Abstract
Administration of a single high dose of methamphetamine (METH) causes a rapid and reversible decrease in the activity of the tryptophan hydroxylase (TPH), the rate-limiting enzyme in the synthesis of 5-hydroxytryptamine. This effect can be reversed completely by exposing the METH-impaired enzyme to a reducing environment, which suggests that the decrease in TPH activity is a reversible oxidative consequence of free radical formation. Consistent with this hypothesis, a single METH administration to male rats increased oxygen radical formation, as demonstrated by increased striatal dihydroxybenzoic acid formation after coadministration of salicylate with METH. Prevention of METH-induced hyperthermia attenuated both the increase in dihydroxybenzoic acid formation and the decrease in TPH activity observed 1 h after METH administration. These data suggest that both reactive oxygen species and hyperthermia contribute to the acute decrease in TPH activity which results from a single METH administration.
Administration of the frequently abused amphetamine analog, METH, has profound effects on central nervous system function. For example, a single high-dose administration of METH to rats causes a rapid and reversible decrease in the activity of the 5HT-synthesizing enzyme, TPH (Hotchkiss and Gibb, 1980; Bakhit and Gibb, 1981; Peat et al., 1985). An apparent reduction in content of 5HT and its metabolite, 5HIAA, follows this acute reduction in enzyme activity (Peat et al., 1985). Because TPH per se is rapidly inactivated by oxidizing factors (Kuhn et al., 1980) and reactive oxygen species appear to be formed after METH administration (Kondo et al., 1994; Giovanni et al., 1995; Hirata et al., 1995), it has been suggested that METH-induced reactive oxygen species may effect the acute decrease in TPH activity. The finding by Stoneet al. (1989b) that the decrease in TPH activity caused by METH is reversed completely by exposing the enzyme to a reducing environment supports the hypothesis that a single METH administration impairs, via oxygen radicals, 5-hydroxytryptaminergic neuronal function.
In addition to causing oxygen radical formation, METH administration rapidly increases core body temperatures. Whereas this drug-induced hyperthermia likely contributes to the neurotoxic effects of multiple administrations of this stimulant (Bowyer et al., 1994; Bronstein and Hong, 1995; Farfel and Seiden, 1995;Albers and Sonsalla, 1995), the role of body temperature in mediating acute effects of METH, including the rapid decrease in TPH activity, is less established. However, the recent finding by Che et al.(1995) that the acute decrease in TPH activity caused by the METH-related drug, MDMA, is attenuated when MDMA-induced hyperthermia is prevented suggests that increased body temperatures contributes to the immediate effects of a single METH administration as well.
Because both oxygen radicals and hyperthermia may play a role in the acute effects of METH, we examined the possibility that the interaction between these factors may effect the acute decrease in TPH activity resulting from a single injection of the stimulant. The results reveal that hyperthermia contributes to both the oxygen radical generation and the decreased TPH activity which result from METH administration. These findings suggest a correlation between METH-induced formation of free radicals and the immediate reduction in TPH activity caused by this stimulant.
Methods
Animals.
Male Sprague Dawley rats (200–300 g; Simonsen Laboratories, Gilroy, CA) were maintained under conditions of controlled temperature and lighting, with food and water providedad libitum. On the day of the experiment, rats (mean rectal temperature of approximately 37.3°C) were injected with drug or saline vehicle, and then housed in groups in plastic cages that were either placed on ice (environmental temperature, 6°C), at room temperature (26°C) or on a heating pad (environmental temperature, 30°C). Where indicated, core body temperatures were measured 10 min before decapitation with use of a digital thermometer (Physiotemp Instruments, Inc., Clifton, NJ). All procedures were conducted in accordance with approved National Institutes of Health guidelines.
Drugs and chemicals.
(±)METH hydrochloride was supplied generously by the National Institute on Drug Abuse. Analytical reference materials for METH determination [+METH and deuterated METH (METH-d8)] were obtained from Radian Corporation (Austin, TX). Sodium salicylate, 2,3-DHBA and 2,5-DHBA were purchased from Aldrich Chemical Co. (Milwaukee, WI). Drugs were administered as indicated in the legends of appropriate figures; with the exception of sodium salicylate, doses were calculated as the respective free bases.
TPH activity.
TPH activity was determined in tissue homogenates using HPLC by measuring 5HTP formation resulting from the hydroxylation in vitro of tryptophan according to a modification of the method described by Johnson et al.(1992). Frozen tissue samples were sonicated in 150 to 300 μl of ice-cold 50 mM HEPES buffer (pH 7.4) containing 0.2% Triton X-100 and 6 mM dithiothreitol; where specified (see legend to fig. 1), 50 μM FeCl2 was included in this buffer. The resulting suspension was centrifuged (16,000 × g for 15 min; 4°C). Unless otherwise specified (see legend to fig. 1), duplicate aliquots (15 μl) of supernatant were then incubated for 10 min (37°C) with 10 μl reaction mixture (52.8 mM HEPES, 50 mM β-mercaptoethanol, 8 mM tryptophan, 1.25 mMm-hydroxybenzylhydrazine (NSD 1015) and 3.38 mMdl-6-methyl-5,6,7,8-tetrahydrobiopterin dihydrochloride). Boiled supernatant was used for blanks. The reaction was terminated by placing the tubes on ice and adding HPLC mobile phase (see below). The resulting mixture was centrifuged (2500 × g for 10 min; 4°C), and the supernatant retained for 5HTP quantification with HPLC coupled with electrochemical detection [C-18 Microsorb column (Rainin, Woburn MA); glassy carbon electrode set at +0.73 V relative to a Ag/AgCl reference electrode]. The HPLC mobile phase (pH 2.7) consisted of 0.05 M sodium phosphate dibasic, 0.03 M citric acid, 0.1 mM ethylenediaminetetraacetic acid, 0.6 mM sodium octyl sulfate and 15% methanol.
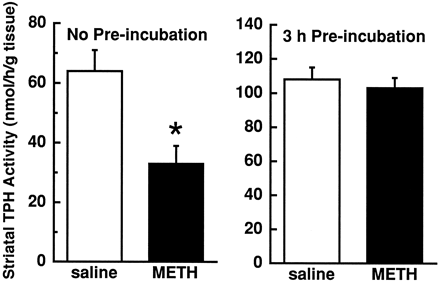
Effects of preincubation in a reducing environment on METH-induced decreases in TPH activity. Rats received METH (15 mg/kg s.c.; solid columns) or saline vehicle (1 ml/kg s.c.; open columns) 1 h before decapitation. Striatal tissue from these rats was sonicated in HEPES-Triton X-100 dithiothreitol buffer containing 50 μM FeCl2, and centrifuged (16,000 ×g 15 min) as described under “Methods.” Quadruplicate aliquots were taken from the resulting supernatants; two aliquots were immediately frozen on dry ice (i.e., “no preincubation”) and two were incubated in an atmosphere of 100% helium for 3 h before placement on dry ice. Frozen samples were then thawed and incubated (37°C; 10 min) with reaction mixture as described under “Methods.” Columns represent means and vertical lines represent 1 S.E.M. of determinations in eight rats. *Value for METH-treated rats that is significantly different from saline-treated controls (P < .05).
METH determination.
Brain tissues (whole brains minus the cerebellum, hippocampi, striatum and some frontal cortex) were each homogenized in 2 ml distilled water and frozen at −70°C. On the day of the assay, these homogenates were equilibrated to room temperature. Three hundred nanograms of deuterated METH (METH-d8) were added as internal standards to each sample. Samples were vortexed for 5 s and then made alkaline (pH > 12) with 100 μl of concentrated ammonium hydroxide. A 500-μl or 1-ml aliquot from each homogenate was extracted into 4 ml butyl chloride/chloroform (4:1, v/v) for 30 min. Samples were centrifuged for 30 min at 1200 × g, and each organic phase (containing analytes of interest) was transferred to a clean tube. This step was repeated twice. Trifluoroacetic acid anhydride (200 μl) was added to each organic phase and heated for 30 min at 70°C. The extracts were cooled to room temperature and then evaporated to dryness at 40°C. A 14-point standard curve ranging from 10 to 5000 ng/ml was prepared with human plasma and extracted as described above. Analytical accuracy and intra-assay precision were verified by concurrent analysis of quality control samples that were prepared in drug-free rat brain homogenate (100, 500 and 2,000 ng/ml). Extracts were reconstituted in 100 μl of chloroform before analysis by gas chromatography/mass spectrometry.
Concentrations of METH were determined with a Finnigan 4500 MAT mass spectrometer operating in positive chemical ionization mode (methane/ammonia reagent gas) coupled to a DB5 MS-30 M-0.25 μ capillary column. Ions monitored were 263 m/z and 271m/z (METH and METH-d8, respectively). Accuracy was within 6 to 17% of spiked METH target values for brain homogenate quality control samples.
DHBA and salicylate determination.
Dihydroxybenzoic acids were quantified by HPLC with electrochemical detection according to a modification of the method of Althaus et al. (1995). This method was selected because, as reported by Chieuh et al.(1994), other HPLC procedures do not readily separate DHBA from dopamine and its metabolites. Because salicylate concentrations can differ among experiments, salicylate concentrations were determined by HPLC with ultraviolet detection (Althaus et al., 1995) in all experiments that used the DHBA technique. Frozen striata were sonicated in modified HPLC mobile phase buffer (14.15 g/l monochloroacetic acid, 10% acetonitrile, 0.05% mannitol, 0.02% deferoxamine; pH 2.4) and the resulting tissue suspension frozen immediately on dry ice. This suspension was then centrifuged (22,000 × g 20 min) and the resulting supernatant split for electrochemical and ultraviolet analysis (mobile phase consisting of 10% acetonitrile, 10% tetrahydrofuran, 14.15 g/l monochloroacetic acid in double-distilled H20; pH 2.4; Hewlett Packard 25-cm C-18 column; 0.65 V for EC and 295 nm for UV detection). The lower limits of sensitivity for salicylate and DHBA detection in tissue were approximately 2 nmol/g tissue and 1 to 2 pmol/g tissue, respectively. DHBA was detected in salicylate-treated control rats (i.e., rats that received salicylate, but not METH): this appearance is inherent in the technique and may reflect scavenging of hydroxyl radicals formed under normal physiological conditions. However, much of the base-line DHBA detected in control samples likely arises from atmospheric exposure of salicylate-containing samples. Hence, samples were kept shielded from light and frozen until immediately before analysis to prevent spontaneous oxidation of salicylate.
Data analysis.
Statistical analyses between two groups were conducted by a two-tailed Student’s t test. Analyses among three or more groups were conducted with analysis of variance followed by Fisher’s test. Differences among groups were considered significant if the probability of error was less than 5%.
Results
Results presented in figure 1 (left panel) demonstrate that a single high-dose (15 mg/kg s.c.) METH injection decreased TPH activity in rat striatum 1 h after administration. METH-inactivated TPH was completely reactivatedin vitro by 3 h of anaerobic preincubation (i.e., incubation before the assay; see “Methods” and legend to fig. 1) with dithiothreitol and iron. Anaerobic preincubationper se increased basal TPH activity in striata obtained from saline-treated rats, similar to that described previously (Stoneet al., 1989a).
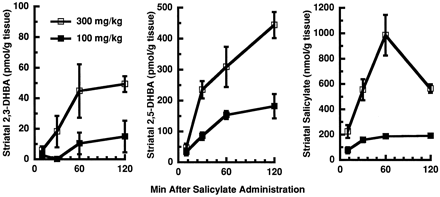
The finding that the METH-induced loss in TPH activity presented in figure 1 is reversed after exposure of METH-induced tissue to a reducing environment suggests that the decrease in enzyme activity was an oxidative consequence of administering the stimulant. To directly assess whether a single administration of METH causes oxygen radical formation, production of the hydroxylated salicylate metabolites, 2,3-DHBA and 2,5-DHBA, was measured by a modification of the method ofAlthaus et al. (1995). Before use of this technique to assess effects of METH, optimal dosing and timing conditions for salicylate administration were determined (fig.2). Similar to that which has been reported previously (Giovanni et al., 1995), intraperitoneal salicylate administration effected a dose- and time-related increase in both salicylate appearance and DHBA formation in rat striatal tissue. DHBA and salicylate concentrations appeared to plateau 60 min after administration of 100 mg/kg salicylate; hence, this dose and time point was chosen for experiments reported below. In preliminary experiments, coadministration of salicylate with 15 mg/kg METH did not affect central METH concentrations significantly (4.6 ± 0.4 and 5.9 ± 1.0 ng/mg tissue for vehicle- and salicylate-treated rats, respectively; n = 6). Moreover, salicylate per se did not affect rectal body temperatures, nor did it alter METH-induced hyperthermia. At doses of up to 300 mg/kg, salicylate did not affect striatal TPH activity 1 h after administration (data not shown).
Time-course effects of salicylate administration on 2,3-DHBA, 2,5-DHBA and salicylate appearance in rat striatum. Rats received 100 mg/kg (solid symbols) or 300 mg/kg (open symbols) salicylate (1 ml/kg i.p.) at various times before decapitation. Symbols represent means and vertical lines represent 1 S.E.M. of determinations in five rats.
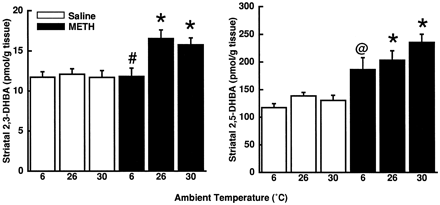
Results presented in figures 3 and4 demonstrate effects of METH on body temperature, TPH activity and DHBA formation 1 h after a single administration in rats maintained at an ambient environmental temperature of 26°C. In these animals, METH increased rectal temperatures by approximately 3°C (fig. 3, left panel). Moreover, this high-dose METH regimen decreased TPH activity (fig. 3, right panel) and increased both 2,3-DHBA and 2,5-DHBA formation (fig. 4). To ascertain the effects of manipulating body temperatures, rats were placed in 6°C or 30°C ambient environments, respectively. Placement of rats in a 6°C environment both prevented METH-induced hyperthermia and attenuated the METH-related reduction in striatal TPH activity (fig. 3). Moreover, such prevention of METH-induced hyperthermia blocked increases in 2,3-DHBA formation (fig. 4, left panel). In contrast, enhancing METH-induced hyperthermia (i.e., by placement of METH-treated rats in an ambient environment of 30°C) did not further decrease TPH activity or increase DHBA formation relative to METH-treated rats maintained at 26°C.
Effects of varying ambient temperatures on METH-altered core body temperature and TPH activity. Rats received METH (15 mg/kg s.c.; solid columns) or saline vehicle (1 ml/kg s.c.; open columns) 1 h before decapitation. Immediately after METH or vehicle administration, rats were placed in 6°C, 26°C or 30°C ambient environment. *Values for METH-treated rats that are significantly different from corresponding saline-treated controls; #values for METH-treated rats placed in a 6°C environment that are significantly different from METH-treated rats placed in a 26°C or 30°C environment. (P < .05).
Effects of varying ambient temperatures on METH-altered DHBA formation. Data were obtained in the same experiment depicted in figure 3. Mean salicylate concentrations in tissues obtained from saline-treated rats placed in 6°C, 26°C and 30°C environments were 80 ± 8, 93 ± 13, and 83 ± 8 nmol/g tissue, respectively. Mean striatal salicylate concentrations in tissues obtained from METH-treated rats placed in 6°C, 26°C and 30°C environments were 94 ± 8, 105 ± 9 and 90 ± 13 nmol/g tissue, respectively. Salicylate concentrations did not differ significantly among the treatment groups. Columns represent means and vertical lines 1 S.E.M. of determinations in seven to eight rats. *Values for METH-treated rats that are significantly different from corresponding saline-treated controls; #value for METH-treated rats placed in a 6°C environment that is significantly different from METH-treated rats placed in 26°C or 30°C environments; @ value for METH-treated rats that is significantly different from METH-treated rats placed in a 30°C environment (P < .05).
Because changes in the pharmacokinetics of either METH or salicylate could contribute to the observed results, levels of METH and salicylate were determined. Striatal content of salicylate assessed 1 h after drug administration was not affected significantly by either METH treatment or variation in rectal temperatures (see legend to fig. 4). Rats with a final rectal temperature of 37.9°C (i.e.,which had been placed in a 6°C environment) had whole brain METH concentrations 1.5-fold greater than that of rats with a final temperature of 42.1°C (i.e., which had been placed in a 30°C environment; 12.8 ± 0.7 and 18.7 ± 2.0 ng/mg tissue for rats exposed to 30°C and 6°C environments, respectively;n = 7–8).
Discussion
Because of the increased frequency of METH abuse, elucidation of its consequent neurochemical impact is important. Of particular interest are reports that monoaminergic systems are especially sensitive to administration of this potent stimulant. For instance, a single METH administration (10–15 mg/kg, as used in the present study) causes a rapid and reversible decrease in the activity of TPH (Bakhit and Gibb, 1981), and a corresponding reduction in 5HT content (Peatet al., 1985). It has been suggested by Stone et al. (1989a, b) that this rapid decrease in TPH activity may be a reversible oxidative consequence of free radical formation. The present findings confirm this assertion by demonstrating that the METH-induced decrease in TPH activity was completely restored by exposure to an anaerobic reducing environment including dithiothreitol under helium gas (fig. 1). It is interesting that only 3 h of exposure to reducing factors were required to restore the METH-induced decrease in TPH activity, because Stone et al. (1989a) reported that complete reversal of the effects of MDMA requires 24 h incubation. This difference suggests that the extent of free radical formation and consequent oxidation of TPH might be greater for MDMA than METH.
To assess more directly whether a single administration of METH causes reactive oxygen species formation, we measured production of the hydroxylated salicylate metabolites, 2,3-DHBA and 2,5-DHBA. Both are used as indices of reactive oxygen species formation, although 2,5-DHBA is less reliable as it can be formed both by interacting with hydroxyl radicals and by nonreactive oxygen species-mediated processes (Halliwell et al., 1991). The results reveal that coadministration of METH with salicylate increased DHBA formation 1 h after drug treatment. These findings are consistent with those of Kondo et al. (1994) and Giovanni et al. (1995)who demonstrated increased DHBA and presumably hydroxyl radical formation, albeit after multiple high-dose administrations of METH. This acute increase in free radical formation observed 1 h after METH administration correlates temporally with the rapid METH-induced decrease in TPH activity described above.
In 1995, Che et al. found that decreases in TPH activity caused by the methylenedioxy analog of METH, MDMA, are attenuated when the drug-induced hyperthermia is prevented. Consistent with this finding, prevention of METH-induced hyperthermia by placement of rats in a 6°C environment attenuated the decrease in TPH activity resulting from a single METH injection. Because prevention of hyperthermia attenuated, but did not abolish, the METH-induced decrease in TPH activity, factors other than body temperature must be important for effecting METH-induced decreases in TPH activity.
Because prevention of METH-induced hyperthermia attenuates the drug-induced decrease in TPH activity, and because the effect of METH on TPH is associated with oxygen radicals, the relationship between body temperature and oxygen radicals was assessed. Results reveal that prevention of METH-induced hyperthermia blocked increases in 2,3-DHBA formation. These data reveal that hyperthermia contributes to the hydroxyl radical generation resulting from a single METH administration.
The finding of Stone et al. (1989b) that METH-induced decreases in TPH activity are completely reversed by exposing METH-inactivated enzyme to a reducing environment suggests that oxidative processes are solely responsible for the METH effect. Nevertheless, results presented in figure 3 demonstrate that TPH activity is still somewhat decreased in the absence of METH-enhanced 2,3-DHBA formation, which suggests that other reactive species, in addition to hydroxyl radicals, likely contribute to the decrease in TPH activity. Consistent with this possibility, superoxide radicals have been demonstrated to mediate METH-induced effects on 5HT neurons, as evidenced by findings that increased superoxide dismutase activity protects against METH-induced neurotoxicity (Hirata et al., 1995).
One possible explanation for our finding that there is an association among METH-related elevated rectal temperature, increased DHBA content and decreased TPH activity is that drug-induced hyperthermia may alter the pharmacokinetics of either METH or salicylates. For example, if elevated body temperature increased the distribution of either or both of these drugs into the striatum, this could increase DHBA content in a temperature-sensitive manner. However, in this experiment, brain levels of salicylate were not elevated significantly in hyperthermic animals. Furthermore, METH content was actually greater in the brains of nonhyperthermic (final core temperature, 37.9°C) versushyperthermic (final core temperature, 42.1°C) rats. These results, although limited in that METH and salicylate levels were assessed at only one time point (i.e., immediately before decapitation), indicate that variations in pharmacokinetic factors caused by changes in body temperature were not likely responsible for our changes in DHBA formation after METH treatment.
It is interesting to speculate about the nature of reactive oxygen species resulting in the acute impairment of TPH that results from a single METH administration. Dopamine, released after METH administration, is a plausible candidate because: 1) depletion of dopamine prevents the acute METH-induced decrease in TPH activity (Schmidt et al., 1985); and 2) dopamine may have oxidative consequences by causing formation of hydrogen peroxide, superoxide, dopamine quinones (Graham, 1978; Baez et al., 1995) or the neurotoxin 6-hydroxydopamine (Seiden and Vosmer, 1984). Hydrogen peroxide and superoxide, in turn, can cause hydroxyl radical formation. 5HT, also released after METH administration, can undergo nonenzymatic oxidation to produce the toxin 5,6-dihydroxytryptamine (Comminset al., 1987) and other toxic species (Wrona et al., 1995) which may contribute to the oxidative consequences of METH treatment. Carrier- or diffusion-mediated uptake of any of these reactive products could conceivably effect the oxidative decrease in TPH activity observed after METH administration. Further experiments identifying the precise reactive species involved in METH-induced effects on monoaminergic neurons are required.
In summary, we have confirmed and extended the observation by Stoneet al. (1989b) that a single high dose of METH causes a reactive oxygen species-mediated decrease in striatal TPH activity which is reversed by 3 h exposure to reducing conditions. Our findings support the hypothesis that this immediate change in enzyme activity is caused by free radical formation by demonstrating that there is a relatively rapid concomitant increase in the production of DHBA metabolites from salicylate after a single METH administration. In addition, we observed that the METH-induced formation of reactive oxygen species is temperature sensitive and is attenuated in nonhyperthermic, METH-treated animals: this finding suggests that hyperthermia facilitates formation of oxidative species resulting from the administration of high-dose METH treatment.
Acknowledgments
The authors acknowledge gratefully the excellent technical assistance of Meisha Beyeler, Keith Elkins and Lloyd Bush.
Footnotes
-
Send reprint requests to: Annette E. Fleckenstein, Ph.D., 112 Skaggs Hall, University of Utah, Salt Lake City, UT 84112.
-
↵1 This research was supported by PHS grants DA00869, DA04222 and DA05780.
- Abbreviations:
- DHBA
- dihydroxybenzoic acid
- METH
- methamphetamine
- 5HT
- 5-hydroxytryptamine
- 5HIAA
- 5-hydroxyindoleacetic acid
- 5HTP
- 5-hydroxytryptophan
- HPLC
- high-performance liquid chromatography
- MDMA
- methylenedioxymethamphetamine
- TPH
- tryptophan hydroxylase
- HEPES
- N-[2-hydroxylethyl]piperazine-N′-[2-ethanesulfonic acid]
- Received September 4, 1996.
- Accepted June 16, 1997.
- The American Society for Pharmacology and Experimental Therapeutics