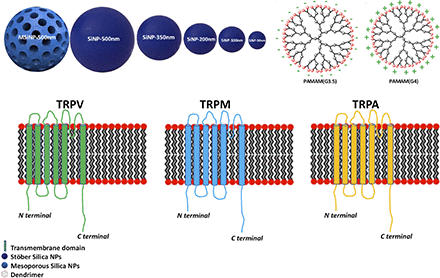
Visual Overview
Abstract
Fundamental to the design and development of nanoparticles for applications in nanomedicine is a detailed understanding of their biologic fate and potential toxic effects. Transient receptor potential (TRP) ion channels are a large superfamily of cation channels with varied physiologic functions. This superfamily is classified into six related subfamilies: TRP canonical, TRP vanilloid (TRPV), TRP melastatin (TRPM), TRP ankyrin (TRPA), TRP polycystin, and TRP mucolipin. TRPA1, TRPM2, and TRPM8 are nonselective Ca2+-permeable cation channels which regulate calcium pathways under oxidative stress, whereas TRPV4 can be activated by oxidative, osmotic, and thermal stress as well as different fatty acid metabolites. Using a series of well characterized silica nanoparticles with variations in size (approximately 50–350 nm in diameter) and porosity, as well as cationic and anionic poly(amido amine) (PAMAM) dendrimers of similar size, we examined the toxicity of these nanoparticles to human embryonic kidney-293 cells overexpressing different TRP channels. The data show that the toxicity of mesoporous silica nanoparticles was influenced by expression of the TRPA1 and TRPM2 channels, whereas the toxicity of smaller nonporous silica nanoparticles was only affected by TRPM8. Additionally, TRPA1 and TRPM2 played a role in the cytotoxicity of cationic dendrimers, but not anionic dendrimers. TRPV4 did not seem to play a significant role in silica nanoparticle or PAMAM toxicity.
Introduction
Silica nanoparticles (SNPs) and poly(amido amine) (PAMAM) dendrimers have shown potential for use in nanomedicine as drug delivery systems, imaging agents, biosensors, and theranostics, among other applications (Sadekar and Ghandehari, 2012; Lu et al., 2017; Hadipour Moghaddam et al., 2018; Saikia et al., 2018; Shao et al., 2018). To fully exploit this potential, a detailed understanding of their potential to cause toxicity as a function of nanoparticle physicochemical properties is needed. A better understanding of the structural components that promote toxicity of these particles will help in the design of safe nanoparticles for use in nanomedicine.
Transient receptor potential (TRP) ion channels are a superfamily of polymodal cellular sensors located on the plasma membranes of many mammalian cell types that nanoparticles can potentially interact with. Different TRPs are involved in different physiologic processes, such as the regulation of local and cellular calcium levels and associated signaling, control of Na2+ and Mg2+ levels, transduction of sensory signals, cell proliferation, cell death, migration, and general homeostasis (Fleig and Penner, 2004; Nilius, 2007; Venkatachalam and Montell, 2007; Gees et al., 2010; Andres et al., 2016; Markó et al., 2017). The TRP superfamily can be divided into seven subfamilies: TRP vanilloid (TRPV), TRP ankyrin (TRPA), TRP canonical, TRP melastatin (TRPM), TRP mucolipin, and TRP polycystin (Polycystic Kidney Disease [PKD] and polycystic kidney disease 2-like [PKDL]) (Fleig and Penner, 2004; Gees et al., 2010; Khalil et al., 2018). Most TRP channels are preferentially calcium permeable, and calcium signals originating from the opening of these channels have been reported to be important in modulating cell metabolism and general homoeostasis (Mulier et al., 2017).
In prior studies, it has been demonstrated that SNP uptake disrupts cellular calcium homoeostasis and alters the expression of calcium channel–related genes (Yang et al., 2009; Ariano et al., 2011; Duan et al., 2016). There have also been a few reports on alterations to TRP canonical and TRPV4 channel activities and C60 fullerene- and silica nanoparticle–induced cytotoxicity (Dryn et al., 2018). A role of the TRPM subfamily channel in SNP-induced oxidative stress and cell death has also been reported. It was shown that the TRPM2 channel was required for reactive oxygen species (ROS) production in human embryonic kidney (HEK)-293 cells and the subsequent increase in intracellular Ca2+ induced by SNPs via changes in NADPH oxidase activity (Yu et al., 2015). However, limited information exists regarding the influence of variation in particle size and surface charge on nanoparticle toxicity via TRP channels. In this manuscript, we report on possible roles for TRPA1, TRPM2, TRPM8, and TRPV4 in regulating the cytotoxicity of SNPs with systematic variations in size and porosity, and of PAMAM dendrimers with variations in surface charge, using HEK-293 cell lines stably overexpressing these human TRPs. We chose the HEK-293 line as a model since these cells have few endogenous TRP channels which allowed for a more clear determination of the precise functions of these channels with respect to SNP and PAMAM toxicity.
Materials and Methods
Chemicals and Reagents.
Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 medium, LHC-8, retinoic acid, and epinephrine were purchased from Invitrogen (Grand Island, NY). Fetal bovine serum was purchased from American Type Culture Collection (Rockville, MD). TrypLE Express was purchased from Gibco (Thermo Fisher, Waltham, MA). Phosphate-buffered saline biotechnology-grade tablets were received from VWR (Radnor, PA). Human TRPA1 cDNA was obtained from Open Biosystems (Huntsville, AL). Human TRPM8 cDNA was obtained from Origene (Rockville, MD). Human TRPV1 cDNA was amplified from fetal brain mRNA (Stratagene, La Jolla, CA). Hydrochloric acid (BDH Aristar grad) was obtained from VWR (Brooklyn, NY). Absolute ethanol (200 proof) and ammonium hydroxide were from Decon Laboratories, Inc. (King of Prussia, PA) and EMD Millipore Corp. (Billerica, MA), respectively. Cetyltrimethylammonium bromide (CTAB, ≥99.0%) and tetraethyl orthosilicate (98%) were purchased from Sigma-Aldrich (St. Louis, MO). Commercially available ethylene diamine-core PAMAM dendrimers were purchased from Dendritech (Midland, MI). The diameter of PAMAM is a theoretical value based on perfect dendrimer synthesis, which is 36°A and 45°A for PAMAM G3.5 (COOH terminus) and G4 (NH2 terminus), respectively. All chemicals were used as received.
Synthesis and Characterization of Nanoparticles.
SNPs with variations in size and porosity were synthesized and characterized by a modified Stöber method, as reported previously (Stöber et al., 1968; Huh et al., 2003; Yu et al., 2011; Saikia et al., 2016; Yazdimamaghani et al., 2018a). In brief, amorphous spherical nonporous SNPs approximately 50, 100, 200, 350, and 500 nm in diameter were prepared by changing tetraethyl orthosilicate, water, and ammonium hydroxide contents, as shown in Table 1. Approximately 500-nm mesoporous SNPs (MSNPs) were synthesized by the CTAB emulsifier templating combined with modified Stöber method (Saikia et al., 2016). Mesoporous SNPs were further subject to CTAB removal using acid extraction at 60°C for 6 hours. The SNPs were fully characterized using transmission electron microscopy (TEM) on a JEOL JEM 1400 microscope (JEOL Ltd., Tokyo, Japan) operating at 120 kV. One drop of colloidal solution of SNP sample was placed on a copper formwar-carbon-coated TEM grid. The excess liquid was removed using a piece of filter paper, the grid was allowed to dry for 5 minutes, and then the SNPs on the dried grid were imaged using a TEM instrument. The surface morphology of nanoparticles was studied using scanning electron microscopy. One drop of colloidal solution of SNP sample was placed on a bilateral tape and gold sputter–coated utilizing an Sputter Coater (Gatan PECS coated with Au, CA). Each sample was observed under an Quanta 650 FE-SEM scanning electron microscope operating at 20 kV (FEI, Hillsboro, OR). Dynamic light scattering and zeta potential measurements were carried out by Zetasizer Nano ZS (Malvern Instruments Ltd., Worcestershire, UK). Nitrogen adsorption−desorption isotherm analysis was conducted on an ASAP 2020 (Micromeritics, Norcross, GA) at −196°C to measure internal and external surface area and pore size.
Synthetic conditions of nonporous and mesoporous SNPs based on molar ratio of solution
Cell Culture TRP Channel Cloning.
Cells were maintained in a humidified cell culture incubator at 37°C with a 95% air:5% CO2 atmosphere. HEK-293 cells (American Type Culture Collection) were cultured in DMEM/F12 medium containing 5% fetal bovine serum and 1× penicillin/streptomycin. Human TRP channel–overexpressing HEK-293 cells (TRPA1, TRPM2, TRPM8, and TRPV4) were generated as previously described (Deering-Rice et al., 2011, 2012) and cultured in DMEM/F12 medium containing 5% fetal bovine serum, 1× penicillin/streptomycin, and geneticin (300 μg/ml).
Cytotoxicity Assays.
The in vitro cytotoxicity of nanoparticles was assessed by a Cell Counting Kit-8 assay (Dojindo Molecular Technology, Rockville, MD). In brief, cells (4 × 103 per well) were seeded in 96-well microliter plates for 24 hours before treatment. Cells were exposed to different concentrations of freshly prepared nanoparticles and incubated for 24 hours. The medium was removed and 100 ml of medium solution containing 10% (v/v) Cell Counting Kit-8 reagent was added. The cells were kept at 37°C for a further 1- to 2-hour incubation. The absorbance of the supernatant was obtained by scanning with a SpectraMax M2 microplate reader (Molecular Devices, Sunnyvale, CA) at 450 nm. Each experiment was performed at least three times in triplicate. Control experiments were performed using cells treated with complete medium without particles as the negative control and 0.01% (v/v) Triton X-100 as the positive control. The difference between values was considered significant at the level of P ≤0.05.
Results and Discussion
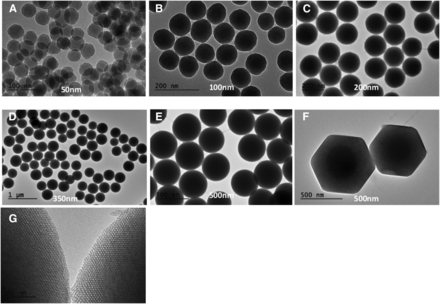
Figures 1 and 2 and Tables 2 and 3 outline the characteristics of the synthesized SNPs. Stöber SNPs (∼50, 100, 200, 350, and 500 nm) with zeta potential values of approximately −40 to −50 mv were prepared to investigate the influence of size. In addition, the effect of porosity was examined by comparing similar-size Stöber SNPs (500 nm) with MSNPs. Porosity increased the surface area of the particles by approximately 40-fold in comparison with nonporous particles. MSNPs had an approximately 3.05-nm pore size, which was confirmed by TEM in at least 50 different particles.
Transmission electron microscopy images of Stöber (A–E) and mesoporous silica nanoparticles (F and G) with variations in size and porosity. Increasing the size of the Stöber particles from 50 to 500 nm results in an increase in the smoothness on the surface of the particle. (F and G) Uniformly aligned mesopores along and perpendicular to the axes of mesoporous SNPs were observed. (G) High-resolution TEM images showed two-dimensional hexagonal mesopores in the close-packing structure for mesoporous SNPs.
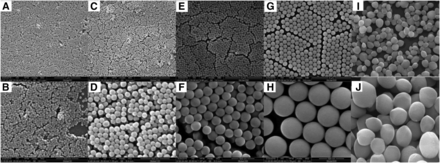
Scanning electron microscopy images of Stöber SNPs with an average diameter of 46 nm (Stöber50) (A and B), Stöber SNPs with an average diameter of 91 nm (Stöber100) (C and D), Stöber SNPs with an average diameter of 205 nm (Stöber 200) (E and F), Stöber SNPs with an average diameter of 432 nm (Stöber 500) (G and H), and mesoporous SNPs with an average diameter of 466 nm (I and J). Data for 50- and 500-nm SNPs and MSNPs were reported previously (Saikia et al., 2016; Yazdimamaghani et al., 2018b).
Physicochemical characterization of Stöber and mesoporous silica nanoparticles in different solutions: water, cell culture medium (RPMI) with serum (fetal bovine serum), and without serum
Values are the mean ± S.D. (n = 3).
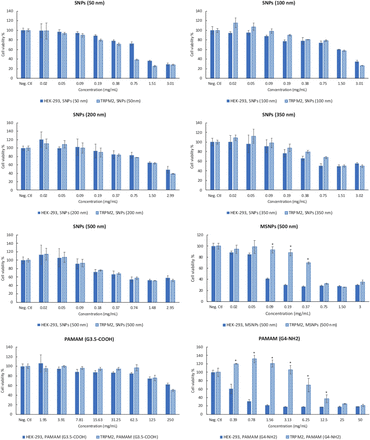
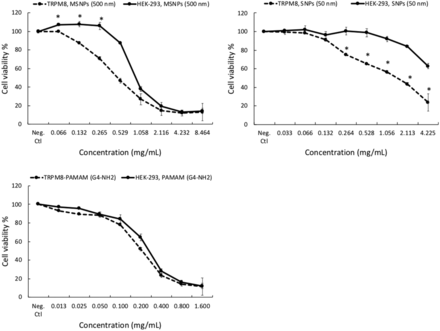
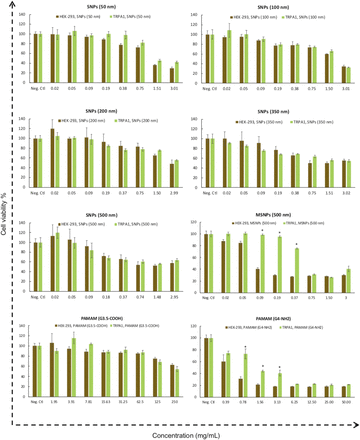
Different TRP ion channel–dependent toxicity of silica nanoparticles and PAMAM dendrimers
Results of the cytotoxicity assay show PAMAM G4-NH2 dendrimers and 500-nm MSNPs have different toxicity in TRPA1- and TRPM2-overexpressed HEK-293 cells. Overexpressing of TRPM8 also changed the toxicity of Stöber 50-nm SNPs and 500-nm MSNPs.
The human TRPM subfamily was named from the founding member melastatin (TRPM1) and consists of eight members that can be grouped into four pairs (TRPM1 and TRPM3, TRPM2 and TRPM8, TRPM4 and TRPM5, and TRPM6 and TRPM7) (Fleig and Penner, 2004). It is well known that TRPM2 channels are expressed by many cell types in the immune system and play a crucial role in producing proinflammatory cytokines in response to oxidative stress and inflammatory stimuli (Jiang et al., 2010; So et al., 2015; Syed Mortadza et al., 2015). TRPM8 is activated by moderately cool temperatures and by compounds that elicit a sensation of coolness, such as menthol (McKemy, 2007; Sabnis et al., 2008; Liu et al., 2013). The cytotoxicity data in Figs. 3 and 4 show that TRPM2 and TRPM8 affect the cytotoxic properties of 500-nm MSNPs, 50-nm SNPs, and PAMAM G4-NH2 dendrimers, albeit in different ways and with some degree of selectivity. Specifically, overexpression of TRPM2 attenuated the cytotoxicity of 500-nm MSNPs and PAMAM G4-NH2 dendrimers in comparison with normal HEK-293 cells. However, the viability of TRPM8-overexpressing HEK-293 cells treated with 500-nm MSNPs and 50-nm SNPs significantly decreased relative to normal TRPM8-deficient HEK-293 cells. Published data suggest that TRPM8 channels form calcium-permeable channels in the Endoplasmic Reticulum (ER) membrane and plasma membrane of prostate cancer cells are involved in proliferation, differentiation, and cell death (Fliniaux et al., 2017). Here, our data showed cell viability was significantly reduced by exposure to SNPs in a concentration-dependent manner in the cells expressing a high level of TRPM8 channels. On the other hand, TRPM2 channels might mediate a defense mechanism in the cells that have taken up the nanoparticles and increase their survival. The increase in cell viability of TRPM2-overexpressing cells in our current studies is consistent with previous reports examining smaller SNPs (Yu et al., 2015). This study showed that the ROS production in HEK-293 cells expressing a low level of TRPM2 resulted in cell death, but high TRPM2 expression led to inhibition of ROS production and attenuated cell death (Yu et al., 2015). We observed the same protective effect of TRPM2 with MSNPs and positively charged PAMAM G4-NH2 dendrimers. Thus, it can be concluded that the protective mechanism of TRPM2 against cell death induced by SNPs and PAMAM dendrimers is highly dependent on physiochemical properties.
Comparison of cytotoxicity between HEK-293 cells and HEK-293 cells overexpressing the human TRPM2 ion channel after treatment with different concentrations of SNPs and PAMAM dendrimers for 24 hours. Data show a significant (P ≤ 0.05) TRPM2-dependent reduction in 500-nm MSNPs and PAMAM G4-NH2 cytotoxicity relative to normal HEK-293 cells. Values are the mean ± S.D. (n = 3).
Comparison of cytotoxicity between HEK-293 cells and HEK-293 cells overexpressing the human TRPM8 ion channel after treatment with different concentrations of SNPs (nonporous 50 and 500 nm; mesoporous 500 nm) and PAMAM G4 dendrimers for 24 hours. Data show a significant increase in the cytotoxicity of the 500-nm mesoporous (MSNP 500) and 50-nm nonporous SNPs (P ≤ 0.05), but not PAMAM G4-NH2 dendrimers in TRPM8-overexpressing cells. Values are the mean ± S.D. (n = 3). Neg. Ctl, negative control.
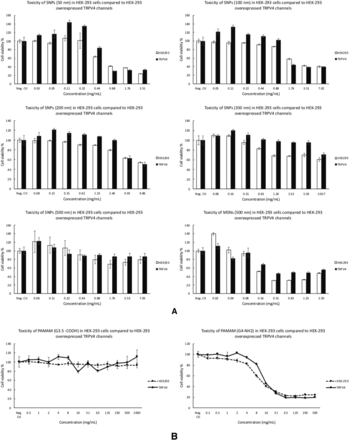
The TRPA1 cation channel is a molecular sensor for electrophiles and oxidants in lung cells (Bessac and Jordt, 2010). TRPA1 is the only TRPA protein present in humans and other mammals (Venkatachalam and Montell, 2007). TRPA1-overexpressing HEK-293 cells were treated with SNPs, MSNPs, and PAMAM dendrimers, and their viability in the presence and absence of nanoparticles was measured. All SNPs showed concentration-dependent toxicity in HEK-293 cells and in HEK-293 cells overexpressing the TRPA1 channel (Fig. 5). Among all of the SNPs studied, HEK-293 cells overexpressing TRPA1 were less susceptible to toxicity caused by 500-nm mesoporous SNP and PAMAM G4-NH2 dendrimers. At a dose range of 0.09–0.37 mg/ml, the degree of toxicity caused by 500-nm mesoporous SNPs in TRPA1-overexpressing HEK-293 cells was significantly (P ≤ 0.05) less than for normal HEK-293 cell MSNPs (Fig. 5). The same trend was observed for the positively charged PAMAM dendrimer in the concentration range of 0.78–3.13 mg/ml. These results suggest that overexpression of TRPA1 on the cell membrane, and perhaps intracellularly, can induce resistance to the toxic effect of MSNPs and PAMAM G4-NH2 dendrimers. To the best of our knowledge, there are no reports on TRPA1 regulating the cytotoxicity of SNPs in any model system. Finally, previous observations showed that TRPA1 may mediate some of the adverse effects of wood smoke nanoparticles in humans (Shapiro et al., 2013). One explanation for our results is that overexpression of TRPA1 in HEK-293 cells can facilitate rescue from cell death signaling, which is triggered by SNP uptake. More mechanistic studies are needed to investigate the molecular pathways related to TRPA1, novel agonists such as SNPs, and modulation of cell viability.
Comparison of cytotoxicity between HEK-293 cells and HEK-293 cells overexpressing the human TRPA1 ion channel after treatment with different concentrations of SNPs and PAMAM dendrimers for 24 hours. Data show a significant (P ≤ 0.05) TRPA1-dependent reduction in 500-nm MSNPs and PAMAM G4-NH2 cytotoxicity relative to normal HEK-293 cells. Values are the mean ± S.D. (n = 3). Neg. Ctl, negative control.
TRPV4 was originally identified to be involved in the regulation of osmotic homeostasis. Involvement of TRPV4 channels was reported for gonadotropin hormone-releasing hormone release by neurons when treated with 50-nm SNPs (Gilardino et al., 2015). Further studies on human bronchial epithelial cell lines demonstrated the inhibition of TRPV4 in the presence of 10.2-nm silica nanoparticles, suggesting that these nanoparticles may impair the positive regulatory action of the stimulation of TRPV4 channels on ciliary function of 16HBE human bronchial epithelial cells (Sanchez et al., 2017). Consistent with previous reports (Gilardino et al., 2015), we did not observe a correlation between the size of SNPs with TRP-related toxicity in TRPV4-overexpressing HEK-293 cells (Fig. 6). Our results showed that there is no significant difference for cytotoxicity between normal HEK-293 cells and TRPV4-overexpressing HEK-293 when exposed to SNPs, MSNPs, or PAMAM dendrimers of either surface charge (Fig. 6). These results suggest that the effect of TRPV4 on nanoparticle effects may be cell-type-specific and/or dependent upon differences in the zeta potential values.
Comparison of cytotoxicity between HEK-293 cells with HEK-293 cells overexpressing the human TRPV4 ion channel after treatment with different concentrations of SNPs (A) and PAMAM dendrimers (B) for 24 hours. Data show no significant difference in cytotoxicity between the two cell lines for any of the nanoparticles tested. Values are the mean ± S.D. (n = 3).
Overall, our data show that, among a series of well characterized SNPs with systematic variations in size and porosity, the toxicity of smaller particles and MSNPs is significantly affected by TRPM2, TRPA1, and TRPM8 channels in HEK-293 cells. On the other hand, TRPA1 and TRPM2 play an important role in the cytotoxicity of amine-terminated PAMAM G4-NH2 dendrimers but not carboxyl-terminated negatively charged PAMAM G3.5-COOH dendrimers of approximately similar size. Finally, TRPV4 does not seem to play a significant role in SNP, MSNP, or PAMAM dendrimer toxicity.
It is known that the cellular uptake of nanoparticles can also induce different stresses to cells. Depending upon the severity, duration, and mode of stress, the cumulative cellular response can range from activation of prosurvival pathways or unprogrammed cell death (Fulda et al., 2010). It has been demonstrated that SNP uptake can disrupt cellular calcium homoeostasis (Yang et al., 2009; Ariano et al., 2011; Duan et al., 2016). Also, calcium can trigger a signaling cascade in which Calmodulin-Dependent Protein Kinase (CAM) kinase activates protein kinase B pathways and protects cells from apoptosis (Yano et al., 1998). Here, our data show that overexpression of different TRP/Ca2+ channels (TRPA1 and TRPM2) on the membranes of epithelial cells may help cells overcome or avoid the cytotoxic effects of certain forms of particles as a function of the nanoparticles’ physicochemical properties. The next challenge is to understand the specific calcium-sensitive pathways that are modulated by the various nanoparticles as a function of the presence or absence of specific TRP channels to understand the related cell survival and death molecular pathways.
We have previously shown that transepithelial transport and toxicity of PAMAM dendrimers depend on their size, surface charge, concentration, and incubation time of these particles (Greish et al., 2012; Sadekar and Ghandehari, 2012; Thiagarajan et al., 2013), and that cellular uptake, in vivo biodistribution, and toxicity of silica nanoparticles depend on their size, porosity, surface functionality, and geometry, among other factors (Yu et al., 2012; Herd et al., 2013; Yazdimamaghani et al., 2018b). However, little is known about the detailed mechanisms of interaction of these nanoparticles with ion channels, such as the TRPs, which have varied physiologic functions such as being cellular redox potential sensors or modulating various phases of immune responses. Although our data do not address these topics, they show that size, porosity, and surface functionality influence the toxicity of SNPs and PAMAM dendrimers via selective interactions that are, in part, related to the expression and function of different TRP channels. As such, these early but enlightening studies have implications with respect to the design of silica and dendritic nanoconstructs for transepithelial transport and their immunotoxicity.
Conclusion
Together, these findings provide the first evidence of differential involvement of TRPA1, TRPM2, and TRPM8 in SNPs and PAMAM dendrimer toxicity in a model system of human HEK-293 cell lines overexpressing TRP channels. Specifically, size and surface properties of the nanoparticles seem to have important roles in the effects of different TRPs on the acute cytotoxicity of these materials in HEK-293 cells. A better understanding of the underlying molecular mechanisms of cation TRP channel–related nanoparticle toxicity will be necessary to further elucidate the critical aspects of how TRPs influence nanoparticle toxicity. However, we anticipate that these novel findings will provide fresh insight into the design of safe and effective nanoparticles for use in nanomedicine applications.
Authorship Contributions
Participated in research design: Mohammadpour, Yazdimamaghani, Reilly, Ghandehari.
Conducted experiments: Mohammadpour, Yazdimamaghani, Reilly.
Performed data analysis: Mohammadpour, Yazdimamaghani, Reilly, Ghandehari.
Wrote or contributed to the writing of the manuscript: Mohammadpour, Yazdimamaghani, Reilly, Ghandehari.
Footnotes
- Received September 18, 2018.
- Accepted November 12, 2018.
Support was provided by the National Institutes of Health National Institute of Environmental Health Sciences of the [Grants R01ES024681, R01ES017431, and R01ES027015]. The authors acknowledge the use of the University of Utah shared facilities of the Micron Microscopy Suite and the University of Utah USTAR shared facilities supported in part by the MRSEC Program of the National Science Foundation (NSF) [Award No. DMR-1121252].
The authors declare no conflicts of interest.
Abbreviations
- CTAB
- cetyltrimethylammonium bromide
- DMEM
- Dulbecco’s modified Eagle’s medium
- HEK
- human embryonic kidney
- MSNP
- mesoporous silica nanoparticle
- PAMAM
- poly(amido amine)
- ROS
- reactive oxygen species
- SNP
- silica nanoparticle
- TEM
- transmission electron microscopy
- TRP
- transient receptor potential
- TRPA
- TRP ankyrin
- TRPM
- TRP melastatin
- TRPV
- TRP vanilloid
- Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics