Abstract
Fenobam [N-(3-chlorophenyl)-N′-(4,5-dihydro-1-methyl-4-oxo-1H-imidazole-2-yl)urea], a potent metabotropic glutamate mGluR5 receptor antagonist, reported to have analgesic effects in animals and anxiolytic effects in humans, also caused adverse events, including psychostimulant-type effects and “derealization phenomena.” Recent electrophysiologic, pharmacologic, and anatomic data show that the mGluR5 antagonists 2-methyl-6-(phenylethynyl)pyridine (MPEP) and (E)-2-methyl-6-styryl-pyridine (SIB-1893) can inhibit NMDA receptor–mediated activity and that mGluR5 receptors are highly expressed in limbic and forebrain regions. The present studies first evaluated the potential of mGluR5 receptor antagonists to cause PCP-like psychoactive effects in a rat drug discrimination procedure and, second, explored and characterized the selective mGluR5 antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) as a discriminative stimulus and compared MTEP with other drugs known to be psychoactive in humans. Additionally, the reinforcing potential of MPEP and MTEP was compared with phencyclidine (PCP) in a rat intravenous self-administration procedure. Dizocilpine [(+)-MK-801] and ketamine caused full PCP-appropriate responding. Memantine and the mGluR5 antagonists caused no or weak partial PCP-appropriate responding. In MTEP-trained rats, MTEP, MPEP, and fenobam caused full and equipotent MTEP-appropriate responding. (+)-MK-801 and memantine caused MTEP-appropriate responding below 70%, whereas PCP, chlordiazepoxide and LSD caused MTEP-appropriate responding below 50%. Δ9-Tetrahydrocannabinol, yohimbine, arecoline, and pentylenetetrazole all caused MTEP-appropriate responding below 20%. Rats self-administered PCP but not MPEP or MTEP, indicating a lack of reinforcing effects of the mGluR5 antagonists. These data suggest that the mGluR5 antagonists appear not to have reinforcing properties, that the discriminative effects of mGluR5 antagonists and PCP are dissimilar, and that mGluR5 antagonists may produce psychoactive effects different from NMDA-antagonists and other drugs with known psychotomimetic properties.
Introduction
Excitatory amino acid (EAA) receptors play a major role in the mediation of synaptic excitation in the central nervous system (CNS), and glutamate is the most abundant EAA in the CNS (Monaghan et al., 1989). Glutamatergic transmission is mediated via three ligand-gated ionotropic receptors (iGluRs) [N-methyl-d-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainate (Monaghan et at., 1989)] and a G-protein–coupled metabotropic receptor (mGluR) (Conn and Pin, 1997). Attenuation of glutamate transmission may be beneficial for CNS disorders, including cerebral ischemia, epilepsy, anxiety, pain, and depression.
For example, anticonvulsant effects were demonstrated with competitive (Chapman et al., 1991a) and uncompetitive NMDA antagonists such as phencyclidine (PCP) (Hayes and Baster, 1985; Chapman and Meldrum, 1989) and the AMPA antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline (NBQX) (Chapman et al., 1991b; Swedberg et al., 1995). NMDA receptor blockade can cause hallucinations and other vivid psychoactive effects in humans (Aniline and Pitts, 1982; Bey and Patel, 2007), mediates PCP-like discriminative effects in primates (Nicholson et al., 2007) and rats (Koek et al., 1990; Willetts and Balster, 1988; Swedberg et al., 1995), and maintains self-administration in animals (Nicholson et al., 2007), whereas the AMPA antagonist NBQX caused no PCP-like discriminative effects (Swedberg et al., 1995).
Recent discoveries of several mGluR receptor subtypes increase the number of potential targets for drug discovery (Conn and Pin, 1997), and mGluR5 receptors are implicated in several CNS functions (Schoepp, 2001) and disorders, including anxiety, depression, pain, Parkinson disease (Spooren et al., 2001), and gastroesophageal reflux disease (Keywood et al., 2009); these receptors are localized in the forebrain and limbic regions in rats (Spooren et al., 2001) and primates (Muly et al., 2003).
Fenobam [[N-(3-chlorophenyl)-N′-(4,5-dihydro-1-methyl-4-oxo-1H-imidazole-2-yl)urea]; McN-3377], recently characterized as an mGluR5 receptor antagonist with anxiolytic (Porter et al., 2005) and analgesic effects in rodents (Montana et al., 2009), had shown anxiolytic effects in humans (Pecknold et al., 1980, 1982; Lapierre and Oyewumi, 1982) and was dissimilar to the classic anxiolytic diazepam in being more of a psychostimulant (Itil et al., 1978; Friedmann et al., 1980) and causing hallucinations (Friedmann et al., 1980). The more selective mGluR5 antagonist 2-methyl-6-(phenylethynyl)pyridine (MPEP) (Gasparini et al., 1999) had anxiolytic-like activity in rats (Ballard et al., 2005) and, similar to (E)-2-methyl-6-styryl-pyridine (SIB-1893), inhibited NMDA receptor activity in electrophysiologic and pharmacologic studies (O’Leary et al., 2000; Movsesyan et al., 2001) and had antagonistic effects on primate cocaine drug discrimination and self-administration (Lee at al., 2005). The more recent mGluR5 antagonist MTEP was more selective toward NMDA and other receptors and more potent in rat anxiety models compared with MPEP (Cosford et al., 2003; Busse et al., 2004).
