Abstract
The global prevalence of neurologic disorders is rising, and yet we are still unable to deliver most drug molecules, in therapeutic quantities, to the brain. The blood brain barrier consists of a tight layer of endothelial cells surrounded by astrocyte foot processes, and these anatomic features constitute a significant barrier to drug transport from the blood to the brain. One way to bypass the blood brain barrier and thus treat diseases of the brain is to use the nasal route of administration and deposit drugs at the olfactory region of the nares, from where they travel to the brain via mechanisms that are still not clearly understood, with travel across nerve fibers and travel via a perivascular pathway both being hypothesized. The nose-to-brain route has been demonstrated repeatedly in preclinical models, with both solution and particulate formulations. The nose-to-brain route has also been demonstrated in human studies with solution and particle formulations. The entry of device manufacturers into the arena will enable the benefits of this delivery route to become translated into approved products. The key factors that determine the efficacy of delivery via this route include the following: delivery to the olfactory area of the nares as opposed to the respiratory region, a longer retention time at the nasal mucosal surface, penetration enhancement of the active through the nasal epithelia, and a reduction in drug metabolism in the nasal cavity. Indications where nose-to-brain products are likely to emerge first include the following: neurodegeneration, post-traumatic stress disorder, pain, and glioblastoma.
Introduction
Neurologic disorders are the largest cause of disability-adjusted life years and the second leading cause of death globally—representing 16.8% of global deaths (GBD 2015 Neurologic Disorders Collaborator Group, 2017). The burden of neurologic diseases is rising, with unipolar and depressive disorders predicted to become the second largest cause of morbidity by 2030 (Mathers and Loncar, 2006). In Europe, the societal cost of neurologic disorders was estimated at €798 billion in 2010, a figure comprising direct medical as well as nonmedical costs (60%) and productivity losses (40%) (Gustavsson et al., 2011). Conditions such as dementia, anxiety, and addiction inflict the greatest costs on European health budgets. There is thus a pressing need for new central nervous system (CNS) medicines. The development of CNS drugs is currently hampered by the fact that these drugs have to cross the blood brain barrier (BBB) in therapeutic quantities. The BBB is a formidable barrier that prevents the passage of most compounds from the blood to the brain and comprises tight endothelial capillary cell junctions, with the capillaries surrounded by astrocyte foot processes, endothelial cells with low transcytotic capacity, efflux pumps on the endothelial cells, and degradative enzymes close to the abluminal surface (Daneman and Prat, 2015). For drugs to cross the BBB, they must be less than 400 Da in molecular weight, be largely apolar, and not multicyclic (Ghose et al., 2012). However, a large number of compounds do not fit within these parameters, imparting serious constraint to the development of CNS actives. In actual fact, 98% of drug molecules do not cross the BBB in therapeutic quantities (Pardridge, 2005).
An alternative method of delivering molecules to the brain is the nose-to-brain route (Uchegbu et al., 2014; Godfrey et al., 2018). This route bypasses the BBB. The nose-to-brain route is gaining in popularity, as demonstrated by both preclinical (Godfrey et al., 2018) and human (Craft et al., 2012) studies. This route of delivery is the subject of this review, and papers quoted are confined to publications that actually demonstrate delivery to the brain via established quantification techniques. We have also highlighted clinical studies in which nose-to-brain delivery was the intended outcome.
Nose-to-Brain Mechanism of Delivery
For the purposes of drug delivery, the nasal cavity is divided into the respiratory area and the olfactory area, with the latter situated high up in the nares and the former closer to the nostrils (Sahin-Yilmaz and Naclerio, 2011). The nasal epithelium is well vascularized (Sahin-Yilmaz and Naclerio, 2011), and, within the olfactory area, olfactory neurons are exposed (Purves et al., 2004), enabling the transport of drug compounds directly into the brain via the olfactory neurons. The exact mechanism by which compounds transfer from the nasal mucosa to the brain is not fully understood. However, it is known that absorption of molecules takes place at the olfactory and respiratory epithelia (Lochhead and Thorne, 2012). The routes of compound transfer through the olfactory area, of the nares, to the olfactory bulb are transcellular through either the sustentacular cells or the exposed olfactory sensory neurons (Thorne et al., 2008; Lochhead and Thorne, 2012). The route of transfer of compounds through the nasal respiratory epithelium to the brain is via the trigeminal nerves (Thorne et al., 2008; Lochhead and Thorne, 2012). Transport to other brain areas after entry to the brain (e.g., to the mid brain from the olfactory bulb or to the brain stem from the trigeminal nerve) is thought to be mainly either by extracellular convective bulk flow (Lochhead and Thorne, 2012) or via perivascular routes (Lochhead et al., 2015). The paracellular route is not thought to be significant. Intranasally dosed nanoparticles have been observed in the olfactory bulb just 5 minutes after dosing (Godfrey et al., 2018), indicating this to be the route of entry for nanoparticle delivery systems. Drug compounds, having crossed the olfactory epithelium, may also be taken up into the general circulation via the nasal vasculature; however, the nasal vasculature is devoid of fenestrations and expresses the tight junction proteins (e.g., zonula occludens 1, occludin, and claudin 5) (Lochhead and Thorne, 2012); thus, significant transport to the general circulation via this route will be limited to low molecular weight apolar compounds. A key advantage of the nose-to-brain route is the possibility of reducing plasma exposure, as has been demonstrated (Hamidovic et al., 2017; Godfrey et al., 2018), thus eliminating peripheral side effects.
