Abstract
Recent studies demonstrated that vesicular dopamine (DA) uptake can be rapidly altered in synaptic vesicles purified from the striata of stimulant-treated rats. Specifically, a single administration of the plasmalemmal DA transporter inhibitor, cocaine, or the DA D2 agonist, quinpirole, increases vesicular DA uptake in vesicles purified from the striata of treated rats. These effects of cocaine are prevented by pretreatment with a D2, but not D1, DA receptor antagonist. The purpose of the present study was to characterize the effect of a mechanistically different psychostimulant, methamphetamine (METH), on vesicular DA uptake. Results demonstrated that a single administration of this DA-releasing agent rapidly and reversibly decreased vesicular DA uptake. The METH-related decrease in vesicular DA uptake was attenuated by pretreatment with the D2 antagonist, eticlopride, but not the D1 antagonist, SCH23390 (R-[+]-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine). Core body temperature did not contribute to the effects of METH on vesicular DA uptake. Neither quinpirole nor cocaine increased vesicular DA uptake when rats were concurrently treated with METH. These studies provide further evidence that psychostimulants rapidly and differentially modify vesicular DA uptake. In addition, these studies demonstrate a complex role for D2 DA receptors in altering vesicular DA transport.
Sequestration of monoamines, such as dopamine (DA), into synaptic vesicles for storage and subsequent release, is mediated by the vesicular monoamine transporter-2 (VMAT-2). Recent studies demonstrate a novel mechanism whereby this sequestration is rapidly regulated by both psychostimulant and D2 DA receptor agonist treatment. Specifically, Brown et al. (2001b) demonstrated that a single injection of cocaine or quinpirole increases vesicular [3H]DA uptake, as assessed in synaptic vesicles purified from the striata of treated rats sacrificed 1 h after treatment. The cocaine-induced effect was not attributable to residual drug in the vesicular preparation, and was associated with an increase in Bmax of binding of the VMAT-2 ligand, [3H]dihydrotetrabenazine ([3H]DHTBZ; Brown et al., 2001a). In addition, this increase was: 1) reversed within 6 h after drug treatment; 2) mimicked by administration of other DA transporter inhibitors (GBR-12935 and amfonelic acid); and 3) dependent on D2, but not D1, receptor activation (Brown et al., 2001a,b).
Recent evidence suggests that not only do cocaine or D2 receptor agonists rapidly alter vesicular DA uptake, but methamphetamine (METH) administration does as well. Specifically, multiple high-dose administrations of METH produce a rapid (within 1 h; Brown et al., 2000, 2001a) and prolonged (persisting at least 24 h; Brown et al., 2000; Hogan et al., 2000) decrease in both vesicular [3H]DA uptake and [3H]DHTBZ binding, as assessed in vesicles purified from the striata of treated animals. A single METH administration decreases vesicular [3H]DA uptake as well (Brown et al., 2001a). The purpose of the present study was to characterize and determine the role, if any, of DA receptors in this latter effect. Results demonstrated that a single METH administration rapidly and reversibly decreased vesicular [3H]DA uptake. The data also suggest a complex role for D2 receptors regulating VMAT-2 function. These findings provide further evidence that drug treatments that alter DA disposition can rapidly alter vesicular DA uptake and provide insight into mechanisms underlying the acute physiological regulation of the VMAT-2.
Materials and Methods
Animals.
Male Sprague-Dawley rats (280–330 g; Simonsen Laboratories, Inc., Gilroy, CA) were maintained under controlled lighting and temperature conditions, with food and water provided ad libitum. Rats were sacrificed by decapitation. All rats were housed in groups of eight in plastic cages the day before the experiment. Mean rectal temperature was determined before drug administration by use of a digital thermometer (Physiotemp Instruments, Inc., Clifton, NJ) where indicated. To restore hyperthermia in treated groups, cages were placed on a heating pad (environmental temperature ∼28.5°C). Blockade of hyperthermia was accomplished by placing cages on ice (environmental temperature ∼6°C). Where indicated, mean rectal temperatures for all treated rats were assessed 10 min before drug treatment and again before decapitation. All experiments were conducted in accordance with National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Drugs and Radioligands.
(±)-METH hydrochloride was supplied by the National Institute on Drug Abuse (Bethesda, MD). SCH23390 and eticlopride were purchased from Sigma-Aldrich (St. Louis, MO). 7,8-[3H]DA (47 Ci/mmol) was purchased from Amersham Biosciences (Piscataway, NJ).
Preparation of Rat Striatal Synaptic Vesicles.
Synaptic vesicles were obtained from synaptosomes prepared from rat striatum as described previously (Fleckenstein et al., 1997). Synaptosomes were resuspended and homogenized in cold distilled deionized water. Osmolarity was restored by the addition of HEPES and potassium tartrate, 25 mM and 100 mM (final concentrations, pH 7.5, at 4°C), respectively. Samples were centrifuged for 20 min at 20,000g(4°C) to remove lysed synaptosomal membranes. MgSO4 (1 mM, final concentration) was added to the supernatant, which was then centrifuged for 45 min at 100,000g (4°C). The resulting vesicular pellet was resuspended in ice-cold wash buffer (see below) at a concentration of 50 mg/ml (original tissue wet weight) for striatal tissue.
Vesicular [3H]DA Uptake.
Vesicular [3H]DA uptake was performed by incubating 100 μl of the resuspended vesicular pellet at 30°C for 3 min in assay buffer (final concentration: 25 mM HEPES, 100 mM potassium tartrate, 1.7 mM ascorbic acid, 0.05 mM EGTA, 0.1 mM EDTA, 2 mM ATP-Mg2+, pH 7.5, at 30°C) in the presence of [3H]DA (30 nM final concentration except in kinetic analyses, wherein 0.8–10 μM [3H]DA was used). The reaction was terminated by addition of 1 ml of cold wash buffer (assay buffer containing 2 mM MgSO4substituted for the ATP-Mg2+, pH 7.5, at 4°C) and rapid filtration through Whatman GF/F filters soaked previously in 0.5% polyethylenimine. Filters were washed three times with ice-cold wash buffer using a Brandel filtering manifold (Brandel, Inc., Gaithersburg, MD). Radioactivity trapped in filters was counted using a liquid scintillation counter. Nonspecific values were determined by measuring vesicular [3H]DA uptake at 4°C in wash buffer (i.e., no ATP present).
Statistical Analysis.
Statistical analyses among three or more groups were performed using analysis of variance followed by Fisher's protected least significant difference post hoc comparison. Analysis between two groups was conducted using a paired Student'st test. Differences were considered significant if probability of error was less than 5%.
Results
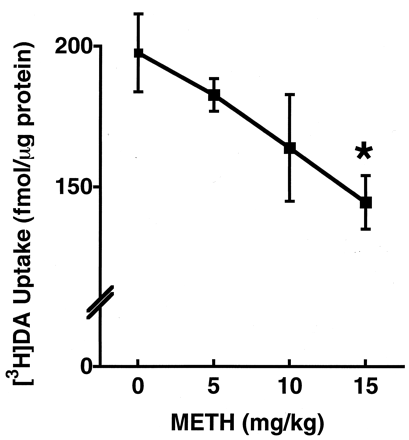
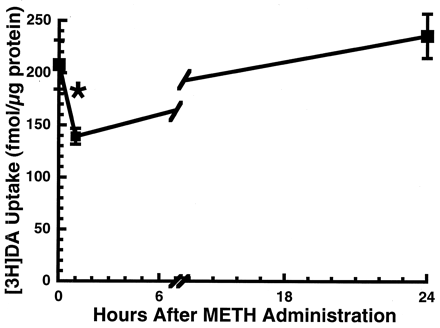
In accordance with a previous report (Brown et al., 2001a), a single METH administration (15 mg/kg s.c.) rapidly (within 1 h) decreased vesicular [3H]DA uptake as assessed in vesicles purified from the striata obtained from treated rats (Fig.1). This decrease was attributable to a decrease in the Vmax, with little change in the Km of vesicular [3H]DA uptake (797 fmol/μg of protein/min and 161 nM after saline treatment versus 607 fmol/μg of protein/min and 187 nM after a single 15 mg/kg METH injection, respectively), as described under Materials and Methods. This effect was dose-related, with a dose of 15 mg/kg effecting a 25% decrease in vesicular [3H]DA uptake (Fig. 1). Higher doses were not administered due to increased mortality. The deficit in vesicular [3H]DA uptake recovered by 24 h after treatment (Fig. 2).
Rats received a single administration of saline vehicle (1 ml/kg s.c.) or METH (5, 10, or 15 mg/kg s.c.) and were decapitated 1 h later. Uptake was determined using a single concentration of [3H]DA as described underMaterials and Methods. Symbols represent the means and vertical lines represent 1 S.E.M. of determinations in six rats. ★, value for treated rats that is significantly different from saline-treated controls (p ≤ 0.05).
Rats received a single administration of METH (15 mg/kg s.c.) and were decapitated 1 or 24 h later. Other rats received saline vehicle (1 ml/kg s.c.) and were decapitated 1 h later. Symbols represent the means and vertical lines represent 1 S.E.M. of determinations in six rats. ★, value for treated rats that is significantly different from saline-treated controls (p ≤ 0.05).
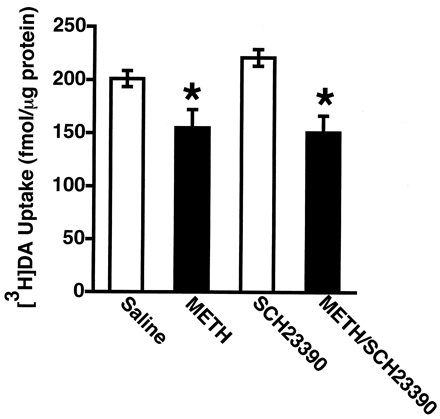
To examine the role of DA D1 receptors in the METH-induced decrease in vesicular [3H]DA uptake, the D1 antagonist SCH23390 (0.5 mg/kg, i.p) was administered 15 min before METH treatment. This dose was selected based on previous studies demonstrating that it prevented cocaine-induced increases in D1-associated parameters such as locomotor activity (Baker et al., 1998) and neuropeptide immunoreactivity (Alburges and Hanson, 1999; Alburges et al., 2000). Data presented in Fig. 3demonstrate that a single METH injection decreased vesicular [3H]DA uptake by 23%, an effect that was not prevented by SCH23390 pretreatment.
Rats received a single administration of SCH23390 (0.5 mg/kg i.p.) or saline vehicle (1 ml/kg i.p.) 15 min before a single administration of either saline vehicle (1 ml/kg s.c.) or METH (15 mg/kg s.c.). All rats were decapitated 1 h after the last drug injection. Columns represent the means and vertical lines represent 1 S.E.M. of determinations in six rats. ★, values for treated rats that are significantly different from saline-treated controls (p ≤ 0.05).
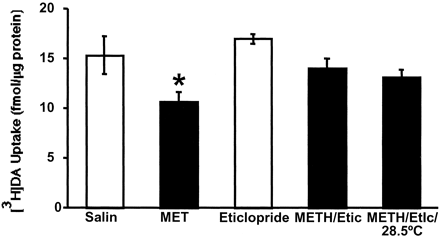
The role of DA D2 receptors in the METH-induced decrease in vesicular [3H]DA uptake was investigated by administering the D2 antagonist, eticlopride (0.5 mg/kg i.p.), 15 min before METH treatment. This dose and time point was selected based on previous studies demonstrating its effectiveness at preventing cocaine-induced increases in vesicular DA uptake (Brown et al., 2001b). Data presented in Fig.4 demonstrate that this pretreatment attenuated the METH-induced deficit. In addition to attenuating the METH-induced decrease in vesicular uptake, eticlopride pretreatment attenuated the hyperthermia caused by METH (i.e., core body temperatures increased from 36.9 ± 0.1°C to 40.1 ± 0.1°C in METH-treated rats versus 36.8 ± 0.1°C to 38.4 ± 0.1°C in METH-treated rats pretreated with eticlopride). Neither saline nor eticlopride pretreatment per se altered rectal temperatures (data not shown). Because METH-induced increases in core body temperature have been implicated in the dopaminergic deficits induced by high-dose METH treatment (Bowyer et al., 1992, 1994; Albers and Sonsalla, 1995), the role of this attenuation was investigated. Specifically, some of the eticlopride-pretreated rats were exposed to a warmer ambient temperature (28.5°C) upon METH treatment to maintain hyperthermia. This manipulation resulted in a body temperature of 39.8 ± 0.1°C, a value similar to that observed after METH treatment in animals exposed to the 24°C ambient environment. Results presented in Fig. 4 demonstrate that the ability of eticlopride to attenuate the decrease in vesicular [3H]DA uptake induced by METH was not reversed by restoring hyperthermia in the eticlopride/METH-treated rats (40.1 ± 0.1°C).
Rats received a single administration of eticlopride (0.5 mg/kg i.p.) or saline vehicle (1 ml/kg) 15 min before a single administration of either METH (15 mg/kg s.c.) or saline vehicle (1 ml/kg s.c.). All rats were maintained in an ambient environment of 24°C, except where indicated, in which case rats were placed in a 28.5°C environment (see Materials and Methods). All animals were decapitated 1 h after the last drug injection. Columns represent the means and vertical lines represent 1 S.E.M. of determinations in six rats. ★, value for treated rats that is significantly different from saline-treated controls (p ≤ 0.05).
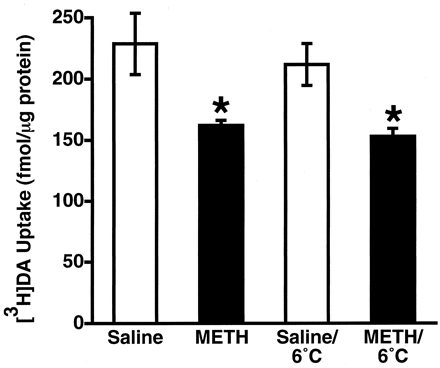
To investigate further the role of hyperthermia in mediating the METH-induced decrease in vesicular [3H]DA uptake, rats were treated with either saline or METH, and hyperthermia was attenuated by placing these animals in a cool environment (6°C). Data presented in Fig. 5 demonstrate that attenuation of hyperthermia per se (36.7 ± 0.1°C to 40.5 ± 0.1°C in METH-treated rats versus 36.8 ± 0.1°C to 38.1 ± 0.1°C in METH-treated rats exposed to the cool environment) did not prevent the METH-induced decrease in vesicular [3H]DA uptake.
Rats received either a single administration of METH (15 mg/kg s.c.) or saline vehicle (1 ml/kg s.c.) and were decapitated 1 h later. All rats were maintained in an ambient environment of 24°C, except where indicated, where rats were place in a 6°C environment (see Materials and Methods). Columns represent the means and vertical lines represent 1 S.E.M. of determinations in six rats. ★, values for treated rats that are significantly different from saline-treated controls (p ≤ 0.05).
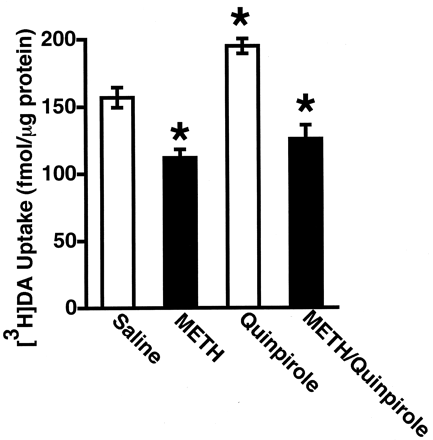
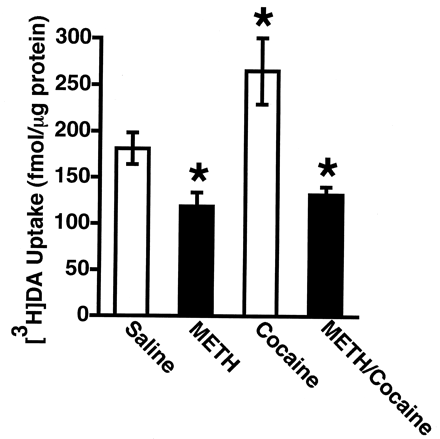
It has been demonstrated previously that administration of either the D2 DA receptor agonist, quinpirole, or the plasmalemmal DA reuptake inhibitor, cocaine, increases vesicular [3H]DA uptake (Brown et al., 2001a,b). Results presented in Figs.6 and 7 demonstrate that neither treatment increased [3H]DA uptake when rats were concurrently treated with METH.
Rats received a single administration of quinpirole (1 mg/kg i.p.) or saline vehicle (1 ml/kg) immediately before a single administration of either METH (15 mg/kg s.c.) or saline vehicle (1 ml/kg s.c.). All animals were decapitated 1 h after the last drug injection. Columns represent the means and vertical lines represent 1 S.E.M. of determinations in six rats. ★, values for treated rats that are significantly different from saline-treated controls (p ≤ 0.05).
Rats received a single administration of cocaine (30 mg/kg i.p.) or saline vehicle (1 ml/kg) immediately before a single administration of either METH (15 mg/kg s.c.) or saline vehicle (1 ml/kg s.c.). All animals were decapitated 1 h after the last drug injection. Columns represent the means and vertical lines represent 1 S.E.M. of determinations in 7 to 13 rats. ★, values for treated rats that are significantly different from saline-treated controls (p ≤ 0.05).
Discussion
Previous studies suggest that amphetamine and, presumably, its analog METH alter vesicular sequestration of DA by incorporation of the drug into synaptic vesicles and disruption of the vesicular pH gradient needed for transport (Sulzer and Rayport, 1990; Sulzer et al., 1995). Results presented herein demonstrate a potential additional mechanism whereby METH rapidly alters vesicular DA uptake. Specifically, a single METH injection rapidly (within 1 h) and reversibly (within 24 h) decreased vesicular DA uptake, an effect associated with a decrease in Vmax and little change inKm for uptake. The METH-induced decrease in uptake was not associated with hyperthermia caused by drug treatment. However, this deficit was mediated, in part, by D2 receptor activation as evidenced by findings that it was attenuated by pretreatment with the D2 DA receptor antagonist, eticlopride.
In contrast to the decreases in vesicular DA uptake induced by METH treatment, cocaine administration increases vesicular [3H]DA uptake. Like the deficit induced by METH treatment, the cocaine-induced increase was prevented by eticlopride pretreatment, suggesting that it, too, is mediated by D2 receptor activation (Brown et al., 2001b). The ability of D2 DA receptor activation to increase vesicular DA uptake was confirmed by the demonstration that administration of the D2 DA receptor agonist, quinpirole, increases vesicular DA transport as well (Brown et al., 2001b). Hence, these data imply that depending upon the circumstances, D2 activation can either increase or decrease vesicular DA uptake.
Further evidence indicating a complex role for D2receptors in affecting VMAT-2 comes from findings that the effect of quinpirole on vesicular DA uptake did not occur if rats were treated concurrently with METH. Moreover, cocaine did not increase vesicular DA uptake when rats were concurrently treated with METH. Taken together, these data demonstrate that METH mediates its effect on VMAT-2 through mechanisms that are very different from those underlying the effects of cocaine and quinpirole, although each involves D2receptor activation. This paradox implies that these drugs 1) stimulate different subsets of D2 receptors (i.e., presynaptic or postsynaptic D2 receptors), and/or 2) activate D2 receptors in such a manner that the downstream signaling pathways of these receptors respond differently. Further studies are needed to resolve these issues.
Noteworthy are recent cyclic voltammetry studies by Schmitz et al. (2001) suggesting that application of the METH analog, amphetamine, inhibits vesicular DA release from striatal slice preparations, in part, by activating D2 DA receptor autoreceptors after induction of the reverse transport of DA. A role for D2 DA receptors in the inhibitory action of amphetamine on vesicular release was also suggested by Wieczorek and Kruk (1994), who employed cyclic voltammetry to demonstrate that amphetamine application reduced DA overflow signal amplitudes by ∼80% and that application of the D2antagonist, sulpiride, attenuated this inhibition. Those results are consistent with present data indicating that METH treatment decreases vesicular DA uptake (and hence perhaps the amount of DA packaged per vesicle) via a D2-associated mechanism.
The nature of the METH-induced decrease in vesicular DA uptake remains unknown. It may represent a rapid D2 DA receptor-mediated degradation of VMAT-2 protein, a conformational change in VMAT-2 such that the transporter is no longer active, alterations of the proton gradient that drives vesicular DA uptake, or a redistribution of VMAT-2 protein within nerve terminals. Support for this latter possibility (i.e., that METH causes a redistribution of VMAT-2 protein within nerve terminals in vivo, causing less to be retained in the pool ultimately purified in our preparations) comes from findings that multiple METH administrations rapidly (within 1 h after the last treatment) decrease DHTBZ binding (Brown et al., 2000) and VMAT-2 immunoreactivity (E. L. Riddle, M. K. Topham, J. W. Haycock, G. R. Hanson, and A. E. Fleckenstein, manuscript submitted for publication), and thus presumably protein levels, in the same purified vesicular preparation employed in the current studies. Furthermore, studies by Hogan et al. (2000) demonstrate similar decreases in DHTBZ binding in purified vesicular preparation but not in whole tissue homogenates, suggesting that the VMAT-2 protein may be redistributed or “trafficked” between vesicle populations or other compartments after METH treatment. Ongoing studies in the laboratory are investigating this phenomenon.
In conclusion, the data demonstrate that METH administration rapidly decreases vesicular DA uptake. This rapid and reversible deficit is dependent on D2 DA receptor activation and is not associated with METH-induced hyperthermia. In addition to the METH-mediated decrease in vesicular DA uptake, a paradoxical effect of D2 DA receptor activation on vesicular DA uptake was also revealed. These data not only provide further evidence that drug treatments that alter DA disposition can rapidly alter vesicular DA uptake, but also provide insight into the acute regulation of the VMAT-2.
Footnotes
-
This research was supported by National Institutes of Health Grants DA04222, DA00869, DA11389, and DA013367.
- Abbreviations:
- DA
- dopamine
- VMAT-2
- vesicular monoamine transporter-2
- DHTBZ
- dihydrotetrabenazine
- GBR-12935
- 1-[2-(diphenylmethoxy)ethyl]-4-(3-phenylpropyl)piperazine
- METH
- methamphetamine
- SCH-23390
- R-[+]-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine
- Received December 11, 2001.
- Accepted April 16, 2002.
- The American Society for Pharmacology and Experimental Therapeutics