Footnotes
- Received April 15, 2019.
- Accepted May 14, 2019.
↵
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
Abbreviations
- CO
- castor oil
- G
- glycolide
- HS
- 12-hydroxystearic acid
- IR
- infrared
- L
- lactide
- PCL
- poly(caprolactone)
- PLGA
- poly(lactic-co-glycolic acid)
- PTMC
- poly(trimethylene carbonate)
- ROP
- ring-opening polymerization
- TMC
- trimethylene carbonate
- Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics