Abstract
The vesicular monoamine transporter-2 is the sole transporter responsible for sequestration of monoamines, including dopamine (DA), into synaptic vesicles. Previous studies demonstrate that agents that inhibit DA transporter function, such as cocaine, increase vesicular [3H]DA uptake and binding of the ligand [3H]dihydrotetrabenazine ([3H]DHTBZ), as assessed in vesicles prepared from treated rats. The present studies examine the role of DA receptors in these cocaine-induced effects. Results demonstrate that administration of the D2 DA receptor antagonist, eticlopride, but not the D1 DA receptor antagonist, SCH23390, inhibited these cocaine-induced increases. Similar to the effects of cocaine, treatment with the D2 agonist, quinpirole, increased both vesicular [3H]DA uptake and [3H]DHTBZ binding. In contrast, administration of the D1 agonist, SKF81297, was without effect on vesicular [3H]DA uptake or [3H]DHTBZ binding. Finally, coadministration of quinpirole and cocaine did not further increase vesicular [3H]DA uptake or [3H]DHTBZ binding when compared with treatment with either agent alone. These data suggest that cocaine-induced increases in vesicular DA uptake and DHTBZ binding are mediated by a D2 receptor-mediated pathway. Furthermore, results indicate that D2 receptor activation, per se, is sufficient to increase vesicular DA uptake.
Synaptic vesicles sequester neurotransmitters for storage and subsequent release. The sequestration of catecholamines, including dopamine (DA), is mediated by the vesicular monoamine transporter-2 (VMAT-2) [formerly referred to as the SVAT or MAT (Erickson et al., 1992)]. Recent data suggest that vesicular DA sequestration is increased by treatment with agents that inhibit the plasmalemmal DA transporter (DAT). Specifically, cocaine and other DA uptake inhibitors, such as GBR12935 and amfonelic acid, increase vesicular [3H]DA uptake and binding of the VMAT-2 ligand [3H]dihydrotetrabenazine ([3H]DHTBZ), as assessed in vesicles purified from the striata of treated rats (Brown et al., 2001). This cocaine effect is probably not attributable to residual drug in the vesicular preparation, and it represents an increase in theVmax, but notKm, of [3H]DA uptake and Bmax, but notKd, for [3H]DHTBZ binding (Brown et al., 2001). The cocaine-mediated increase in vesicular uptake and binding occurs rapidly (within 1 h) and is reversed 6 h after drug treatment (Brown et al., 2001).
Although mechanism(s) contributing to the cocaine-mediated increases in [3H]DHTBZ binding and [3H]DA uptake remain to be elucidated, it is well established that cocaine inhibits the activity of the plasmalemmal DAT (Wilson and Schuster, 1972; Roberts and Koob, 1982; Ritz et al., 1987). Such inhibition greatly increases synaptic concentrations of DA (Hurd and Ungerstedt, 1989; Nakachi et al., 1995) that, in turn, activate DA receptors. Accordingly, the purpose of the present studies was to determine the role of D1 and D2 DA receptors in the cocaine-induced increases in vesicular [3H]DA uptake and [3H]DHTBZ binding. The results indicate that cocaine-induced increases in vesicular DA uptake are mediated by D2, but not D1, receptor activation. Furthermore, results reveal that D2receptor activation, per se, is sufficient to increase vesicular DA uptake in our purified vesicular preparation.
Materials and Methods
Animals.
Male Sprague-Dawley rats (280–330 g; Simonsen Laboratories, Gilroy, CA) were maintained under controlled lighting and temperature conditions, with food and water provided ad libitum. Rats were sacrificed by decapitation. All experiments were conducted in accordance with National Institutes of Health guidelines for the care and use of laboratory animals.
Drugs and Radioligands.
(−)-Cocaine hydrochloride was supplied by the National Institute on Drug Abuse (Bethesda, MD). SKF81297, quinpirole, SCH23390, and eticlopride were purchased from Sigma Chemical Co. (St. Louis, MO). 7,8-[3H]DA (46 Ci/mmol) was purchased from Amersham Pharmacia Biotech, Inc. (Arlington Heights, IL), and α-[2-3H]DHTBZ (15 Ci/mmol) was purchased from American Radiolabeled Chemicals Inc. (St. Louis, MO). Tetrabenazine (TBZ) was kindly donated by Drs. Jeffrey Erickson, Helene Varoqui (Louisiana State University Health Sciences Center, New Orleans, LA), and Erik Floor (University of Kansas, Lawrence, KA). Drugs were administered as indicated in figure legends; doses were calculated as the respective free base.
Preparation of Rat Striatal Synaptic Vesicles.
Synaptic vesicles were obtained from synaptosomes prepared from rat striatum as described previously (Fleckenstein et al., 1997). Synaptosomes were resuspended and homogenized in cold distilled deionized water. Osmolarity was restored by addition of HEPES and potassium tartrate (final concentrations: 25 mM and 100 mM; pH 7.5 at 4°C), respectively. Samples were centrifuged for 20 min at 20,000g(4°C) to remove lysed synaptosomal membranes. MgSO4 (final concentration: 1 mM) was added to the supernatant, which was then centrifuged for 45 min at 100,000g (4°C). The resulting vesicular pellet was resuspended in ice-cold wash buffer (see below) at a concentration of 50 mg/ml (original tissue wet weight).
Vesicular [3H]DA Uptake and [3H]DHTBZ Binding.
Vesicular [3H]DA uptake was performed by incubating 100 μl of synaptic vesicle samples (∼2.5 μg of protein) at 30°C for 3 min in assay buffer (final concentration, in mM: 25 HEPES, 100 potassium tartrate, 1.7 ascorbic acid, 0.05 EGTA, 0.1 EDTA, 2 ATP-Mg2+; pH 7.5 at 30°C) in the presence of [3H]DA (final concentration: 30 nM). The reaction was terminated by addition of 1 ml of cold wash buffer (assay buffer containing 2 mM MgSO4 substituted for the ATP-Mg2+, pH 7.5 at 4°C) and rapid filtration through Whatman GF/F filters soaked previously in 0.5% polyethylenimine (Whatman, Maidstone, UK). Filters were washed three times with ice-cold wash buffer using a Brandel filtering manifold (Brandel Inc., Gaithersburg, MD). Radioactivity trapped in filters was counted using a liquid scintillation counter. Nonspecific values were determined by measuring vesicular [3H]DA uptake in wash buffer (i.e., no ATP present) at 4°C.
Binding of [3H]DHTBZ was performed as described by Teng et al. (1998). Briefly, 200 μl of the synaptic vesicle preparation (∼6 μg of protein) was incubated in wash buffer in the presence of [3H]DHTBZ (final concentration: 2 nM) for 10 min at 25°C. The reaction was terminated by addition of 1 ml of cold wash buffer and rapid filtration through Whatman GF/F filters soaked in 0.5% polyethylenimine. Filters were washed three times with ice-cold wash buffer. Nonspecific binding was determined by coincubation with 20 μM TBZ. All protein concentrations were determined by a Bio-Rad protein assay (Bio-Rad Inc., Hercules, CA).
Statistical analyses were performed using an analysis of variance followed by a Fisher protected least significant difference post hoc comparison. Differences were considered significant if probability of error was less than 5%.
Results
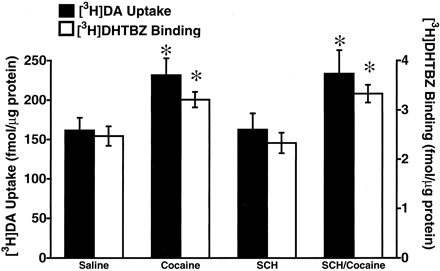
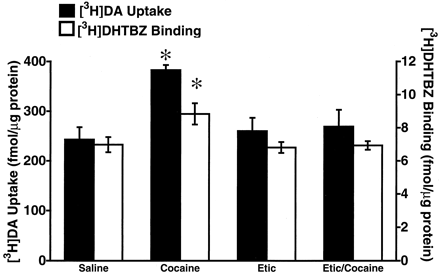
In accordance with previously published data (Brown et al., 2001), results presented in Fig. 1 demonstrate that a single injection of cocaine (30 mg/kg, i.p.) rapidly increased vesicular [3H]DA uptake and [3H]DHTBZ binding as assessed in purified striatal synaptic vesicles obtained from rats decapitated 1 h after treatment. Administration of the D1antagonist, SCH23390 [administered at a dose demonstrated previously to prevent cocaine-induced increases in D1-associated parameters including locomotor activity (Baker et al., 1998), and neuropeptide (i.e., substance P and neurotensin) immunoreactivity (Alburges and Hanson, 1999; Alburges et al., 2000)] did not prevent these increases in vesicular [3H]DA uptake or [3H]DHTBZ binding induced by cocaine administration (Fig. 1). In contrast, administration of the D2 antagonist, eticlopride, completely blocked the cocaine-induced increase in vesicular [3H]DA uptake and [3H]DHTBZ binding (Fig.2). Administration of either SCH23390 or eticlopride per se had no effect on vesicular [3H]DA uptake or [3H]DHTBZ binding (Figs. 1 and 2).
