Abstract
It has been hypothesized that high-dose methamphetamine treatment rapidly redistributes cytoplasmic dopamine within nerve terminals, leading to intraneuronal reactive oxygen species formation and well characterized persistent dopamine deficits. We and others have reported that in addition to this persistent damage, methamphetamine treatment rapidly decreases vesicular dopamine uptake, as assessed in purified vesicles prepared from treated rats; a phenomenon that may contribute to aberrant intraneuronal dopamine redistribution proposedly caused by the stimulant. Interestingly, post-treatment with dopamine transporter inhibitors protect against the persistent dopamine deficits caused by methamphetamine; however, mechanisms underlying this phenomenon have not been elucidated. Also of interest are findings that dopamine transporter inhibitors, including methylphenidate, rapidly increase 1) vesicular dopamine uptake, 2) vesicular monoamine transporter-2 (VMAT-2) ligand binding, and 3) VMAT-2 immunoreactivity in a vesicular subcellular fraction prepared from treated rats. Therefore, we hypothesized that methylphenidate post-treatment might protect against the persistent striatal dopamine deficits caused by methamphetamine by rapidly affecting VMAT-2 and vesicular dopamine content. Results reveal that methylphenidate post-treatment both prevents the persistent dopamine deficits and reverses the acute decreases in vesicular dopamine uptake and VMAT-2 ligand binding caused by methamphetamine treatment. In addition, methylphenidate post-treatment reverses the acute decreases in vesicular dopamine content caused by methamphetamine treatment. Taken together, these findings suggest that methylphenidate prevents persistent methamphetamine-induced dopamine deficits by redistributing vesicles and the associated VMAT-2 protein and presumably affecting dopamine sequestration. These findings not only provide insight into the neurotoxic effects of methamphetamine but also mechanisms underlying dopamine neurodegenerative disorders, including Parkinson's disease.
High-dose methamphetamine administration causes persistent dopamine deficits in rodents, nonhuman primates, and humans (for review, see Fleckenstein et al., 2000). Dopamine, per se, likely contributes to this damage, because it is attenuated by pretreatment of rats with the dopamine synthesis inhibitor α-methyl-p-tyrosine (Gibb and Kogan, 1979; Wagner et al., 1983). Intraneuronal dopamine has been suggested to be of particular importance, because methamphetamine application causes oxygen radical formation within ventral midbrain culture-containing dopamine neurons (Cubells et al., 1994).
Intraneuronal dopamine levels are regulated largely by the vesicular monoamine transporter-2 (VMAT-2), because this carrier transports dopamine into synaptic vesicles for storage. Amphetamine analogs, including methamphetamine, alter VMAT-2 function. For instance, Sulzer and Rayport (1990) demonstrated that amphetamine disrupts the proton gradient necessary for vesicular uptake of neurotransmitter in the intracellular compartments in midbrain dopaminergic neurons. In addition, Brown et al. (2000) reported that multiple methamphetamine injections rapidly (within 1 h) decrease vesicular dopamine uptake as assessed in purified striatal vesicles obtained from treated rats. Similar findings were reported by Hogan et al. (2000). The rapid decrease in uptake observed 1 h post-treatment is associated with a redistribution of VMAT-2, and presumably associated vesicles, within nerve terminals (Riddle et al., 2002). Accordingly, it can be hypothesized that methamphetamine, in part by redistributing synaptic vesicles and in part by disrupting vesicular dopamine storage, causes dopamine to accumulate in an extravesicular intracellular oxidizing environment. This, in turn, promotes the generation of oxygen radicals and reactive metabolites within dopamine neurons, thereby triggering dopamine terminal loss.