The average volume of the human nasal cavity has been measured using magnetic resonance imaging as 16,449.81 ± 4288.42 mm3, with the area of the nostril opening being 357.83 ± 108.09 mm2 (Schriever et al., 2013). Nostril opening correlates positively with nasal cavity volume (Schriever et al., 2013). No difference between the average volume of the nasal cavity was observed between men and women.
In human studies, intranasal insulin has been located within the cerebrospinal fluid (CSF) of human subjects (Born et al., 2002) and found to improve cognitive performance in Alzheimer’s disease (AD) patients (Craft et al., 2012). Studies with intranasal insulin show that there is no increase in blood insulin levels (Hamidovic et al., 2017), indicating that preferential brain delivery of peptides in humans is possible via this route. These studies demonstrate the utility of the nose-to-brain route in humans, especially if peripheral drug activity should be avoided.
Limitations
There are limitations to the use of the nose-to-brain route, and these must be acknowledged when developing new therapeutics to be administered via this route. There is a limitation on the dose volume for liquids of 100–250 μl (Davis, 1999; Djupesland et al., 2014; Santos-Morales et al., 2017) and powders 20–50 mg (depending on the bulk density of the powder) (Davis, 1999; Tepper and Johnstone, 2018; Shrewsbury et al., 2019), making the route only possible for potent drugs. Drugs that are metabolized by nasal cavity enzymes will also need to be protected from degradation, and drug formulations must be non-irritant to the nasal cavity. Furthermore, from a drug development point of view, a nasal delivery device is required to deliver drugs via the nose-to-brain route.
Drug Formulations
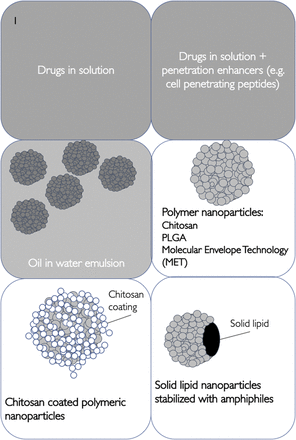
Although clinical studies have predominantly involved the use of drugs in solution (Craft et al., 2012), in preclinical studies a variety of formulation types have been tested (Fig. 1; Table 1), such as both solutions (Thorne et al., 2008) and particulate dispersions (Godfrey et al., 2018). Most animal studies have been conducted in rodents, and clinical studies have usually involved the use of a nasal drug delivery device.
Schematic representation of nose-to-brain formulations.
Nose-to-brain formulations
Solutions
Simply dissolving the drug molecule in an aqueous phase has been used to administer molecules via the nose-to-brain route (Born et al., 2002; Thorne et al., 2008; Craft et al., 2012; Parker et al., 2017). The vast majority of clinical studies, which report pharmacological effects, have involved a solution of the drug in aqueous media delivered using a nasal delivery device (Born et al., 2002; Craft et al., 2012; Parker et al., 2017). One of the first reports on the delivery of peptides to the brain involved the intranasal delivery of insulin to the brain in an insulin solution (Sigurdsson et al., 1997). Pharmacological activity has been observed in clinical studies, yet preclinical studies reveal just how little of the applied dose is actually delivered to the brain. Thorne et al. (2008) delivered a Cmax of 0.0064% of the dose of radiolabeled interferon-β1b to a monkey brain using an aqueous solution of the drug and speculated that delivery would be improved with the addition of absorption enhancers in the formulation. In all cases where the Cmax has been reported as a percentage of the total dose, brain weight was assumed to be 1% of the animal’s average body weight (Herculano-Houzel et al., 2011). Where a range of body weights is given, a midpoint is taken as the representative body weight. Oxytocin has also been delivered to the brain via the nasal route using a solution with a Cmax of 0.003% of a 10 μg dose being found in the brain (Tanaka et al., 2018). A solution of the human immunodeficiency virus replication inhibitor DB213 delivered the drug to the rat brain with a Cmax that was estimated at no more than 0.007% of the administered dose (Wang et al., 2017). These Cmax values are extremely low when compared with similar computations following oral dosing where the Cmax is 0.24%–4.3% of the administered dose (Siew et al., 2012), i.e., 100–1000 times greater.
The addition of functional excipients to these solution formulations improves brain delivery via the nasal route. This approach is exemplified by multiple studies. When using a solution of Serpin B2 and Activin A via the nose to brain route, neuroprotective activity was only seen in a mouse brain injury model (middle cerebral artery occlusion) when a penetration enhancer (tetradecyl-β-D-maltoside) was added to the protein solutions (Buchthal et al., 2018). The addition of a penetrating peptide (CPP, L-penetratin, RQIKIWFQNRRMKWKK) to a solution of exendin-4, a glucagon-1 receptor agonist, resulted in delivery of exendin-4 to the hypothalamus and hippocampus on nasal delivery to normal mice and the activation of insulin signaling, with the plain exendin-4 solution and exendin-4 plus the inactive D-penetratin, showing no brain delivery (Kamei et al., 2018). In a senescence-accelerated mouse model of cognitive dysfunction, intranasal exendin-4/CPP solutions plus supplemental insulin resulted in a therapeutic response against severe cognitive dysfunction (Kamei et al., 2018). The response was evaluated using the Morris Water Maze test after daily insulin and exendin-4 doses were administered for 4 weeks.
Conjugation of a CPP to an active also promotes the brain transport of said active, as demonstrated with the conjugation of low molecular weight protamine (with the peptide sequence: VSRRRRRRGGRRRR) to bovine serum albumin, β-galactosidase, or horseradish peroxidase (demonstrator proteins) (Lin et al., 2016). Although the majority of the protein was seen in the olfactory bulb, some brain delivery was indicated by enzyme activity assays of the latter two proteins and the detection of fluorescently labeled bovine serum albumin within the brain. The feasibility of administering arginine- and lysine-containing CPPs via the nasal route needs to be established to ensure adequate tolerability if they are to be used in clinical evaluations. It is known that arginine-containing CPPs are less toxic than lysine-containing CPPs (Saar et al., 2005), and hence, arginine molecules should be prioritized for evaluation if a CPP is added to the nose-to-brain formulation.
In an effort to increase the nasal residence time of nasal solutions, and thus increase drug transport through the olfactory neurons, others have added viscosity-increasing agents such as carboxymethylcellulose (Shingaki et al., 2010). When methotrexate solution containing carboxymethylcellulose was administered intranasally in combination with oral acetazolamide, significant tumor regression was observed in a rat 9L glioma model when compared with an intraperitoneal dose of the drug (Shingaki et al., 2010).
Nanoparticles
To address the very low drug transfer levels seen with conventional solution nasal formulations, drug delivery experiments have been conducted with nanoparticulate formulations (nanoemulsions, lipids, or polymer particles). Essentially these formulations offer the possibility of penetration enhancement or a longer nasal cavity residence time (Ahmad et al., 2017), with good evidence that nanoparticulates result in improved delivery of the cargoes, but limited quantitative evidence of delivery of the actual nanosystems (Ahmad et al., 2017; Godfrey et al., 2018). Actually, Ahmad et al. (2017) found that nanoemulsion particles of 100 nm penetrated the olfactory bulb and could be found in the brain to a small extent, whereas particles of 900 nm did not penetrate the brain at all. The nanoemulsion cargo was distributed throughout the brain with the 100 nm emulsion droplets. These data indicate that a particle size cutoff may be operational for the delivery of nanoformulations beyond the olfactory bulb.
Converting the solution formulation to a particulate formulation often has a transformational effect on the level of drug detected in the brain following intranasal delivery. The delivery of a solution of leucine-5-enkephalin (LENK), a δ-selective opioid agonist, to rat brains via the nose-to-brain route resulted in virtually undetectable levels of LENK in the brain (Godfrey et al., 2018). Delivery was enhanced when LENK was formulated in an absorption-enhancing chitosan-based nanoparticle (Godfrey et al., 2018). The formulation of rivastigmine (a cholinesterase inhibitor) being studied as a dementia treatment within a chitosan-containing emulsion increased the brain exposure 5-fold when dosed intranasally, when compared with an intranasal dose of the drug in solution (Shah et al., 2018). The intranasal delivery of quetiapine (an antipsychotic drug) resulted in a Cmax that was estimated at 0.035% of the dose when dosed as a solution and a Cmax that was estimated at 0.09% when dosed as chitosan–tripolyphosphate nanoparticles (Shah et al., 2016). From a commercial perspective, solution-based formulations are less appealing as their shelf life is likely to be limited and more prone to formulation microbial contamination.
Nanosystems may be divided into nanoparticles prepared from lipids (Eskandari et al., 2011) (usually solid lipid nanoparticles) and nanoparticles prepared from polymers such as chitosan derivatives (Godfrey et al., 2018), chitosan (Van Woensel et al., 2017), or poly(L-lactide-co-glycolide) (Seju et al., 2011) (Figs. 1 and 2).
(a) Chitosan and (b) poly(L-lactic acid-co-glycolic acid).
Lipid Nanoparticles.
Lipid nanoparticles, also known as solid lipid nanoparticles, consist of a lipid core stabilized by a surfactant, and they differ from oil-in-water emulsions in that the lipids are solids at room temperature and the formulation is prepared by melting the lipid, followed by a form of size reduction and then surfactant stabilization of the resulting particles in an aqueous disperse phase (Muller et al., 2000). These formulations may be loaded with hydrophobic drugs, and on application via the nasal route have been shown to deliver drugs to the brain. Valproic acid lipid nanoparticles when administered intranasally delivered significantly more drug to the brain, when compared with the drug in solution, and protected animals against seizures in a maximal electric shock seizure model, with the protection being to a similar extent as that seen on administration of intraperitoneal phenytoin (Eskandari et al., 2011). The model used mimics generalized tonic–clonic partial seizures. It is speculated that these lipid formulations protect the drug from degradation in the nasal cavity and may indeed promote drug transport by unspecified mechanisms. The lipid formulation was prepared from octyldodecanol, soy lecithin S100, cetyl palmitate, and the nanoparticles stabilized with Poloxamer 188.
Nanoparticles Containing Chitosan and Chitosan Derivatives.
Chitosan (Fig. 2a) has been incorporated into a number of nose-to-brain nanoformulations as chitosan solution, and chitosan nanoparticles (prepared by physical cross-linking of chitosan with tripolyphosphate) have been shown to act as penetration enhancers by temporarily opening intercellular tight junctions (Artursson et al., 1994; Vllasaliu et al., 2010). However, whereas studies have shown superior nose-to-brain delivery using chitosan nanoparticles (Shah et al., 2016), the mechanism of brain delivery enhancement is not completely understood. The application of quetiapine chitosan nanoparticles, with the nanoparticles formed by chitosan–tripolyphosphate, resulted in 34% more drug being delivered to the brain when compared with an intranasal solution of the drug (Shah et al., 2016). The brain Cmax was estimated at 0.056% of the administered dose of 2.3 mg kg−1 with the nanoparticle formulation and 0.03% of the administered dose with the solution of the drug. The use of intranasal chitosan nanoparticles containing pramipexole corrected motor deficits in a rotenone model of Parkinson’s disease, and pharmacodynamic effects were superior in the nanoparticle-administered animals when compared with a nasal solution or an oral dosage form of the drug (Raj et al., 2018). In all of these preclinical studies, the demonstration of drug delivery to the brain with pharmacokinetics data plus pharmacodynamic responses provides confidence in the approach (Godfrey et al., 2018; Raj et al., 2018).
The delivery of biologics via the nose-to-brain route is an area where the route is theoretically able to really offer the most impact. Solid evidence for the delivery of biologics exceeding a mol. wt. of 10 kDa to the brain is relatively rare. However, there are a few preclinical studies in the literature, as a few groups have reported evidence of gene silencing via the nose-to-brain route. Chitosan–Tripolyphosphate short interfering RNA (siRNA) nanoparticles, on intranasal administration, have been shown to silence the galectin-1 gene, a gene that drives chemoresistance and immune therapy resistance, resulting in increased survival in a mouse tumor model when treated concurrently with temozolamide (Van Woensel et al., 2017). Others have also reported gene silencing with chitosan nanoparticles made with a chitosan–mangafodipir electrostatic complex, where mangafodipir (a manganese dipyrydoxyl diphosphate chelate) is used to physically cross-link chitosan (Sanchez-Ramos et al., 2018), and siRNA delivery to the olfactory bulb, using a chitosan derivative, N-ethylamino-6-O-glycolchitosan, has been reported (Simao Carlos et al., 2017).
Gene silencing of the reporter GFP gene was observed on intranasal application of chitosan–mangafodipir nanoparticles in Tg GFP+ mice, with gene silencing observed in the olfactory bulb, striatum, hippocampus, and cortex (Sanchez-Ramos et al., 2018). Gene expression was also reported in the striatal region when the red fluorescent protein gene was administered intranasally within chitosan–mangafodipir nanoparticles (Sanchez-Ramos et al., 2018). The delivery of nucleic acids to the brain using the nose-to-brain route is an important breakthrough. However, further studies are needed to confirm the real potential and possible wider applicability of the nose-to-brain route for the delivery of nucleic acids. Along with genes and siRNA, a chitosan amphiphile has been used to deliver a labile peptide to the brain (Godfrey et al., 2018). On intranasal administration, N-palmitoyl-N-monomethyl-N,N-dimethyl-N,N,N-trimethyl-6-O-glycolchitosan [Nanomerics’ molecular envelope technology (MET)] nanoparticles encapsulating LENK (a δ opioid receptor agonist) produced analgesia in all animal models tested (acute, chronic, and spontaneous pain models), with exclusive central activity and no peptide detected in the periphery after nasal dosing (Godfrey et al., 2018). The administration of a solution of LENK resulted in drug appearing in the olfactory bulb, minimal levels appearing in the brain, and no analgesic response. MET–Propofol formulations also produced sedation in a healthy rat model on intranasal administration (Uchegbu et al., 2014). The MET is known to be mucoadhesive, but does not open tight junctions (Siew et al., 2012), and mucoadhesion within the nasal cavity would prolong the residence time of the formulation within the nares, providing the opportunity for an extended duration of action. The MET is also a penetration enhancer, demonstrating penetration enhancement in the gut epithelium via particle uptake mechanisms (Garrett et al., 2012; Serrano et al., 2015). MET nanoparticles were detected in the brain parenchyma; however, the extent of brain uptake and the influence of particle uptake on peptide delivery are not well understood (Godfrey et al., 2018). What is clear is that Nanomerics’ MET delivers labile peptides to the brain via the olfactory bulb pathway, and there is drug biodistribution and pharmacological evidence of transport into the deeper parts of the brain via perivascular pathways (Godfrey et al., 2018).
Studies have been conducted with chitosan-containing emulsions in which the presence of chitosan significantly improved the deposition of drug in the brain following intranasal delivery. Chitosan (mol. wt. = 100–300 kDa) at a concentration of 0.3% w/v significantly increased the brain deposition of zomitriptan when administered in an oil-in-water emulsion, with the mucoadhesion of the drug-containing formulation being implicated in this improved bioavailability (Abdou et al., 2017). The formulation consisted of a Capryol propylene glycol monocaprylate oil phase stabilized with Kolliphor polyoxyl 40 hydrogenated castor oil and Transcutol P (diethylene glycol monoethyl ether).
The chitosan coating of lipid microparticles also dramatically improved the distribution of reservatrol to the CSF on intranasal administration of the 60 μm lipid particles coated with chitosan to a rat model (Trotta et al., 2018). The microparticles consisted of a core of tristearin, glyceryl behenate, and stearic acid stabilized with phosphatidyl choline and further coated with chitosan. In vivo studies demonstrated that no resveratrol was detected in the rat CSF after an intravenous infusion of the drug alone, whereas the nasal delivery of resveratrol in a chitosan suspension or encapsulated in uncoated lipid microparticles, dispersed in water, achieved distribution of resveratrol to the CSF. Additionally, a dramatic increase in CSF levels of over 6-fold was achieved on the administration of the reservatrol lipid microparticles with a chitosan coating when compared with the uncoated nanoparticles (Trotta et al., 2018). This marked increase in the CSF levels was achieved without any detectable systemic exposure, demonstrating a direct and specific nose-to-brain pathway.
Chitosan (Fig. 2a) and its derivatives have been clearly shown to enhance delivery of actives via the nose-to-brain route.
Poly(L-lactide-co-glycolide) Nanoparticles.
Poly(L-lactide-co-glycolide) (PLGA) (Fig. 2B) is a polymer approved for human use in the world’s largest markets (Danhier et al., 2012). It is approved for use in drug delivery systems, and this means that it is the polymer of choice for preparing medicinal products as it is biodegradable and demonstrates no toxicity concerns when used in humans. PLGA may be used to protect drugs from degradation in the nasal cavity and may be loaded with hydrophobic drugs (Danhier et al., 2012). These properties have been exploited for nose-to-brain delivery. Olanzapine, when loaded onto PLGA nanoparticles, resulted in delivery to the brain that was 10 times more efficient than nose-to-brain delivery with olanzapine solution, resulting in a Cmax of 0.049% of the dose and a Cmax of 0.0045% of the dose with the nanoparticle and solution formulations, respectively (Seju et al., 2011). As well as pharmacokinetics evidence of nose-to-brain transport, pharmacodynamics evidence of nose-to-brain transport has been recorded in the form of a reduction in seizures in a rat seizure model, using PLGA nanoparticles (Musumeci et al., 2018). PLGA nanoparticulate oxcarbazepine reduced seizures in a rat seizure model (seizures induced by intraperitoneal pentylene tetrazole) on intranasal administration, and the nanoparticles were superior to the drug in solution in protecting against seizures (Musumeci et al., 2018). A PLGA-poly(ethylene glycol) copolymer nanoparticle, conjugated with Solanum tuberosum lectin (a lectin that binds to N-acetyglucosamine receptors on the nasal respiratory epithelium) and loaded with basic fibroblast growth factor, improved cognition in a mouse AD model, on intranasal administration (Zhang et al., 2014). It is interesting to note that PLGA nanoparticles have not been reported to be penetration enhancers or to be mucoadhesive, and yet delivery to the brain is enhanced through the nasal route. Adding chitosan to the surface of PLGA nanoparticles did alter their brain transport, as the resulting positively charged chitosan-coated PLGA nanoparticles appeared to transport from the caudal to the rostral regions of the brain more slowly, when compared with plain negatively charged PLGA nanoparticles (Bonaccorso et al., 2017). However, the impact of the chitosan coating on actual drug deposition in the brain was not studied in the report. It is apparent that different transport pathways may be involved in the transport of positively charged and negatively charged nanoparticles. The addition of a specific targeting ligand aimed at a receptor expressed on both neuronal surfaces and the nasal respiratory epithelium (lactoferrin) plus a N,N,N-trimethylchitosan (TMC) coating resulted in delivery of huperzine A (a reversible cholinesterase inhibitor being developed as an AD treatment) to the olfactory bulb, cerebellum, and hippocampus (Meng et al., 2018). By comparing TMC- and lactoferrin-coated nanoparticles with both plain PLGA- and TMC-only–coated nanoparticles, TMC was found to promote brain delivery, increasing brain exposure to huperzine A when coated onto the PLGA nanoparticles, and lactoferrin was found to further increase brain exposure to huperzine A (Meng et al., 2018). It is clear from these data that a positive charge seems to promote brain accumulation, whereas targeting ligands that promote cellular uptake further promote brain delivery. All of this evidence suggests that for the nose-to-brain route, the particle transport mechanisms are governed by the particle surface chemistry. Clarification of the different biologic mechanisms at play will assist with product design of nose-to-brain dosage forms.
Other Delivery Systems
Physical interventions aimed at increasing drug localization in particular areas are an emerging area. Focused ultrasound with the administration of microbubbles has been used to deliver gold nanoclusters to specific brain regions (Ye et al., 2018). 64Cu-labeled or Texas Red–labeled gold nanoclusters were delivered to the brain stem using focused ultrasound and microbubbles to localize the nanoclusters to the brain stem. The focused ultrasound causes localized microbubble cavitation at the target region and thus enables cellular uptake, with minimal delivery to the peripheral circulation (Ye et al., 2018). No histologic-level tissue damage was detected in the nose, trigeminal nerve, and brain.
Clinical Use of Nose-to-Brain Delivery
It is clear from the foregoing account that utilizing the nose-to-brain route is a suitable method of achieving brain delivery of actives. As such, a variety of clinical trials have been reported that use this route. The first report of nose-to-brain delivery was made in 2002 by Born et al. (2002), in which insulin along with melanocortin(4–10) and vasopressin was administered as intranasal solutions to humans, and elevated levels of all three drugs detected in the CSF 10 minutes after dosing with peak levels were observed 80 minutes after dosing. This breakthrough study has paved the way for a variety of clinical studies using the nose-to-brain route (Chapman et al., 2013) for various disease indications.
Insulin
Alzheimer’s Disease.
AD is characterized by cognitive degeneration and is a disease of ageing (Lane et al., 2018). The disease is also associated with insulin dysregulation, and AD patients have lower CSF insulin levels, higher plasma insulin levels, and a reduced CSF, plasma insulin ratio when compared with healthy adults (Craft et al., 1998). The intranasal administration of 20 IU insulin daily results in increased CSF insulin (Born et al., 2002) and an improved delayed story recall (recalling a story 20 minutes after it was read to participants) in AD patients (Craft et al., 2012). The same study reported improved partner-rated ability to carry out daily functions when patients were administered a 20 or 40 IU daily dose of insulin (Craft et al., 2012). Insulin was administered as a solution in the study over 4 months. Craft et al. (2017) also compared intranasal long-acting insulin—insulin detemir (insulin with a C14 fatty acid chain at the proline residue at position 29 of the B chain) with regular insulin in a 4-month study, in which patients received a daily dose of 40 IU insulin, and found memory improvements at months 2 and 4 only in the regular insulin group and not in the insulin detemir group. The regular insulin solution group was also associated with a decrease in changes in brain volume in AD-affected areas (Craft et al., 2017). This is evidence that insulin that is immediately available in solution appeared to translocate to interact with the appropriate brain receptors more efficiently than its lipidized analog. The efficacy of insulin to translocate and interact with the relevant brain regions was further examined by using rapidly-acting insulin: namely insulin aspart, in which a proline is replaced by aspartic acid, as insulin aspart does not form hexamers (Benedict et al., 2007). Regular insulin forms hexamers that have to dissociate into monomers prior to pharmacological activity (Kahn, 1985; Benedict et al., 2007). Insulin aspart, when given at a daily dose of 160 IU over 8 weeks, was superior to regular insulin, administered at the same dose, in improving memory in a word recall test (Benedict et al., 2007). These data further demonstrate that nonaggregated insulin available as monomers and not as hexamers or the lipidized analog is more efficient at locating relevant brain receptors when dosed via the nose-to-brain route.
Other Conditions.
Due to intranasal insulin’s clear benefits on memory (Craft et al., 2012), intranasal insulin has also been studied in pediatric patients with 22q13 deletion syndrome (Phelan–McDermid syndrome), a syndrome characterized by developmental delay and both cognitive and motor deficits (Schmidt et al., 2009). The administration of 40 IU insulin daily for 1 year resulted in an improvement in cognitive function and improvements in both fine and gross motor function. Some nose bleeding was observed in one patient. Intranasal insulin has also been shown to reduce nicotine cravings in smokers when given as a single 60 IU dose, and although there was no increase in peripheral insulin levels, there was a slight decrease in blood glucose in this study (Hamidovic et al., 2017). The single dose of intranasal insulin even reduced the cravings when participants were subjected to a stressful experience. Nasal irritation (a burning sensation) was the most common side effect reported (Hamidovic et al., 2017).
Oxytocin
Oxytocin, a peptide that has been studied for its psychologic effects (Shin et al., 2015), has been dosed intranasally in human nose-to-brain experiments for the treatment of post-traumatic stress disorder (PTSD) (van Zuiden et al., 2017), autistic spectrum disorder (ASD) (Parker et al., 2017), and schizophrenia (Shin et al., 2015). The data on oxytocin use in the treatment of PTSD have been replicated. In one study, oxytocin was found to reduce a provoked PTSD reaction (provoked by a script-reading challenge) in female PTSD patients when given intranasally at a dose of 20 IU oxytocin in a solution (Sack et al., 2017). The reduced PTSD response was seen despite an increase in heart rate being observed during the script-reading challenge. A further study examined the effect of intranasal oxytocin in PTSD patients admitted to an accident and emergency department (van Zuiden et al., 2017). Patients were given 40 IU oxytocin intranasally or placebo in a randomized controlled study, and only patients with high acute clinician-rated PTSD symptom severity showed beneficial effects to the nose-to-brain administration of oxytocin.
In the case of ASD, the response to intranasal oxytocin was dose related in adult patients, in that a single dose of 8 IU oxytocin intranasally did improve the ability to emotionally rate faces, whereas a dose of 40 IU did not, when compared with placebo (Quintana et al., 2017). Further data from a pediatric study, in which children were dosed with 24 IU oxytocin intranasally daily for 4 weeks, demonstrated that ASD children did benefit from an intranasal dose of oxytocin, especially when pretreatment levels of oxytocin were low in the blood (Parker et al., 2017). The ASD patient responders showed an enhanced social ability in the treatment arm. In this study, children in the placebo arm with high endogenous levels of oxytocin also showed an enhanced social ability during the study. This demonstrates that a careful titration of oxytocin doses with reference to pretreatment blood levels may need to be undertaken for pediatric patients to benefit from intranasal oxytocin.
Finally, intranasal oxytocin has been found to decrease amygdala activity to fearful and neutral faces in schizophrenic patients, when compared with effect of intranasal oxytocin in healthy controls (Shin et al., 2015). These data provide a possible route to control the response to emotional faces in schizophrenic patients and thus moderate the behavior of schizophrenic patients.
Other Drugs
Brain tumors are especially difficult to treat due to a combination of the BBB (Groothuis, 2000) and the fact that the tumors are sometimes diagnosed late (Dobrovoljac et al., 2002). Delivering drugs via the nose to the brain may enable high drug concentrations to be present in the vicinity of the tumor. In a long-term study involving 117 men and 81 women with primary glioblastoma multiforme (n = 154), grade III astrocytoma (n = 26), and anaplastic oligodendroglioma (n = 5), the intranasal administration of perillyl alcohol, an antitumor agent, resulted in 19% survival in the cohort 4 years after dosing (Da Fonseca et al., 2013). Patients received 267–534 mg daily in four doses. Side effects included nasal soreness, but the therapy was well tolerated with adherence to the protocol recorded at 95%. These data are encouraging, and nose-to-brain treatment of intracranial tumors requires further investigation.