Psychoactive and hallucinatory effects of fenobam in humans (Friedmann et al., 1980; Pecknold et al., 1982), MPEP antagonism of NMDA receptor–mediated effects (O’Leary et al., 2000; Movsesyan et al., 2001), and effects on cocaine self-administration and drug discrimination (Lee et al., 2005) warrant further characterization of behavioral effects of mGluR5 antagonists.
In contrast to the hyperactivity and ataxia often observed with NMDA antagonists such as PCP (Swedberg et al., 1995), mGluR5 antagonists in our discovery program reduced spontaneous activity and caused flat body posture in rodents at higher doses in the Irwin screen (Irwin, 1968) or on spontaneous locomotor activity but caused no other systematic behavioral effects or interacted with pentylenetetrazole convulsion thresholds (unpublished data).
In the development of CNS active drugs, regulatory and safety aspects include investigation of the potential for abuse liability, and by applying a proactive abuse liability assessment strategy (Swedberg, 2013), the present studies were designed to assess NMDA antagonist–like discriminative effects of mGluR5 receptor antagonists in rat PCP drug discrimination and to investigate the feasibility of establishing MTEP drug discrimination and assessing the selectivity of MTEP discrimination using other mGluR5 antagonists, NMDA antagonists, and other drugs with psychoactive or hallucinogenic properties in humans. Cannabinoid receptor agonists like (−)-Δ9-THC are psychoactive in humans (D’Souza et al., 2004) and animals (Wiley, 1999). LSD is a well-known hallucinogenic (Passie et al., 2008) with discriminative effects in animals (Appel et al., 2004). Arecoline, a cholinergic agonist and active ingredient in the betel nut, is a CNS stimulant in humans (Bhat et al., 2010) and is psychoactive in animals (Meltzer and Rosecrans, 1981). Yohimbine, an α2-adrenergic receptor antagonist, can cause CNS excitation and dizziness and reinstatement of drug self-administration (Fitzgerald, 2013). Pentylenetetrazole is a nonspecific CNS stimulant (Bloom, 1990), and chlordiazepoxide, a sedative anxiolytic (Rall, 1990), is both a psychostimulant and sedative-anxiolytic in humans and animals, respectively. Additionally, MTEP was compared with MPEP and PCP in a rat intravenous self-administration procedure to evaluate the reinforcing potential of mGluR5 antagonism.
Materials and Methods
All animal experiments were performed in accordance with the guidelines of The Swedish National Board for Laboratory Animals under a protocol approved by the Ethics Committee of Southern Stockholm, Sweden. Studies were carried out in accordance with the Declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health.
Drug Discrimination Experiments
Subjects.
Forty male Wistar rats (Scanbur BK AB, Sollentuna, Sweden; and Moellegaard, Ry, Denmark) weighing 240–250 g at the beginning of the experiments were housed in pairs, or group housed up to eight rats per cage, in a colony room with water accessible at all times and lights on between 6:00 AM and 6:00 pm; by restricting access to food, animals were kept at approximately 80% of free-feeding weight.
Apparatus.
Sixteen operant chambers enclosed in sound-attenuating cubicles with exhaust fans providing “white noise” and equipped with two response levers, cue lights, a house light, and a food magazine were used (Med Associates Inc., St. Albans, VT). Food pellets used were 45-mg Dustless Precision Pellets (Bio-Serv, Frenchtown, NJ). Experiments were run and data collected by a PC with DRDIWin PC Software (Ellegaard Systems A/S, Faaborg, Denmark) and LOIS DRDI software (R&D IS, AstraZeneca R&D Södertälje).
Discrimination Training and Testing.
Training and testing procedures were similar to those described earlier (Swedberg et at., 1995). In brief, an autoshaping procedure was used to train rats to approach the response levers and learn that food pellets were available. Before any drug administration, the rats were trained to press either response lever (left or right) once [a fixed ratio (FR)1 schedule] to produce a food pellet. The production of one food pellet constituted the completion of one trial. After learning that pressing any of the two levers would produce food, a “drug lever” and a “no-drug” lever were assigned to each rat in a balanced fashion. From this point and on, each animal received either an injection of the training drug (“drug”) or no injection (“no drug”) before the training sessions and were required to press the lever appropriate to the pretreatment received (“correct lever”) to produce a food pellet. Animals were run on a single alternation schedule with an increase in the FR response requirement every other day until an FR10 was reached. Presses on the incorrect lever had no programmed consequences other than to reset the FR value on the correct lever, thus requiring the rats to emit 10 correct responses consecutively to obtain a food pellet. At FR10, a double-alternation schedule was introduced so that pairs of two consecutive drug (D) and no-drug (N) sessions alternated (D, D, N, N, D, D, N, N, and so forth). A session lasted until 50 food pellets had been earned (equaling 50 trials) or until 15 minutes had elapsed, whichever occurred first. Rats were then trained to reach a criterion of 90% correct responding with no more than nine responses on the incorrect lever before the first reinforcement in each session for eight consecutive sessions. The two sessions immediately after the eighth criterion session were acquisition test sessions in which the training conditions (D and N) were tested. Ten consecutive responses on either lever would produce a food pellet (i.e., both levers were “correct”), and the same performance criteria were applied.
During testing, animals were run according to a single-alternation schedule (D, N, D, N, etc.), and tests were interspersed between the training sessions (D, N; formally: D, T, N, D, T, N, T, D, N, T, etc.). Test sessions were identical to training sessions except during tests, both levers were correct and 10 consecutive presses on any of the levers would produce a food pellet. Test sessions were typically run on Tuesdays and Fridays, provided that the animals performed according to the criteria on the training days. If training day performance fell below criteria for any rat on a single training day, the upcoming test was postponed for that rat, and the rat was tested again only after completing two consecutive training sessions during which the criteria were met. During testing, the appropriate vehicle of each drug was tested as the “no-drug” condition.
Several groups (n = 8/group) of male Wistar rats were trained to discriminate 2.8 mg/kg PCP administered intraperitoneally 30 minutes before training or 2 mg/kg i.p. MTEP 30 minutes before training from no drug.
Data Analysis.
Drug discrimination results are expressed as the mean (±S.E.M.) of the individual percentages of drug responding during drug and no drug sessions, respectively, in rats completing at least 10 trials. Rates of responding are expressed as the mean (±S.E.M.) number of responses per second in all rats tested. ED50 values for the discriminative effects were determined as the dose producing 50% training drug-appropriate responding. Response rate–effects are shown as percentage of vehicle control rates. ED50 values and 95% confidence limits were calculated by use of analysis of variance and linear regression techniques (GraphPad Prism version 5; GraphPad Software, Inc., La Jolla, CA) (Snedecor and Cochran, 1967). SigmaPlot for Windows version 12.5 (Systat Software Inc., Chicago, IL) was used for graphics.
Drugs and doses producing less than 20% training drug–appropriate responding are considered not to produce discriminative effects similar to those of the training drug. Drugs and doses producing between 20% and 80% training drug–appropriate responding are considered to produce discriminative effects that are partly similar to those of the training drug. Drugs and doses causing 80% or more training drug–appropriate responding are considered to share fully the discriminative effects of the training drug; and drugs producing full training drug–like discriminative effects and no significant effects (<50% reduction relative to vehicle alone) on response rates are considered to produce discriminative effects identical to those of the training drug.
Drug Self-Administration Experiments
Animals.
Male Wistar rats (n = 24, 8/test drug; Taconic Europe A/S, Ry, Denmark) were housed in a temperature-controlled environment on a 12-hour light/dark cycle (lights off at 6:00 PM) with ad libitum access to food and water. The rats were allowed to acclimate in their new environment for at least 1 week before the start of the experiment.
Surgery.
Intravenous catheters (CamCaths, Ely, UK) were implanted into the right jugular vein under isoflurane (Forene Abbott, Solna, Sweden) anesthesia. Rats were administered postoperative analgesia [0.06 mg/kg s.c. buprenorphine (Temgesic); Schering-Plough, Brussels, Belgium] and antibiotic [amoxicillin (Bimoxyl Veterinary); Ceva Animal Health, Dublin, Ireland]. The catheters were flushed with a heparin solution (50 U/ml; Heparin LEO; LEO Pharmaceuticals, Copenhagen, Denmark) before and after every session, and a heparinized glycerol lock solution (500 U/ml heparin LEO, 50:50 heparin/glycerol) was used over weekends. Catheter patency was tested before the start of the study with an infusion of the short-acting anesthetic propofol (Rapinovet; 10 mg/ml; Schering-Plough Animal Health, Ballerup, Denmark). The patency test was also performed during the course of the study in the event there were signs of nonsatisfactory catheter function. Failure to respond to propofol resulted in recatheterization in the left jugular vein (n = 4).
Apparatus.
Eight operant chambers (Med Associates Inc.), each equipped with two retractable response levers, two cue lights, a house light, and an infusion pump (PHM-100, 3.33 rpm; Med Associates Inc.), were used. Each chamber was enclosed in a sound-attenuating cubicle, and a ventilating fan that operated throughout the sessions served as “white noise” (Med Associates, Inc). A 10-ml plastic syringe placed in the pump was connected to the implanted catheter through CoEx tubing (Harvard Apparatus, Kent, UK) and protected by a flexible metal leash (CamCaths). Experiments were run and data collected by SARAWin PC Software (Ellegaard Systems A/S).
Self-Administration Training and Testing.
Rats were food-trained on an FR1 schedule of reinforcement. After catheter implantation, intravenous self-administration was initiated with cocaine (0.5 mg/kg per infusion), available on an FR5 schedule during daily 3-hour sessions. Once stable self-administration behavior was achieved (>10 delivered infusions and <15% variation in the number of infusions per rat during three consecutive sessions), vehicle was substituted for cocaine to cause extinction of responding (≤10 delivered infusions for 3 consecutive days). Dose-response curves were generated for PCP (0.03–1.0), MPEP (0.1–3.0), and MTEP (0.01–3.0; all doses in mg/kg per infusion) in separate groups of animals (n = 8). Each dose was given until stable self-administration behavior or extinction was reached and was preceded by a cocaine session to re-establish responding if the animal did not respond to the previous dose.
Data Analysis.
Self-administration data from FR5 sessions are presented as the mean (±S.E.M.) of the number of delivered infusions every 3 hours and of intake (mg/kg) every 3 hours, respectively. Statistical analysis on group values was performed using a one-way analysis of variance followed by Dunn’s multiple comparisons test (GraphPad Prism, GraphPad Software Inc.) when appropriate.
Drugs
MTEP (AstraZeneca R&D, Mölndal, Sweden) was dissolved in 20% hydroxypropyl-β-cyclodextrin (HPβCD) and 80% water, MPEP (AstraZeneca R&D) was dissolved in 5% HPβCD and 95% water, and fenobam (AstraZeneca R&D) was dissolved in 40% -HPβCD and 60% sterile water. (−)-Δ9-THC (100 mg/ml in ethanol; Lipomed AG, Arlesheim, Switzerland) was pipetted into vials and exposed to a slow flow of nitrogen gas to evaporate the ethanol. A mixture of PEG and Tween 80 was added to the (−)-Δ9-THC such that in the planned final solution the concentration of PEG and Tween 80 reached 3% to 5%. (+)-MK-801 hydrogen maleate (Research Biochemicals, Inc., Natick, MA), phencyclidine (PCP; Lipomed), arecoline HBr (Sigma-Aldrich), pentylenetetrazole (PTZ; Sigma-Aldrich), chlordiazepoxide HCl (Sigma-Aldrich), ketamine HCl (Sigma-Aldrich), and memantine HCl (Research Biochemicals, Inc.) were dissolved in saline. Yohimbine HCl (Sigma-Aldrich) was dissolved in sterile water. Lysergic acid diethylamide (LSD; Lipomed) was dissolved in 5% acetic acid and 5% mannitol, and pH was adjusted to ∼5 by adding NaOH). MTEP was administered in volumes of 10 ml/kg. MPEP was administered at 4 ml/kg (PCP study) or 10 ml/kg (MTEP study); fenobam was administered at 5 ml/kg (PCP study) or 10 ml/kg (MTEP study), and all other drugs were given at 2 ml/kg. Doses given refer to the weight of the base. The following routes of administration and pretreatment times were used: PCP (i.p., 15 minutes), (+)-MK-801 (i.p., 15 minutes), ketamine (i.p., 15 minutes), fenobam (p.o., 30 minutes), MPEP (p.o., 15 minutes), memantine (i.p., 15 minutes), MTEP (p.o., 15 minutes, PCP study), MTEP (i.p., 30 minutes, MTEP study), LSD (s.c., 30 minutes and i.p., 15 minutes), chlordiazepoxide (i.p., 30 minutes), yohimbine (s.c., 30 minutes), arecoline (s.c., 30 minutes), PTZ (i.p., 30 minutes) and (−)-Δ9-THC (i.p., 20 minutes). Drug discrimination and self-administration studies are key pharmacologic tools to determine psychoactive and reinforcing effects of drugs as part of abuse liability assessment, requiring exposures including and exceeding estimated therapeutic levels to cover nonintended use, typically self-medication beyond recommended doses (European Medicines Agency, 2006; International Conference on Harmonization, 2009; Food and Drug Administration, 2010).
Results
Rats were successfully trained to discriminate PCP at 2.8 mg/kg i.p., 15 minutes, or MTEP at 2 mg/kg i.p., 30 minutes, from no drug. The number of sessions required to reach the criterion for testing (see Materials and Methods), including shaping sessions (FR1–FR7) and training sessions (FR10) for two representative groups (n = 8/group) of rats were 43.38 (range, 29–59) for PCP, and 55.63 (range, 37–71) for MTEP (Table 1). Further scrutiny of the results show that the difference between the two groups lies in the FR10 phase in which the MTEP group required 12 more sessions to reach the testing criteria (Table 1).
Sessions required to reach the criteria in the different phases of the acquisition of phencyclidine (PCP, 2.8 mg/kg) and MTEP (2.0 mg/kg) discrimination
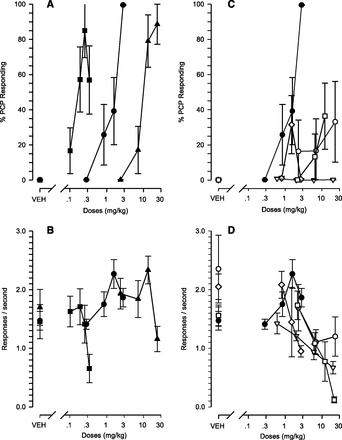
PCP caused a dose-dependent increase in PCP-appropriate responding with an ED50 of 1.55 mg/kg in rats trained to discriminate 2.8 mg/kg i.p. PCP, 15 minutes, from no drug (Fig. 1, A and C; Table 2). Rates of responding showed a small increase with dose (Fig. 1, B and D; Table 2). (+)-MK-801 caused a full and dose-dependent increase in PCP-appropriate responding with an ED50 of 0.18 mg/kg and a decrease at the highest dose (Fig. 1A; Table 2), at which dose rates of responding had decreased to 45% of vehicle-alone rates (Fig. 1B; Table 2). Ketamine caused a full and dose-dependent increase in PCP-appropriate responding with an ED50 of 10.1 mg/kg (Fig. 1A; Table 2) and a reduction in rates of responding to 68% of vehicle alone at the highest dose (Fig. 1B; Table 2).
Percent PCP-appropriate responding (mean ± S.E.M.) (A and C) and responses per second (mean ± S.E.M.) (B and D) in male Wistar rats trained to discriminate PCP (2.8 mg/kg, 15 minutes after i.p. administration) from no drug. (A and B) PCP (i.p., 15 minutes, filled circles), (+)-MK-801 (i.p., 15 minutes, squares) and ketamine (i.p., 15 minutes, triangles). (C and D) PCP (i.p., 15 minutes, filled circles), fenobam (p.o., 30 minutes, diamonds), MPEP (p.o., 15 minutes, open circles), MTEP (p.o., 15 minute, triangles), and memantine (i.p., 15 minutes, squares). Note: At the highest dose of memantine only one of eight rats produced nine or more reinforcements.
Phencyclidine (PCP) discrimination, maximal % PCP responding ED50 and confidence levels (CL), and maximal effects on response rates
Memantine caused a partial and dose-dependent increase in PCP-appropriate responding with a maximal effect of 36.37% at 12.08 mg/kg; at the highest dose, 21.58 mg/kg, only one rat produced more than nine food pellets and 88.5% of the responses were emitted on the PCP-appropriate lever (Fig. 1C; Table 2). Rates of responding after memantine decreased dose-dependently, causing a maximal decrease to 8% of vehicle alone (Fig. 1D; Table 2).
Fenobam caused partial PCP-appropriate responding (31.52%) (Fig. 1C; Table 2) at a dose that reduced rates of responding to 71% of vehicle-alone rates (Fig. 1D; Table 2), whereas at the next higher dose, 2.69 mg/kg, PCP-appropriate responding was reduced to 0.45% (Fig. 1C; Table 2), and response rates were reduced to 46% of vehicle-alone rates (Fig. 1D; Table 2).
MPEP caused increased PCP responding with a maximal effect of 33.1% at a dose of 22.97 mg/kg (Fig. 1C; Table 2), reducing the rates of responding to 51% of vehicle-alone rates (Fig. 1D; Table 2). MTEP caused no PCP-appropriate responding (<1% at all doses; Fig. 1C; Table 2) up to a dose-reducing rates of responding to 43% of vehicle-alone rates (Fig. 1D; Table 2).
MTEP caused a dose-dependent increase in MTEP-appropriate responding with an ED50 of 0.42 mg/kg and a maximal effect of 99.01% at 2.0 mg/kg (Fig. 2A; Table 3), whereas rates of responding were not affected (Fig. 2B; Table 3).
Percent of MTEP-appropriate responding (mean ± S.E.M.) (A, C, and E) and responses per second (mean ± S.E.M.) (B, D, and F) in male Wistar rats trained to discriminate MTEP (2.0 mg/kg, 30 minutes after i.p. administration) from no drug. (A and B) MTEP (i.p., 30 minutes, filled circles), MPEP (p.o., 15 minutes, triangles up), fenobam (p.o., 30 minutes, triangles down), (+)-MK-801 (i.p., 15 minutes, squares), and memantine (i.p., 15 minutes, black diamonds). (C and D) MTEP (i.p., 30 minutes, filled circles), PCP (i.p., 15 minutes, squares), LSD (s.c., 30 minutes, upward triangles), LSD (i.p., 15 minutes, downward triangles), chlordiazepoxide (i.p., 30 minutes, open circles). (E and F) MTEP (i.p., 30 minutes, filled circles), yohimbine (s.c., 30 minutes, squares), arecoline (s.c., 30 minutes, open circles), PTZ (i.p., 30 minutes, hexagons), and (−)-Δ9-THC (i.p., 20 minutes, diamonds).
MTEP discrimination, maximal % MTEP responding ED50 and confidence levels, and maximal effects on response rates
MPEP caused a dose-dependent increase in MTEP-appropriate responding with an ED50 of 0.62 mg/kg and a maximal effect of 99.97% at 6.89 mg/kg (Fig. 2A; Table 3) and a maximal response-rate decrease to 91% of vehicle alone (Fig. 2B; Table 3).
Fenobam caused a dose-dependent increase in MTEP-appropriate responding with an ED50 of 0.96 mg/kg and a maximal effect of 100% at 8.06 mg/kg (Fig. 2A; Table 3) and a maximal response-rate decrease to 87% of vehicle alone (Fig. 2B; Table 3).
(+)-MK-801 caused maximal MTEP appropriate responding of 67.3% at 0.19 mg/kg (Fig. 2A; Table 3), with an ED50 of 0.15 mg/kg, and a maximal response-rate decrease to 74% of vehicle-alone rates (Fig. 2B; Table 3). (+)-MK-801 at 0.1 mg/kg caused 25.58% MTEP-appropriate responding (Fig. 2A), and rates of responding were not affected (Fig. 2B).
Memantine caused a dose-dependent partial increase in MTEP-appropriate responding with an ED50 of 11.42 mg/kg and a maximal effect of 54.25% at 12.95 mg/kg (Fig. 2A; Table 3) and a maximal response rate decrease to 47% of vehicle alone (Fig. 2B; Table 3). At 6.47 mg/kg memantine, MTEP responding was 33.3% (Fig. 2A), and rates of responding were not affected (Fig. 2B).
PCP caused a maximal MTEP-appropriate responding of 27.55% at 2.8 mg/kg (Fig. 2C; Table 3), and rates of responding at this dose was 90% of vehicle-alone rates (Fig. 2D; Table 3).