In addition to the VMAT-2, the dopamine transporter has been implicated in effecting methamphetamine-induced dopamine deficits. For instance, pretreatment with amfonelic acid, a dopamine transporter reuptake inhibitor, prevents the persistent dopamine deficits caused by methamphetamine treatment (Schmidt and Gibb, 1985). Similar findings were reported by others for amphetamine (Fuller and Hemrick-Luecke, 1980, 1982). These researchers speculated that this neuroprotection was due to the ability of dopamine transporter reuptake inhibitors to prevent methamphetamine entry into nerve terminals. However, this interpretation is confounded by the fact that amphetamine analogs diffuse across nerve terminal plasma membranes (Mack and Bonisch, 1979;Liang and Rutledge, 1982; Zaczek et al., 1991), and reports that dopamine transporter reuptake inhibitors can prevent methamphetamine-induced neurotoxicity when administered up to 8 h after methamphetamine treatment (Marek et al., 1990). Consequently, the mechanism whereby dopamine transporter inhibitors prevent the methamphetamine-induced dopaminergic deficits is not known.
Recent reports demonstrate that dopamine transporter reuptake inhibitors, including amfonelic acid, increase vesicular dopamine uptake (Brown et al., 2001). In addition, another dopamine transporter reuptake inhibitor, methylphenidate, increases vesicular dopamine uptake, possibly by causing a redistribution of VMAT-2 within nerve terminals (Sandoval et al., 2002). Accordingly, we hypothesize that dopamine transporter reuptake inhibitors such as methylphenidate may protect against methamphetamine-induced neurodegeneration by preventing or compensating for the methamphetamine-induced alterations in dopamine disposition. The present results confirm this hypothesis. Accordingly, these data provide insight into causes underlying methamphetamine neurotoxicity and may suggest novel therapeutic strategies for treating dopamine neurodegenerative disorders such as Parkinson's disease.
Materials and Methods
Animals.
Male Sprague-Dawley rats (280–340 g; Simonsen Laboratories, Gilroy, CA) were maintained under controlled light and temperature conditions, with food and water provided ad libitum. All procedures were conducted in accordance with National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the University of Utah Institutional Animal Care and Use Committee.
Drugs and Chemicals.
(±)-Methylphenidate hydrochloride and (±)-methamphetamine hydrochloride were supplied by the National Institute on Drug Abuse (Bethesda, MD). 7,8-[3H]Dopamine (42 Ci/mmol) was purchased from Amersham Biosciences Inc. (Piscataway, NJ) and α-[2-3H]dihydrotetrabenazine (20 Ci/mmol) was purchased from American Radiolabeled Chemicals (St. Louis, MO). Tetrabenazine was kindly donated by Drs. Jeffrey Erickson and Helene Varoqui (Louisiana State University Health Sciences Center, New Orleans, LA). VMAT-2 antibody was purchased from Chemicon International (Temecula, CA). Doses were calculated as the respective free base and drugs were dissolved in 0.9% saline.
Preparation of Striatal Synaptic Vesicles.
Synaptosomes were prepared from rat striatum as described previously (Fleckenstein et al., 1997). Synaptosomes were then resuspended and homogenized in cold, distilled, deionized water. Osmolarity was restored by addition of HEPES and potassium tartrate (final concentration 25 and 100 mM, respectively; pH 7.5). Samples were centrifuged for 20 min at 20,000g (4°C) to remove lysed synaptosomal membranes. MgSO4 (1 mM, final concentration) was added to the supernatant, which was then centrifuged for 45 min at 100,000g (4°C). The resulting vesicular pellet was resuspended in wash buffer (see below) at a concentration of 50 mg/ml (original tissue wet weight).
Vesicular [3H]Dopamine Uptake and [3H]Dihydrotetrabenazine Binding.
Vesicular [3H]dopamine uptake was performed by incubating 100 μl (∼2.5 μg of protein) of synaptic vesicle samples at 30°C for 3 min in assay buffer (final concentration 25 mM HEPES, 100 mM potassium tartrate, 1.7 mM ascorbic acid, 0.05 mM EGTA, 0.1 mM EDTA, and 2 mM ATP-Mg2+; pH 7.5) in the presence of [3H]dopamine (30 nM final concentration). The reaction was terminated by addition of 1 ml of cold wash buffer (assay buffer containing 2 mM MgSO4 substituted for the ATP-Mg2+; pH 7.5) and rapid filtration through GF/F filters (Whatman, Maidstone, UK) soaked previously in 0.5% polyethylenimine. Filters were washed three times with cold wash buffer using a filtering manifold (Brandel, Inc., Gaithersburg, MD). Radioactivity trapped in filters was counted using a liquid scintillation counter. Nonspecific values were determined by measuring vesicular [3H]dopamine uptake at 4°C in wash buffer.
Binding of [3H]dihydrotetrabenazine was performed as described previously (Brown et al., 2000; Sandoval et al., 2002). Briefly, 200 μl (∼5 μg of protein) of the synaptic vesicle preparation was incubated in wash buffer in the presence of [3H]dihydrotetrabenazine (2 nM final concentration) for 10 min at 25°C. The reaction was terminated by addition of 1 ml of cold wash buffer and rapid filtration through GF/F filters (Whatman) soaked in 0.5% polyethylenimine. Filters were washed three times with cold wash buffer. Radioactivity trapped in filters was counted using a liquid scintillation counter. Nonspecific binding was determined by coincubation with 20 μM tetrabenazine. All protein concentrations were determined by a protein assay (Bio-Rad, Hercules, CA).
Preparation of Striatal Subcellular Fractions.
Fresh striatal tissue was homogenized in ice-cold 0.32 M sucrose and centrifuged (800g for 12 min; 4°C). The resulting supernatant (S1) was then centrifuged (22,000g for 15 min; 4°C), and the pellets [P2; whole synaptosomal fraction (plasmalemmal membrane plus vesicular subcellular fractions)] were resuspended in cold, distilled, deionized water at a concentration of 50 mg/ml (original wet weight of tissue). Resuspended tissue was aliquoted into two test tubes. One aliquot was centrifuged (22,000g for 20 min; 4°C) to separate plasmalemmal membranes from the synaptic vesicle-enriched fraction. The resulting supernatant (S3) contained the vesicular subcellular fraction of interest, and the pellets (P3; plasmalemmal membrane fraction) were resuspended in cold, distilled, deionized water.
Western Blot Analysis.
Binding of VMAT-2 antibody was performed using 60 μl of whole synaptosomal, plasmalemmal membrane, or vesicle subcellular fractions. Samples were added to 20 μl of loading buffer (final concentration: 2.25% SDS, 18% glycerol, 180 mM Tris base pH 6.8, and 10% β-mercaptoethanol and bromophenol blue). Approximately 60 μg of protein of the whole synaptosomal fraction, 40 μg of protein of the plasmalemmal membrane fraction, or 20 μg of protein of the vesicle subcellular fraction was loaded per well in a 10% SDS-polyacrylamide gel. After electrophoresis, samples were transferred to polyvinylidene difluoride hybridization transfer membrane (PerkinElmer Life Sciences, Boston, MA). All subsequent incubation steps were performed at room temperature while shaking. Each membrane was first blocked for 2 h in 100 ml of Tris buffer saline with Tween (TBST; 250 mM NaCl, 50 mM Tris pH 7.4, and 0.05% Tween 20) containing 5% nonfat dry milk. Each membrane was then incubated with anti-VMAT-2 antibody (1:1000 dilution) in 13 ml of TBST with 5% milk for 1 h and then washed five times (2 × 1-min wash; 3 × 5-min wash) in 70 ml of TBST with 5% milk. The membranes then were incubated for 1 h with the goat F(ab′)2anti-rabbit immunoglobulin antibody (BioSource International, Camarillo, CA) at a 1:2000 dilution in TBST with 5% milk. This secondary antibody had been affinity-isolated, preabsorbed with human immunoglobulin, and conjugated with horseradish peroxidase. The membranes were then washed five times (2 × 1-min wash; 3 × 5-min wash) with 70 ml of TBST, and then developed with the Renaissance Western blot chemiluminescence reagent plus (PerkinElmer Life Sciences), according to manufacturer's specification. Multiple exposures of blots were obtained to ensure development within the linear range of the film (Biomax MR; Eastman Kodak, Rochester, NY). Bands on blots were quantified by densitometry measuring net intensity (the sum of the background-subtracted pixel values in the band area) using Kodak 1D image analysis software.
Vesicular Dopamine Content.
Purified striatal vesicles were prepared as described above. The resulting vesicular pellet was sonicated for approximately 5 s in cold tissue buffer [0.05 M sodium phosphate/0.03 M citric acid buffer with 15% methanol (v/v); pH 2.5] at a concentration of 100 mg/ml (original wet weight of tissue), and centrifuged for 15 min at 22,000g. Tissue pellets were retained and protein was determined according to the method of Lowry et al. (1951). Supernatant (40 μl) was injected onto a high-performance liquid chromatograph system coupled to an electrochemical detector (+0.73 V) for separation and quantitation of dopamine levels using the method of Chapin et al. (1986).
Tissue Dopamine Content.
On the day of the assay, frozen tissue samples were thawed, sonicated for 3 to 5 s in tissue buffer [0.05 M sodium phosphate/0.03 M citric acid buffer with 15% methanol (v/v); pH 2.5], and centrifuged for 15 min at 22,000g. Tissue pellets were retained and protein determined according to the method ofLowry et al. (1951). The supernatant was centrifuged a second time for 10 min at 22,000g. Supernatant (20 μl) was injected onto a high-performance liquid chromatograph system coupled to an electrochemical detector (+0.73 V) for separation and quantitation of dopamine levels by using the method of Chapin et al. (1986).
Data Analysis.
Statistical analyses among three or more groups were performed using an analysis of variance followed by Fisher's protected least significant difference post hoc comparison. Differences were considered significant if probability of error was less than 5%.
Results
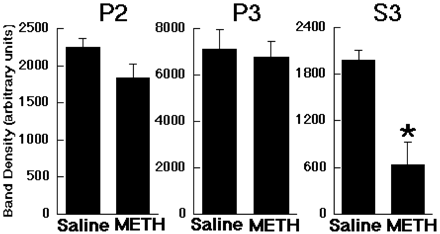
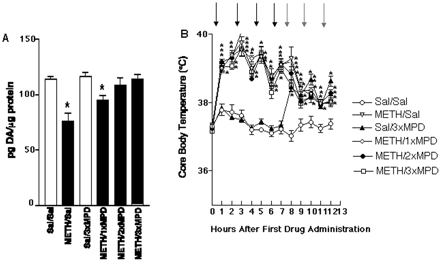
Results presented in Fig. 1demonstrate that multiple administrations of methamphetamine (4 × 7.5 mg/kg s.c.; 2-h intervals) rapidly decreased VMAT-2 immunoreactivity in a vesicular subcellular fraction (S3), with no change in the whole synaptosomal fraction (P2) or in the plasmalemmal membrane fraction (P3) as assessed in sample prepared 1 h after the final methamphetamine injection. In addition, administration of this same methamphetamine regimen decreased striatal dopamine levels with respect to the saline/saline-treated group 7 days after treatment (Fig. 2A). Post-treatment with a single methylphenidate injection 1 h after the last methamphetamine administration partially reversed the 7-day striatal dopamine depletions caused by the methamphetamine treatment. Two or three injections of methylphenidate (1 and 3 h, or 1, 3, and 5 h, respectively) after the last methamphetamine administration completely prevented the persistent methamphetamine-induced striatal dopamine depletions (Fig. 2A). Methylphenidate post-treatment per se did not alter total dopamine levels 7 days after treatment, nor did it prevent the hyperthermia induced acutely by methamphetamine administration (Fig. 2B).
Multiple administrations of methamphetamine (METH) decrease VMAT-2 immunoreactivity. Rats received methamphetamine (four injections; 7.5 mg/kg s.c.; 2-h intervals) or saline (1 ml/kg s.c.). All animals were decapitated 1 h after the last methamphetamine or saline injection. VMAT-2 immunoreactivity was assessed in a whole synaptosomal fraction (P2), a plasmalemmal membrane fraction (P3), and a vesicular subcellular fraction (S3). Columns represent the mean band density, and error bars represent the S.E.M. of determinations in six treated rats. ∗, value for methamphetamine-treated rats that are significantly different from saline-treated controls (p ≤ 0.05).
Post-treatment with methylphenidate attenuates the methamphetamine (METH)-induced dopaminergic deficits. A, rats received methamphetamine (four injections; 7.5 mg/kg s.c.; 2-h intervals) or saline (Sal; 1 ml/kg s.c.). Rats received one injection of methylphenidate (MPD; 5 mg/kg s.c.) 1 h, two injections of methylphenidate 1 and 3 h, three injections of methylphenidate or saline (1 ml/kg s.c.) 1, 3, and 5 h after the last methamphetamine or saline injection. Rats were decapitated 7 days after the last methamphetamine, methylphenidate or saline administration. Columns represent the mean dopamine (DA) concentrations and vertical lines 1 S.E.M. of determinations in 8 to 12 rats. ∗, values that are significantly different from Sal/Sal-treated group. B, time course of core body temperatures. Rectal temperatures were recorded before the first methylphenidate, methamphetamine, or saline injection (t = 0 h), and every hour thereafter (t = 0–13 h). Gray inverted arrows represent time points of methylphenidate or saline administrations and black inverted arrows represent time points of methamphetamine or saline administrations. Symbols represent means, and vertical lines 1 S.E.M. of determinations in 8 to 12 rats. ∗, values that are different from Sal/Sal-treated group (p ≤ 0.05).
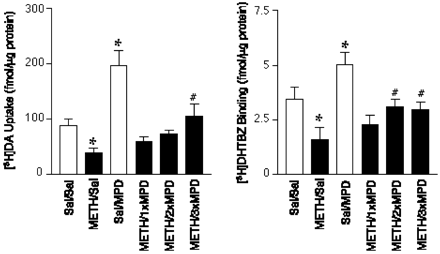
Results presented in Fig. 3 demonstrate that, as has been reported previously (Brown et al., 2001), multiple administrations of methamphetamine rapidly decreased vesicular dopamine uptake and dihydrotetrabenazine binding, as assessed in purified striatal vesicles prepared 1 h after the last methamphetamine injection. These effects persisted at least 6 h after treatment (Fig. 4). Post-treating animals with one, two, or three injections of MPD administered, as described for Fig. 2, during the initial 6-h period after methamphetamine treatment partially reversed these rapid methamphetamine-induced decreases in vesicular dopamine uptake and dihydrotetrabenazine binding (Fig. 3). As reported previously (Sandoval et al., 2002), MPD treatment per se increased vesicular dopamine uptake and dihydrotetrabenazine binding (Fig. 3).
Post-treatment with methylphenidate (MPD; 5 mg/kg/injection s.c.) attenuates the acute methamphetamine-induced decrease in striatal vesicular dopamine (DA) uptake and dihydrotetrabenazine (DHTBZ) binding. Rats received methamphetamine (METH; four injections; 7.5 mg/kg s.c.; 2-h intervals) or saline (Sal; 1 ml/kg s.c.). Some rats received one injection of methylphenidate 1 h after the final methamphetamine injection (e.g., METH/1XMPD). Others received two injections of methylphenidate 1 and 3 h after the final methamphetamine injection (e.g., METH/2XMPD). A third group of rats received three injections of methylphenidate or saline (1 ml/kg s.c.) 1, 3, and 5 h after the last saline or methamphetamine injection (Sal/3xMPD and METH/3xMPD, respectively). Rats were decapitated 1 h after the last methylphenidate, methamphetamine, or saline injection. Columns represent the means and vertical lines 1 S.E.M. of determinations in six rats. ∗, values that are significantly different from the Sal/Sal-treated group; #, values that are significantly different from the METH/Sal-treated group (p ≤ 0.05).
Multiple administrations of methamphetamine (METH) decreased vesicular dopamine (DA) uptake and dihydrotetrabenazine (DHTBZ) binding. Rats received methamphetamine (four injections; 7.5 mg/kg s.c.; 2-h intervals) or saline (1 ml/kg s.c.). Rats were decapitated 1, 2, 4, or 6 h after the last methamphetamine administration or 1 h after the last saline administration (zero-time controls). Symbols represent the means and vertical lines 1 S.E.M. of determinations in six rats. ∗, values that are significantly different from the saline controls.