Other peptides that have been administered to humans via the nose include arginine–vasopressin (AVP) for the treatment of tension headaches and migraine (Yang et al., 2012). AVP when dosed at 100–400 ng to such headache patients resulted in partial or complete headache remission in 96% of patients (27 of 28). Relief was recorded 60–180 minutes after dosing, and headache patients had higher plasma and CSF levels of AVP (Yang et al., 2012), indicating a possible endogenous role for AVP in these headaches. Nonhematopoietic erythropoietin, which has been found to be neuroprotective in animal studies, was well tolerated in humans on intranasal dosing at a dose of 1.5–3.0 mg/day for 4 days (Santos-Morales et al., 2017). Side effects included headache, raised hepatic enzymes, and nasopharyngeal itching, but all side effects resolved after treatment had ended.
Nasal Delivery Devices
For nose-to-brain delivery, the dose must be deposited in the olfactory region, and thus, a special delivery device is required (Lochhead and Thorne, 2012). These devices are either propellant activated in the case of Kurve Technologies’ Vianase (Craft et al., 2017), Impel Neuropharma’s Precision Olfactory Device (Shrewsbury et al., 2019), and Alchemy Pharmatech’s Naltos Device (http://wwwalchemypharmatechcom/indexhtml), or breath activated in the case of the Optinose device (Quintana et al., 2017). Although nose-to-brain delivery is well established in the clinical trial space, it appears that devices that offer nose-to-brain delivery are still not associated with licensed products. Optinose’s sumatriptan product Onzetra is not specifically designated as a nose-to-brain product, but as a nasal product (https://wwwonzetracom/sites/default/files/onzetra_xsail_prescribing_informationpdf). The Vianase device is an electronic atomizer that delivers liquid droplets of 15–20 μm in size to the entire nasal cavity, including the olfactory region (Craft et al., 2012, 2017; http://www.kurvetech.com/nasaltechnology.asp). The Precision Olfactory Device delivers liquids and powders to the olfactory region of the nasal cavity using an inert liquid (hydrofluoalkane) that forms a gas propellant (http://impelnpcom/pod-technology/). Alchemy Pharmatech’s Naltos device (Fig. 3) works by means of an inert gas that is actuated by the device to propel the powder through the nares (http://wwwalchemypharmatechcom/indexhtml). Finally, Optinose exploits the patient’s own exhalation, which propels the dose deep into the nose while simultaneously isolating the oral cavity from the nasal cavity (Djupesland, 2018). Only the Optinose, Precision Olfactory Delivery, and Vianase devices have been used in human nose-to-brain studies to date.
The Naltos device (Alchemy Pharmatech).
Summary
Although the BBB limits the delivery of certain drugs to the brain and, as such, hampers the treatment of certain CNS disorders, accessing the brain via the nose-to-brain route has been demonstrated by scores of preclinical studies and about a dozen clinical trial results. Solution forms of the active have been found to be effective clinically, whereas both nanoparticulate formulations and solutions have been used in animal experiments. The use of nanoparticles and solution penetration enhancers improves the delivery to the brain via the nose-to-brain route, and, because there are limitations in dose volume, these technologies are likely to be very important in the future. The amount of drug delivered is estimated at up to 0.09% of the dose at the Cmax, and yet clear pharmacological effects have been observed in human and animal studies. A device is needed for human studies, and a number of device manufacturers have now entered the market. The route may become important for indications such as pain, AD, PTSD, and intracranial tumors.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Uchegbu, Wang, Xiong, Tsang, Schätzlein.
Footnotes
- Received March 17, 2019.
- Accepted May 21, 2019.
Abbreviations
- AD
- Alzheimer’s disease
- ASD
- autism spectrum disorder
- AVP
- arginine vasopressin
- BBB
- blood brain barrier
- CNS
- central nervous system
- CPP
- cell-penetrating peptide
- CSF
- cerebrospinal fluid
- LENK
- leucine-5-enkephalin
- MET
- molecular envelope technology
- PLGA
- poly(L-lactic acid-co-glycolic acid)
- PTSD
- post-traumatic stress disorder
- siRNA
- short interfering RNA
- TMC
- N,N,N-trimethylchitosan
- Copyright © 2019 by The Author(s)
This is an open access article distributed under the CC BY-NC Attribution 4.0 International license.