LSD after subcutaneous administration caused maximal MTEP-appropriate responding of 47.3% at 0.3 mg/kg (Fig. 2C; Table 3), and rates of responding at this dose were 33% of vehicle-alone rates (Fig. 2D; Table 3), whereas LSD at 0.1 mg/kg caused 6.98% MTEP responding (Fig. 2C), and rates of responding were not affected (Fig. 2D). LSD after intraperitoneal administration caused maximal MTEP-appropriate responding of 35.44% at 0.3 mg/kg (Fig. 2C; Table 3), and rates of responding at this dose were 46% of vehicle-alone rates (Fig. 2D; Table 3), whereas LSD at 0.1 mg/kg after intraperitoneal administration caused 24.59% MTEP responding (Fig. 2C), and rates of responding were not affected (Fig. 2D).
Chlordiazepoxide caused a dose-dependent increase in MTEP appropriate responding with a maximal effect of 40.04% at the highest dose, 18.8 mg/kg (Fig. 2C; Table 3), and rates of responding at this dose were reduced to 49% of vehicle-alone rates (Fig. 2D; Table 3).
(−)-Δ9-THC caused maximal MTEP-appropriate responding of 16.67% at 0.09 mg/kg (Fig. 2E; Table 3), and rates of responding were reduced to 38% of vehicle-alone rates at 0.94 mg/kg (Fig. 2F; Table 3). Yohimbine caused MTEP-appropriate responding of <9% at any dose (Fig. 2E; Table 3), and a maximal reduction in rates of responding to 91% of vehicle alone occurred at 3 mg/kg (Fig. 2F; Table 3). Arecoline at 5.6 mg/kg caused maximal MTEP-appropriate responding of 19.74% (Fig. 2E; Table 3) and a response-rate reduction to 41% of vehicle alone (Fig. 2F; Table 3), and at 10 mg/kg arecoline none of the rats was able to respond.
PTZ caused MTEP-appropriate responding of <1% at any dose (Fig. 2E; Table 3), and a maximal reduction in rates of responding to 71% of vehicle-alone rates occurred at 17.5 mg/kg (Fig. 2F; Table 3).
When cocaine at 0.5 mg/kg per infusion was available, rats self-administered a total mean (±S.E.M.) of 46.6 (7.8), 35.9 (1.6), and 34.8 (1.1) infusions in the PCP, MPEP, and MTEP groups, respectively (Fig. 3, A–E), whereas the same groups self-administered saline at a mean (±S.E.M.) of 5.2 (0.5), 4.4 (0.6), and 5.8 (0.9) infusions, respectively. PCP (0.03–1.0 mg/kg per infusion) maintained self-administration, and the number of infusions over the 3-hour session yielded an inverted U-shaped dose-response curve with a maximal mean (±S.E.M.) intake of 31.0 (6.5) infusions at 0.1 mg/kg per infusion (statistically significant difference from vehicle, P = 0.01; Fig. 3A). The total amount of PCP self-administered increased with dose (Fig. 3B), reaching a maximal mean (±S.E.M.) total intake of 10.6 (2.7) mg/kg over the 3-hour session. MPEP (0.1–3 mg/kg per infusion; Fig. 3C) and MTEP (0.01–3 mg/kg per infusion; Fig. 3E) did not maintain self-administration, as the infusion rates were less than 7.5 and 6.5, respectively, over the 3-hour session. The MPEP and MTEP groups reached maximal mean (±S.E.M.) total intakes of 13.9 (1.6) and 6.0 (1.7) mg/kg, respectively, over the 3-hour session at the highest doses (Fig. 3, D and F).
Infusions delivered on a FR5 schedule of reinforcement (A, C, and E) and total drug intake over the 3-hour session (B, D, and E) in rats trained to self-administer cocaine (0.5 mg/kg per infusion). (A and B) PCP. (C and D) MPEP. (E and F) MTEP.
Discussion
In-house observations suggest contrasting in vivo effects of NMDA and mGluR5 antagonists (unpublished results). Whereas uncompetitive NMDA antagonists typically cause dose-dependent symptoms such as hyperlocomotion and ataxia, mGluR5 antagonists at higher doses typically cause sedative effects, including reduced locomotor activity and flat body posture, consistent with other observations (Pietraszek et al., 2005). Other data show mGluR5 antagonist–enhanced NMDA antagonist effects on prepulse inhibition (Pietraszek et al., 2005). Clinical findings of hallucinations caused by fenobam (Friedmann et al., 1980; Pecknold et al., 1982) and in vitro electrophysiology data showing modulation of functional effects of NMDA in rat neuronal cells by MPEP (Movsesyan et al., 2001) suggested the need to characterize the psychoactive effects of mGluR5 antagonists.
Since PCP causes psychotomimetic effects in man and is readily discriminated by animals and humans, PCP drug discrimination procedures are used to predict psychotomimetic effects of novel glutamate antagonists (e.g., Koek et al., 1990; Swedberg et al., 1995). Koek (1999) concluded that NMDA antagonists producing intermediate or full PCP-like discriminative effects in animals exert PCP-like effects in humans, whereas NMDA antagonists lacking PCP-like discriminative effects in animals appear not to produce PCP-like effects in humans. Our data on NMDA antagonists in PCP trained rats are consistent with those and other findings, as is the lack of PCP-like discriminative effects of the AMPA antagonist NBQX (Swedberg et al., 1995).