Vesicular dopamine content is a functional consequence of vesicular dopamine uptake. Accordingly, we investigated the impact of stimulant treatment on vesicular dopamine content. As a preliminary experiment to validate our assay, rats were treated with reserpine (10 mg/kg i.p.) 6 and 1 h before decapitation. Predictably, reserpine caused >98% depletion in total striatal tissue dopamine levels, and striatal vesicular dopamine levels were below the detection limit of our assay. In another experiment, multiple methamphetamine administrations (4 × 7.5 mg/kg s.c., 2-h intervals) decreased vesicular dopamine levels by 49% (i.e., 43.2 ± 6.0 and 2.30 ± 2.5 pg/μg protein for saline and methamphetamine-treated rats, respectively; n = 6, p ≤ 0.05) as assessed 1 h after treatment. Further results reveal that multiple methamphetamine administrations decreased both vesicular and whole tissue dopamine content by ∼60%, as assessed 6 h after drug treatment (Fig. 5, A and B). In contrast, administration of three injections of methylphenidate (administered over a 5-h period as described for Fig. 2) increased vesicular dopamine levels by ∼140%, without altering total tissue dopamine concentrations (Fig. 5, A and B), as assessed 1 h after the last methylphenidate injection. Finally, post-treatment with three injections of methylphenidate immediately after the multiple methamphetamine regimen (i.e., both agents administered as described for Fig. 2) reversed (or perhaps compensated for) the methamphetamine-induced decrease in vesicular dopamine content observed 6 h after methamphetamine treatment.
Post-treatment with methylphenidate (MPD) does not alter total striatal tissue dopamine (DA) content (A), but attenuates the methamphetamine (METH)-induced decrease in vesicular dopamine content (B). Rats received methamphetamine (four injections; 7.5 mg/kg s.c.; 2-h intervals) or saline (Sal; 1 ml/kg s.c.). Rats received three injections of methylphenidate (5 mg/kg s.c.) or saline (1 ml/kg s.c.) after the last methamphetamine or saline injection. Rats were decapitated 6 h after the last methamphetamine, 1 h after the last methylphenidate, or 1 h after the last saline administration. Columns represent the means and vertical lines 1 S.E.M. of determinations in six rats. ∗, values that are significantly different from Sal/Sal-treated groups (p ≤ 0.05).
Discussion
As described above, high-dose methamphetamine administration causes persistent dopamine deficits in rodents, nonhuman primates, and humans. The majority of studies in rodents and nonhuman primates indicates that dopamine, per se, contributes to these deficits because these are prevented by depleting this monoamine before methamphetamine treatment or by administration of dopamine antagonists. A possible explanation is that dopamine, per se, can cause formation of highly reactive neurotoxic reactive species (for review, see Stokes et al., 1999). The VMAT-2 is a critical regulator of intraneuronal dopamine content. Accordingly, one hypothesis as to mechanisms underlying methamphetamine toxicity is that by decreasing vesicular dopamine uptake, dopamine may accumulate within nerve terminals, and cause formation of neurotoxic reactive oxygen species. Support for this hypothesis comes from findings by Fumagalli et al. (1999) that heterologous VMAT-2 knockout mice were more susceptible to methamphetamine-induced deficits compared with wild-type mice. Additional support for the hypothesis is that alterations in VMAT-2 activity may be neuroprotective; thus, VMAT-2 sequesters the neurotoxin 1-methyl-4-phenylpyridinium, and thereby protects against neuronal damage (Reinhard et al., 1987; Liu et al., 1992; Takahashi et al., 1997; Gainetdinov et al., 1998; Speciale et al., 1998; German et al., 2000; Staal and Sonsalla, 2000).
Of relevance to the present studies are findings that in addition to the VMAT-2, the dopamine transporter has been implicated in effecting the persistent dopamine deficits caused by methamphetamine treatment. Specifically, both pre- and post-treatment with dopamine reuptake inhibitors attenuate the persistent dopamine deficits caused by methamphetamine treatment. The later finding (i.e., that post-treatment with dopamine reuptake inhibitors can protect against methamphetamine toxicity) is of particular importance, because it suggests the existence of a reversible process occurring in the first few hours after methamphetamine treatment that contributes to the long-term dopamine deficits caused by the stimulant. Accordingly, the purpose of this study was to investigate the nature of this acute neurotoxic phenomenon, with a particular emphasis on the roles of dopamine and VMAT-2.
Consistent with the hypothesis that acute alterations in VMAT-2 function contribute to the long-term dopamine deficits caused by methamphetamine treatment, studies have demonstrated that methamphetamine treatment rapidly decreases dopamine uptake in vesicles prepared from the striata of treated rats (Brown et al., 2000, 2002). The vesicles in this preparation apparently are not attached to heavy membranes and likely are present in the cytosol. Also consonant with the hypothesis are results presented in Fig. 1 demonstrating that multiple methamphetamine injections (four injections of 7.5 mg/kg/injection; 2-h intervals) rapidly (within 1 h) decrease VMAT-2 protein levels in this preparation. Slight decreases in VMAT-2 immunoreactivity were also observed in the P2 (synaptosomal) fraction from which the vesicles were obtained, with no change in the membrane fraction (P3). A similar phenomenon was reported previously with higher doses of methamphetamine (four injections of 10 mg/kg/injection; 2-h intervals), except that in this previous report, methamphetamine treatment decreased significantly VMAT-2 immunoreactivity in the P2 fraction by 25% (Riddle et al., 2002). The deficits reported in these studies may reflect a rapid degradation of VMAT-2, although this seems unlikely given that total homogenate VMAT-2 protein levels are not reduced 1 day after multiple methamphetamine treatments (Hogan et al., 2000) and that the turnover of receptor and transporter proteins is typically greater than 1 day (Norman et al., 1987; Battaglia et al., 1988; Fleckenstein et al., 1996). Alternatively, the decrease in VMAT-2 protein in the preparations may reflect a redistribution of VMAT-2 (and associated vesicles) such that at the highest drug doses (e.g., 10 mg/kg/injection), VMAT-2 protein is lost from the P2 fraction altogether (i.e., trafficked out of a portion of nerve terminal retained in the synaptosomal preparation). Further studies are necessary to clarify these issues.
Results presented in Fig. 2 demonstrate that in addition to causing rapid alterations in VMAT-2, methamphetamine treatment causes the expected persistent dopamine deficits. As was reported for amfonelic acid, this long-term consequence was inhibited by post-treatment with another dopamine reuptake inhibitor, methylphenidate. Methylphenidate was selected for study because it is an agent with a wide margin of safety that is often used as treatment for attention deficit hyperactivity disorder (for review, see Challman and Lipsky, 2000). It is well documented that agents that attenuate methamphetamine-induced hyperthermia prevent the long-term dopamine deficits caused by its treatment (Bowyer et al., 1992, 1994), likely because hyperthermia facilitates a methamphetamine-induced formation of reactive oxygen species (Fleckenstein et al., 1997; LaVoie and Hastings, 1999). However, results presented in Fig. 2B demonstrate that methylphenidate did not prevent the hyperthermia caused by methamphetamine treatment. In fact, methylphenidate increased core body temperature per se and accordingly would not have been predictably protective. Hence, another mechanism must account for its neuroprotective effects.
In addition to preventing the persistent dopamine deficits caused by methamphetamine treatment, results presented in Fig. 3 demonstrate that post-treating animals with methylphenidate reversed the acute decreases in vesicular dopamine uptake and dihydrotetrabenazine binding that occur in the first hours after methamphetamine treatment. Because methylphenidate per se increased vesicular dopamine uptake, one possible explanation for these data are that methylphenidate compensated for the methamphetamine-induced deficit in uptake by recruiting vesicles other than those affected by methamphetamine treatment. An important issue in interpreting these data are that uptake and binding in the vesicle preparations are normalized to amount of protein, which to some degree reflects the number of vesicles, thereby raising the possibility that the changes in uptake and binding reflect increases and decreases, respectively, in the number of VMAT-2 per vesicle. However, support for a “trafficking” hypothesis comes from a recent report that methylphenidate rapidly redistributes VMAT-2 protein from a membrane-associated synaptosomal fraction (e.g., the P3 fraction described above) to a nonmembrane (perhaps cytoplasmic)-associated fraction (e.g., the S3 fraction described above). This phenomenon is mediated by both D1 and D2 receptor activation (Sandoval et al., 2002). Accordingly, it can be speculated that the neuroprotective effect of methylphenidate resulted from trafficking of VMAT-2 and associated vesicles to a subcellular region left devoid of VMAT-2 activity because of methamphetamine treatment. Thus, methylphenidate would increase vesicular dopamine sequestration in that region and perhaps compensate for any consequent methamphetamine-associated accumulation of cytoplasmic dopamine. This is supported by the finding that methylphenidate increases vesicular dopamine content as assessed in vesicles prepared from the striata of treated rats without altering total tissue dopamine concentrations (Fig. 5). This suggests that methylphenidate treatment redistributed dopamine within the terminals, presumably as a consequence of the redistribution of vesicles. In contrast, methamphetamine treatment decreased both tissue and vesicular dopamine content, likely because of a deficit in vesicular dopamine sequestration and an inhibition of tyrosine hydroxylase after the multiple methamphetamine injection treatment regimen. Noteworthy are findings that high-dose methamphetamine treatment regimens have been demonstrated to decrease whole tissue striatal tyrosine hydroxylase activity acutely (Morgan and Gibb, 1980; Hanson et al., 1987). Methylphenidate did not prevent the methamphetamine-induced decrease in tissue dopamine content, presumably because it did not prevent the decrease in tyrosine hydroxylase activity. Importantly and in contrast, the methamphetamine-induced decrease in vesicular dopamine content was reversed by the same methylphenidate post-treatment regimen that reversed: 1) the acute (1 h) methamphetamine-induced decrease in vesicular dopamine uptake and dihydrotetrabenazine binding; and 2) the persistent (and likely neurotoxicity-related) dopamine deficits caused by methamphetamine treatment. Hence, these data support the hypothesis that methylphenidate reverses or compensates for the acute methamphetamine-induced redistribution of intraneuronal dopamine and VMAT-2 and may thereby afford neuroprotection.
A role for aberrant VMAT-2 activity as a contributor to neurodegenerative processes has been postulated by several groups (Liu and Edwards, 1997; Miller et al., 1999; Fleckenstein et al., 2000). The present data now provide evidence that by altering VMAT-2 function and subsequently dopamine sequestration, dopamine reuptake inhibitors may reverse or prevent degenerative processes associated with decreased VMAT-2 function. One important application of this concept may be in treatment of Parkinson's disease. Numerous investigators have suggested that dopamine-associated reactive oxygen species formation contribute to the loss of nigrostriatal dopamine neurons underlying this disorder (for review, see Adams et al., 2001; Fahn and Cohen, 1992). Accordingly, pharmacological manipulations that augment the ability to sequester dopamine and prevent its oxidation may be of therapeutic benefit. The therapeutic efficacy of methylphenidate or other agents that modify vesicular dopamine uptake to slow the neurodegenerative processes in Parkinson's disease remains to be determined.
In summary, the present data reveal that methylphenidate post-treatment 1) prevents the persistent dopamine deficits caused by high-dose methamphetamine treatment; 2) rapidly reverses the acute methamphetamine-induced decrease in vesicular dopamine uptake and VMAT-2 ligand binding; and 3) reverses the acute decreases in cytoplasmic vesicular dopamine content caused by methamphetamine treatment. These findings suggest that methylphenidate prevents persistent methamphetamine-induced dopamine deficits by redistributing VMAT-2 protein and enhancing dopamine sequestration. These findings not only provide insight into the neurotoxic effects of methamphetamine but also may enhance our understanding of mechanisms underlying dopamine neurodegenerative disorders such as Parkinson's disease, suggesting a novel therapeutic strategy.
Footnotes
-
This research was supported by National Institutes of Health Grants DA04222, DA00869, DA11389, DA11367, and DA14475.
-
DOI: 10.1124/jpet.102.045005
- Abbreviations:
- VMAT-2
- vesicular monoamine transporter-2
- TBST
- Tris-buffered saline/Tween 20
- Received October 3, 2002.
- Accepted December 4, 2002.
- The American Society for Pharmacology and Experimental Therapeutics