Rats received a single administration of SCH23390 (SCH; 0.5 mg/kg, i.p) or saline vehicle (1 ml/kg, i.p.) 15 min prior to a single administration of either cocaine (30 mg/kg, i.p.) or saline vehicle (1 ml/kg, i.p.). All animals were sacrificed by decapitation 1 h after the last injection. Columns represent the mean vesicular [3H]DA uptake (filled columns) or [3H]DHTBZ binding (open columns), and error bars represent the S.E.M. of determinations in six treated rats. ∗, values for treated rats that are significantly different from saline-treated controls (P < 0.05).
Rats received a single administration of eticlopride (Etic; 0.5 mg/kg, i.p.) or saline vehicle (1 ml/kg, i.p.) 15 min prior to a single administration of either cocaine (30 mg/kg, i.p.) or saline vehicle (1 ml/kg, i.p.). All animals were sacrificed by decapitation 1 h after the last injection. Columns represent the mean vesicular [3H]DA uptake (filled columns) or [3H]DHTBZ binding (open columns), and error bars represent the S.E.M. of determinations in five to six treated rats. ∗, values for treated rats that are significantly different from saline-treated controls (P < 0.05).
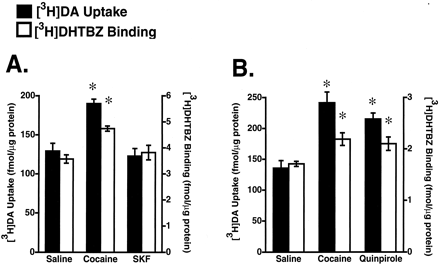
To elucidate further the role of D1 and D2 receptors in affecting vesicular [3H]DA uptake, the effects of the D1 agonist, SKF81297, and the D2 agonist, quinpirole, were examined. Results presented in Fig. 3A demonstrate that administration of SKF81297, administered at a dose shown previously in drug discrimination studies to be a selective agonist at D1 receptors (Haile et al., 2000), did not alter either vesicular [3H]DA uptake or [3H]DHTBZ binding. In contrast, similar to the effects of cocaine, administration of quinpirole increased both vesicular [3H]DA uptake and [3H]DHTBZ binding (Fig. 3B). To determine whether the effects of cocaine and quinpirole were additive, the effects on combined administrations of cocaine and quinpirole were assessed. Coadministration of quinpirole and cocaine did not increase vesicular [3H]DA uptake and [3H]DHTBZ binding to a greater degree than quinpirole or cocaine administered alone (Fig.4).
A, rats received either a single administration of saline vehicle (1 ml/kg, i.p.), cocaine (30 mg/kg, i.p.), or SKF81297 (SKF; 1 mg/kg, i.p.). B, rats received either a single administration of saline vehicle (1 ml/kg, i.p.), cocaine (30 mg/kg, i.p), or quinpirole (1 mg/kg, i.p.). All animals were sacrificed by decapitation 1 h after the last injection. Columns represent the mean vesicular [3H]DA uptake (filled columns) or [3H]DHTBZ binding (open columns), and error bars represent the S.E.M. of determinations in six treated rats. ∗, values for treated rats that are significantly different from saline-treated controls (P < 0.05).
Rats received a single administration of saline vehicle (1 ml/kg, i.p.), cocaine (30 mg/kg, i.p.), quinpirole (1 mg/kg, i.p.), or quinpirole and cocaine (1 and 30 mg/kg, i.p., respectively). All animals were sacrificed by decapitation 1 h after treatment. Columns represent the mean vesicular [3H]DA uptake (filled columns) or [3H]DHTBZ binding (open columns), and error bars represent the S.E.M. of determinations in six treated rats. ∗, values for treated rats that are significantly different from saline-treated controls (P < 0.05).
Discussion
Cocaine is a psychostimulant of abuse that is thought to exert its psychomotor and addictive effects principally by inhibition of the plasmalemmal DAT (Wilson and Schuster, 1972; Roberts and Koob, 1982;Ritz et al., 1987). In addition, recent studies suggest a previously unreported effect of this stimulant: specifically, cocaine increases vesicular DA sequestration. In particular, cocaine, as well as other agents that inhibit the DAT (i.e., GBR12935 and amfonelic acid), increase vesicular [3H]DA uptake and [3H]DHTBZ binding, as assessed in purified vesicles prepared from treated rats (Brown et al., 2001).
One well characterized consequence of cocaine-mediated plasmalemmal DAT inhibition is elevation in extracellular DA and activation of DA receptors. Hence, the present studies examined the role of DA receptors in mediating these cocaine-induced increases in vesicular [3H]DA uptake and [3H]DHTBZ binding. Results reveal that cocaine increases vesicular [3H]DA uptake and [3H]DHTBZ binding via a D2-dependent mechanism since eticlopride prevented these stimulant-mediated effects. A role for D2 receptors was further supported by findings that quinpirole, a D2 agonist, increased vesicular [3H]DA uptake and [3H]DHTBZ binding. Additionally, coadministration of cocaine and quinpirole did not increase vesicular [3H]DA uptake or [3H]DHTBZ binding to a greater extent than administration of either agent alone.
The mechanism(s) whereby D2 receptor activation influences vesicular [3H]DA uptake and [3H]DHTBZ binding remains to be determined. One possibility is that these agents may be altering the phosphorylation state of the VMAT-2 protein or a protein that regulates the VMAT-2. Accordingly, consensus sequences for phosphorylation by protein kinase A (PKA) have been reported in the cytoplasmic loops of VMAT-2 (Liu et al., 1992; Surratt et al., 1993). Because D2 DA receptor activation can decrease cAMP formation (Onali et al., 1985) and therefore may subsequently decrease PKA activity, a D2 receptor-mediated reduction in PKA activity may alter phosphorylation of the VMAT-2 protein and thus enhance its activity. Additionally, D2 receptor activation and a subsequent decrease in PKA activity may alter VMAT-2 activity indirectly (i.e., altering the activity of other enzymes via phosphorylation that in turn may affect vesicular DA sequestration). For instance, it is well established that D2 receptor activation decreases tyrosine hydroxylase activity. Pothos et al. (1998) demonstrated that incubation of PC12 cells with quinpirole (a D2 receptor agonist) decreased tyrosine hydroxylase activity by 59%. Moreover, cocaine administration has been shown to decrease striatal DA synthesis (Galloway, 1990). Data by Brown et al. (2001) suggest that depletion of DA with the tyrosine hydroxylase inhibitor α-methyl-p-tyrosine increases vesicular DA uptake. Therefore, D2 receptors activation may influence vesicular DA uptake through a mechanism involving inhibition of tyrosine hydroxylase activity and depletion of intracellular DA levels.
Relevant to the present findings is a report by Pothos et al. (1998)demonstrating that treatment with quinpirole reduces DA quantal size by approximately 50%. Noteworthy is the difference in preparations (i.e., purified striatal vesicles in the present study versus PC12 cells in the study by Pothos et al.). Still, it is interesting to speculate that even though the Vmax of DA uptake may be increased as reported by Brown et al. (2001), the amount of DA available for sequestration may be decreased due to a D2-mediated decrease in tyrosine hydroxylase activity, as described above, thereby resulting in a decrease in quantal size as reported by Pothos et al. (1998). Interestingly, Pothos et al. also reported a decrease in frequency of stimulation-evoked quantal release after quinpirole treatment. It can be speculated that this decrease may be the result of fewer vesicles at the plasma membrane available for subsequent release. This might occur if D2 receptor activation causes a redistribution of vesicles within nerve terminals (i.e., away from the plasma membrane). Accordingly, the present data demonstrate that cocaine or D2 receptor activation increases theBmax of DHTBZ binding in our vesicular preparation, a preparation largely devoid of plasma membrane. Hence, it is possible that in our vesicular preparation, we purify vesicles that have been trafficked away from plasma membrane. Further investigation into the nature of the changes in vesicular uptake and trafficking are warranted.
In conclusion, the data presented confirm previous reports that cocaine rapidly increases vesicular DA uptake and DHTBZ binding in a purified vesicular preparation. Furthermore, these data demonstrate that activation of D2 receptors mediates the cocaine-induced increases in vesicular DA uptake and that D2 receptor activation, per se, is sufficient to alter vesicular DA uptake. The present studies extend previous findings that D2 receptor activation may be critical for regulating monoamine transporter function (Meiergerd et al., 1993;Parsons et al., 1993; Rothblat and Schneider, 1997). For example,Mayfield and Zahniser (2001) demonstrated that D2receptor activation increases DA translocation into oocytes expressing both the DAT and D2 receptors. Whether the presently reported increase in vesicular DA sequestration represents 1) a redistribution of vesicles so that more vesicles containing VMAT-2 are detached from the plasmalemmal membranes and are included in the purified vesicular preparations, or 2) enhanced VMAT-2 activity per se remains to be determined. Nonetheless, the ability to alter DA sequestration may represent a novel therapeutic target for the treatment of disorders in which normal DA disposition has been disrupted. Additional investigation into these areas is warranted.
Acknowledgments
We thank Raul Weston for technical assistance with these experiments.
Footnotes
-
This work was supported by USPHS Grants DA00869, DA04222, and DA11389 from the National Institute on Drug Abuse.
- Abbreviations:
- DA
- dopamine
- VMAT-2
- vesicular monoamine transporter-2
- DHTBZ
- dihydrotetrabenazine
- DAT
- dopamine transporter
- PKA
- protein kinase A
- Received January 8, 2001.
- Accepted May 15, 2001.
- The American Society for Pharmacology and Experimental Therapeutics