PCP in the present study yielded an ED50 of 1.55 mg/kg and a response-rate increase to 127% of vehicle-alone rates at the lowest dose, causing a 90% or greater PCP-appropriate responding (2.8 mg/kg), in agreement with Swedberg et al. (1995), reporting a PCP ED50 of 1.75 mg/kg and a response rate increase to 118% of vehicle-alone rates at the lowest dose causing 90% or greater PCP-appropriate responding. Additionally, in the present study, (+)-MK-801 yielded an ED50 of 0.18 mg/kg and maximal PCP-appropriate responding of 85% at 0.25 mg/kg with a response rate of 97% of vehicle alone compared with an ED50 of 0.15 mg/kg and a maximal PCP-appropriate responding of 99% at 0.2 mg/kg with a response rate of 64% of vehicle alone (Swedberg et al., 1995). These comparisons demonstrate the robustness and reproducibility of the assay with minimal differences in methods. Swedberg et al. (1995) used a PCP training dose of 3 mg/kg, whereas currently it was 2.8 mg/kg.
Ketamine, similar to PCP and (+)-MK-801, a drug with known NMDA antagonist action (Koek et al., 1990; Ellison, 1995; Swedberg et al., 1995) caused a dose-dependent increase and full PCP-appropriate responding at a dose causing no significant reduction in response rate compared with vehicle-alone levels, consistent with Swedberg et al. (1995). Memantine, an uncompetitive and low- to moderate-affinity NMDA antagonist caused severe disruption in responding such that only one of eight rats responded at the highest dose (21.58 mg/kg), consistent with previous findings in rats trained to discriminate (+)-MK-801 from no drug (Grant et al., 1996) and in rats trained to discriminate PCP from no drug (Nicholson et al., 1998), whereas in monkeys memantine caused full PCP-appropriate responding without severe response-rate suppression (Nicholson et al., 1998). At the next lower dose of memantine (12.08 mg/kg) in the present study, seven of eight rats responded and the mean PCP-appropriate responding was 36%.
The mGluR5 antagonists fenobam and MPEP caused non–dose-dependent partial PCP-appropriate responding with maximal effects below 34% up to doses causing significant response-rate reduction, whereas MTEP caused no PCP-appropriate responding up to a dose causing significant response-rate reduction. These findings suggest that the partial PCP-like effects of fenobam and MPEP could be attributable to their lower selectivity with regard to NMDA antagonist activity (O’Leary et al., 2000; Movsesyan et al., 2001) and the lack of PCP-like effects of MTEP attributed to its increased selectivity (Cosford et al., 2003).
O’Leary et al. (2000) reported a MPEP concentration-dependent decrease in NMDA-induced lactate dehydrogenase release, observing statistically significant effects of MPEP at 20 µM (∼70% reduction) and at 200 µM (∼90% reduction). Extrapolating from available observations on dose to brain-exposure relations, 5 mg/kg and 0.15 µM (Nagel et al., 2007) and 3 mg/kg and 0.83 µM (Cosford et al., 2003), brain-exposure levels at the high dose of MPEP in this study (22.97 mg/kg) would be predicted to 0.7–6.4 µM, 30- to 3-fold below the active NMDA antagonist concentration (20 µM) reported by O’Leary et al. (2000). In vitro profiling (Cosford et al., 2003) shows MPEP (18 µM) bound more potently than MTEP (>300 µM) to NR2B receptors, which have been reported to mediate PCP-like discriminative effects in rats (Nicholson et al., 2007). These findings would not be inconsistent with NMDA or NR2B antagonism mediating the partial PCP-like discriminative effects of MPEP in this study; however, it should be emphasized that the MPEP dose (22.97 mg/kg) causing partial (33.12%) PCP-like effects is three times higher than the dose (6.98 mg/kg), causing full (99.97%) MTEP-like effects, suggesting that psychoactive effects in humans would reflect mGluR5 antagonist mechanisms before and if any NMDA antagonist effects develop.
The co-occurrence of partial or no PCP-appropriate responding with severe suppression of response rates clearly demonstrates that drug formulation or pharmacokinetic factors such as absorption, distribution, metabolism, or excretion are not attributable for the lack of consistent PCP-like discriminative effects of mGluR5 antagonists. Rats readily learned to discriminate MTEP from no drug within 55.63 sessions compared with 44.38 sessions for PCP, with overlapping ranges showing a comparable speed of acquisition.
Fenobam, MPEP, and MTEP had similar potencies and efficacies both in terms of MTEP-appropriate responding and effects on response rates, fenobam being somewhat less potent than MPEP and MTEP, consistent with [3H]MPEP and [3H]fenobam binding data (Porter et al., 2005). MPEP, MTEP, and fenobam inhibited [3H]MPEP binding to rat mGluR5 receptor transfected HEK-293 cell membranes with mean Ki values of 3.1, 5.4, and 50.8 nM and inhibited [3H]fenobam binding in the same assay with mean Ki values of 8.7, 17.8, and 56.3 nM (Porter et al., 2005). The corresponding in vitro and in vivo potencies and efficacies strengthen the conclusion that the MTEP-like discriminative effects of these compounds are attributable to mGluR5 receptor antagonism.
PCP caused approximately 27% MTEP-appropriate responding at the highest dose, 2.8 mg/kg, but not at lower doses, whereas (+)-MK-801 caused a partial, dose-related increase with a maximum of 67% at the highest dose tested (0.19 mg/kg) and reduced response rates to 74% of vehicle alone. Likewise, memantine caused a partial dose-related increase with a maximum of 54% MTEP-appropriate responding at a dose (12.95 mg/kg) causing response rate reduction to 47% of vehicle-alone rates. These findings suggest that (+)-MK-801 and memantine and, to some degree PCP in addition to their NMDA antagonist properties, may to a lesser degree share some similar downstream functional effects consistent with notions that subtle differences in NMDA antagonist mechanisms may strongly impact neuronal effects (Johnson and Kotermanski, 2006).
Chlordiazepoxide caused a partial, dose-related increase in MTEP-appropriate responding peaking at 40% at a dose (56 mg/kg) lowering response rates to 49% of vehicle rates, and, although inconclusive, not inconsistent with observations of anxiolytic activity of MTEP.
LSD caused non–dose-related partial MTEP-like discriminative effects peaking at 35 to 47% depending on the route of administration but at a dose (0.3 mg/kg) that reduced response rates to 33% and 46% of vehicle alone. Speculatively, the LSD findings may relate to the prominent and robust discriminative effects caused by MTEP and suggest some commonalities in the psychoactive properties, but further studies based on these findings need to be undertaken for any conclusions to be made.
Yohimbine, arecoline, (−)-Δ9-THC, and PTZ caused less than 20% MTEP-appropriate responding up to doses causing substantial lowering of response rates, further demonstrating the selectivity of the MTEP drug discrimination assay.
We conclude that mGluR5 antagonist discriminative effects are selective and clearly dissimilar to other classes of psychoactive drugs, even though in some cases weak partial non–dose-dependent effects occurred, and we re-emphasize that all non-mGluR5 antagonists causing MTEP-appropriate responding did so at doses substantially suppressing response rates.
PCP, but not MPEP or MTEP, maintained self-administration, suggesting different reinforcing properties of mGluR5- and NMDA-antagonists. Results suggest that whereas mGluR5 antagonists produce distinct psychoactive effects, mGluR5 antagonism may not cause abuse as reflected in lack of self-administration. However, as per a proactive strategy for assessment of abuse liability (Swedberg, 2013), mGluR5 antagonists should also be tested in primates (Weerts et al., 2007) to further explore and characterize these compounds.
In conclusion, the present data show that mGluR5 receptor antagonists and NMDA receptor antagonists produce distinctly different discriminative effects, demonstrating that mGluR5-antagonism may produce psychoactive and psychotomimetic effects different from those caused by NMDA antagonism and other known mechanisms mediating psychotomimetic effects. We conclude that the reported psychostimulant and hallucinogenic effects of mGluR5 antagonists in humans are most likely not caused by NMDA antagonism but by a specific antagonism of mGluR5 receptors.
Acknowledgments
The authors thank Maria Ståhlberg, Charlotte Velasquez, and Pernilla Hammar for technical support and Dr. Samantha Budd for the publication of these data. All authors were employees of AstraZeneca at the time these studies were conducted.
Authorship Contributions
Participated in research design: Swedberg, Ellgren, Raboisson.
Conducted experiments: Swedberg, Ellgren.
Performed data analysis: Swedberg, Ellgren.
Wrote or contributed to the writing of the manuscript: Swedberg, Ellgren.
Footnotes
- Received November 9, 2013.
- Accepted January 28, 2014.
↵1 Current affiliation: Independent Consultant, Drug Discovery Pharmacology, Trosa, Sweden.
↵2 Current affiliation: Neuropharmacology, Addiction & Behaviour, Department of Pharmaceutical Biosciences, Uppsala University, Uppsala, Sweden.
↵3 Current affiliation: Galderma R&D SNC, Sophia Antipolis, France.
Portions of these data were previously presented at the following: 72nd Annual Meeting of the College of Problems of Drug Dependence; 12–17 June 2010; Scottsdale, AZ; the 10th Annual Meeting of the Safety Pharmacology Society; 20–23 Sept 2010; Boston, MA (Ellgren et al., 2011; Swedberg, 2011); and the 11th Annual Meeting of the Safety Pharmacology Society; 19–22 Sept 2011; Innsbruck, Austria (Swedberg et al., 2012).
Abbreviations
- AMPA
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CNS
- central nervous system
- EAA
- excitatory amino acid
- McN-3377
- (fenobam) N-(3-chlorophenyl)-N′-(4,5-dihydro-1-methyl-4-oxo-1H-imidazole-2-yl)urea
- FR
- fixed ratio
- HPβCD
- hydroxypropyl-β-cyclodextrin iGluR, ionotropic glutamate receptors
- LSD
- lysergic acid diethylamide
- mGluR
- metabotropic glutamate receptor
- (+)-MK-801
- dizocilpine
- MPEP
- 2-methyl-6-(phenylethynyl)pyridine
- MTEP
- 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine
- NBQX
- 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline
- NMDA
- N-methyl-d-aspartate
- PCP
- phencyclidine
- PTZ
- pentylenetetrazole
- SIB-1893
- (E)-2-methyl-6-styryl-pyridine
- (−)-Δ9-THC
- (−)-Δ9-tetrahydrocannabinol
- Copyright © 2014 by The American Society for Pharmacology and Experimental Therapeutics