Abstract
Antitumor drugs may cause asymptomatic diastolic dysfunction that introduces a lifetime risk of heart failure or myocardial infarction. Cardio-oncology is the discipline committed to the cardiac surveillance and management of cancer patients and survivors; however, cardio-oncology teams do not always attempt to treat early diastolic dysfunction. Common cardiovascular drugs, such as β blockers or angiotensin-converting enzyme inhibitors or others, would be of uncertain efficacy in diastolic dysfunction. This perspective describes the potential value of ranolazine, an antianginal drug that improves myocardial perfusion by relieving diastolic wall tension and dysfunction. Ranolazine acts by inhibiting the late inward sodium current, and pharmacological reasonings anticipate that antitumor anthracyclines and nonanthracycline chemotherapeutics might well induce anomalous activation of this current. These notions formed the rationale for a clinical study of the efficacy and safety of ranolazine in cancer patients. This study was not designed to demonstrate that ranolazine reduced the lifetime risk of cardiac events; it was designed as a short term proof-of-concept study that probed the following hypotheses: 1) asymptomatic diastolic dysfunction could be detected a few days after patients completed antitumor therapy, and 2) ranolazine was active and safe in relieving echocardiographic and/or biohumoral indices of diastolic dysfunction, measured at 5 weeks or 6 months of ranolazine administration. These facts illustrate the translational value of pharmacology, which goes from identifying therapeutic opportunities to validating hypotheses in clinical settings. Pharmacology is a key to the success of cardio-oncology.
Introduction
Cardio-oncology is the discipline that cares for the heart of cancer patients; in fact, conventional chemotherapeutics and some of the newer “targeted” drugs may cause cardiovascular toxicities that call for preventive or curative measures (Minotti et al., 2010). Cardio-oncology rests with an in-depth understanding of the mechanisms of cardiotoxicity and a good collaboration between oncologists and cardiologists. Unfortunately, the mechanisms of toxicity are only in part understood, and the timing and appropriateness of cardiovascular medications in cancer patients remain highly debated. Pharmacologists may help cardiologists and oncologists by providing mechanistic insight and by identifying cardiovascular drugs that are proved safe and effective in patients at risk for cardiotoxicity. This perspective describes how pharmacological reasonings identified ranolazine as a valuable option and formed the rationale for a proof-of-concept phase IIB study with this drug.
General Concepts on Cardiotoxicity from Antitumor Drugs
Doxorubicin and other anthracyclines induce both acute and chronic cardiotoxicity. Acute cardiotoxicity occurs shortly after initiation of an anthracycline regimen and consists of arrhythmias, hypotension, and mild depression of contractile function. Acute cardiotoxicity is relatively infrequent and usually reversible. In contrast, chronic cardiotoxicity develops in a dose-related manner and manifests as potentially life-threatening cardiomyopathy and heart failure (HF) (Minotti et al., 2004). Nonanthracycline chemotherapeutics (alkylators, antimetabolites, tubulin-active agents) may cause coronary endothelial dysfunction and spasm that manifest as ischemia or arrhythmias, but the dose-dependence of such events is less obvious. Targeted agents (antibodies, kinase inhibitors) tend to induce transient cardiac dysfunction, but some of them may render the heart more vulnerable by anthracyclines. With inhibitors of angiogenesis, cardiotoxicity is aggravated by class-related effects like microvasculature dysfunction, hypertension, and thromboembolism (Kamba and McDonald, 2007; Schmidinger et al., 2008). Mechanisms and manifestations of cardiotoxicity from conventional chemotherapeutics or newer drugs have been reviewed elsewhere (Menna et al., 2012; Lal et al., 2013; Suter and Ewer, 2013).
Cardiotoxicity and the Risk/Benefit of Antitumor Drugs.
In patients without preexisting cardiovascular risk factors, cumulative doses of 400 mg/m2 doxorubicin introduce a 5% risk of HF (Swain et al., 2003). Cumulative doses of 240–360 mg/m2 doxorubicin cause much lower risk but retain life-saving effects (Gianni et al., 2008). More than 3 million Americans are entering their 5th to 10th year of survival since cancer diagnosis, and many of them received low dose anthracycline regimens (American Cancer Society, 2012). Nevertheless, studies of breast cancer survivors show that HF may develop 5–10 years after completing chemotherapy (Pinder et al., 2007). Studies of long-term childhood cancer survivors show that >250 mg/m2 anthracycline was high enough to increase the lifetime risk of HF (Mulrooney et al., 2009), whereas 100 mg/m2 anthracycline was high enough to cause asymptomatic cardiac dysfunction (Hudson et al., 2007). Survivors of childhood or adult cancer also show higher incidence of myocardial infarction (MI), which correlates with exposure to anthracyclines (Minotti et al., 2010). These observations suggest that there is no completely “safe” dose of anthracyclines in children (Barry et al., 2007) or adults (Menna et al., 2012). In other studies, the risk of delayed MI correlated with patient’s exposure to alkylators like platinum (Altena et al., 2009) or tubulin-active agents like vincristine (Swerdlow et al., 2007). These findings denote that with current treatment protocols, anthracyclines and nonanthacycline chemotherapeutics are life-saving but introduce a lifetime risk of cardiac events.
Antitumor Drugs and Early Diastolic Dysfunction.
Asymptomatic cancer survivors often present echocardiographic indices of diastolic dysfunction characterized by altered relaxation or restrictive pattern (stiffness) (Carver et al., 2007; Altena et al., 2009). In nononcologic settings, persistent altered relaxation or stiffness cause left ventricle (LV) remodeling that makes diastolic dysfunction progress to HF with preserved left ventricle ejection (LVEF) and eventually to HF with reduced LVEF (Borlaug and Paulus, 2011). Diastolic dysfunction can also cause and be caused by ischemia. Myocardial stiffness and remodeling increase interstitial pressure and diminish coronary conductance, eventually inducing ischemia that aggravates myocardial stiffness by cytoplasmic Ca2+ overload and other mechanisms (Hale et al., 2008). In oncologic settings, the progression of cardiotoxicity from asymptomatic diastolic dysfunction to HF or ischemic disease may be driven by several factors. Cancer survivors are more susceptible to develop comorbidities that induce or aggravate diastolic dysfunction (hypertension, diabetes, dyslipidemia) (Armstrong et al., 2012). It follows that asymptomatic diastolic dysfunction may progress toward HF or ischemic disease by synergizing with risk factors (“hits”) that matured after completing chemotherapy. Unintentional hits may also come from targeted therapies or mediastinal irradiation administered concomitant with or after chemotherapy. The so-called “multiple hits hypothesis of cardiotoxicity” recapitulates such a broad spectrum of possibilities (Menna et al., 2008).
Unmet Needs: Drugs against Diastolic Dysfunction and Proof-of-Concept Studies
The available evidence suggests that diastolic dysfunction could be detected a few months after ending chemotherapy (Tassan-Mangina et al., 2006), but the possibility that it developed early during the course of chemotherapy was not investigated. This calls for proof-of-concept studies that characterized diastolic dysfunction as the earliest consequence of antitumor therapies and probed drugs that could safely prevent it.
Dexrazoxane is the only drug approved for preventing anthracycline-related cardiotoxicity. Dexrazoxane chelates redox-active iron and can also divert topoisomerase IIβ from causing DNA double-strand breaks associated with altered mitochondrial biogenesis and function. In either case, dexrazoxane diminished formation of reactive oxygen species (ROS) in the relatively unprotected heart (Lyu et al., 2007; Menna et al., 2012; Zhang et al., 2012). Clinical use of dexrazoxane has been limited by unconfirmed reports that it could interfere with anthracycline activity in tumors (Swain et al., 1997); therefore, current guidelines recommend using dexrazoxane only in patients who received 300 mg/m2 doxorubicin and may benefit from continued anthracycline treatment (Schuchter et al., 2002).
Common cardiovascular drugs [β blockers, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), Ca2+ antagonists] prevented chemotherapy-induced LVEF decreases in some limited studies, but this reflected their ability to reduce heart rate-pressure products rather than their ability to prevent an earlier diastolic dysfunction (Menna et al., 2012). Many doctors also believe that discomforts from chemotherapy (fatigue, nausea, vomiting) should not be aggravated by discomforts from β blockers, ACEIs, ARBs, or Ca2+ antagonists (bradycardia, hypotension, cough, fluid retention). There is an unmet need for drugs that act on diastolic dysfunction in a specific manner (Paulus and van Ballegoij, 2010).
Ranolazine
The orally available piperazine derivative, ranolazine, was approved by the US Food and Drug Administration as a first-line or top-on-therapy agent for the treatment of stable angina. In Europe, ranolazine was approved for the treatment of stable angina in patients who are inadequately controlled by, or intolerant to, other antianginal drugs (β blockers, ACEIs, ARBs, calcium antagonists). Ranolazine reduces angina episodes, nitrate consumption, and uptitration of other antianginal drugs, and importantly, it does so without reducing heart rate-pressure products (Stone, 2008).
In ischemia-reperfusion or anoxia-reoxygenation, fatty acid oxidation prevails over glucose oxidation and causes decreased recovery of cardiac energy during reperfusion. Ranolazine was originally believed to protect the heart by inhibiting fatty acid oxidation and by shifting metabolism toward glucose oxidation; in preclinical models, however, inhibition of fatty acid oxidation occurred at concentrations of ranolazine that were much higher than those associated with the beneficial effects of ranolazine in ischemia-reperfusion or anoxia-reoxygenation (≥100 µM versus ≤20 µM, respectively) (Matsumura et al., 1998; MacInnes et al., 2003). The preponderance of evidence now shows that ranolazine acts by inhibiting the late inward sodium current (INa,Late) (Belardinelli et al., 2006; Hale et al., 2008).
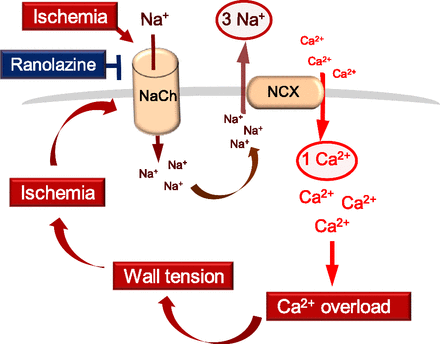
In the repolarizing ischemic LV, there is delayed and/or incomplete inactivation of INa,Late. This causes elevation of intracellular Na+, which exchanges with extracellular Ca2+ via the reverse mode Na+-Ca2+ exchanger. Excess Ca2+ entry activates myofilaments, increases diastolic wall tension, and reduces coronary conductance (Hale et al., 2008). The ischemic heart therefore hosts a vicious cycle in which ischemia begets ischemia by activating INa,Late and by causing diastolic wall tension. Ranolazine interrupts this vicious cycle by inhibiting INa,Late (Fig. 1).
Ranolazine-inhibitable INa,Late and its role in diastolic dysfunction and ischemia. In the ischemic myocardium, delayed and/or incomplete inactivation of INa,Late causes elevated intracellular Na+ that exchanges with extracellular Ca2+ via the reverse mode Na+-Ca2+ exchanger. Excess Ca2+ entry causes diastolic wall tension, and the latter causes ischemia that perpetuates INa,Late activation. By inhibiting INa,Late, ranolazine interrupts the vicious cycle between ischemia and diastolic dysfunction. NaCh, Na+ channel; NCX, Na+-Ca2+ exchanger.
Ranolazine inhibits INa,Late in a concentration-, voltage-, and frequency-dependent manner, and it does so with an IC50 value (approximately 6 µM) that compares well with its therapeutic plasma levels (2–6 µM). Ranolazine does not inhibit early peak INa (IC50 ≥300 µM) but it can marginally inhibit the delayed rectifier K+ current (IKr) or the late inward Ca2+ current (ILate,Ca2+) with IC50 values of 12 or 50 µM, respectively (Antzelevitch et al., 2004; Stone, 2008). The net balance of the effects of ranolazine on inward depolarizing currents (INa,Late, ILate,Ca2+) or outward repolarizing currents (IKr) translates into modest changes of the action potential duration [approximately 5–10 milliseconds prolongation of corrected QT interval in patients treated with 1000 mg BID of ranolazine] (Scirica et al., 2007). Different Na channel isoforms have been characterized and codenamed Nav1.1 to Nav1.8. The majority of studies identified Nav1.5 as the cardiac channel isoform associated with INa,Late (Maltsev and Undrovinas, 2008).
In preclinical models, ranolazine improved diastolic relaxation (“positive lusitropic effect”) in isolated rat hearts exposed to ischemia-reperfusion (Hwang et al., 2009) and in isometrically contracting ventricular muscle strips from end-stage failing human hearts (Sossalla et al., 2008).
Pharmacologic Rationale to Probe Ranolazine in Diastolic Dysfunction from Antitumor Drugs.
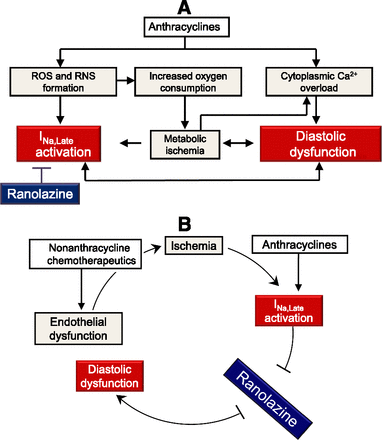
In patients with chronic angina, INa,Late is activated by hypoxia, accumulation of ischemic metabolites, and overproduction of ROS and reactive nitrogen species (Stone, 2008). In patients treated with anthracyclines, oxygen- and nitrogen-centered reactive species accumulate after redox activation of anthracyclines in cardiomyocytes and endothelial cells (Minotti et al., 2004). By continuously forming ROS, anthracyclines can also diminish oxygen tension and set the stage for cardiac hypoxia and metabolic ischemia (Ganey et al., 1991). Moreover, anthracyclines increase cytoplasmic Ca2+ by mechanisms that go from an increased sarcoplasmic release of Ca2+ to an impaired sequestration of Ca2+ in mitochondria or sarcoplasmic reticulum (Minotti et al., 2004). Anthracycline-induced diastolic dysfunction should be a good target for drugs, like ranolazine, that inhibited INa,Late and prevented cytoplasmic Ca2+ overload (Fig. 2A). It was in keeping with this rationale that ranolazine prevented elevations of LV end diastolic pressure, an index of diastolic dysfunction, in isolated rat heart perfused with doxorubicin (L. Belardinelli, personal communication).
Chemotherapy-induced diastolic dysfunction and the role of ranolazine-inhibitable INa,Late. (A) Anthracyclines induce multiple mechanisms of diastolic dysfunction, possibly mediated or amplified by ranolazine-inhibitable INa,Late. (B) Ranolazine inhibition of INa,Late mitigates diastolic dysfunction induced by vicious cycles between anthracyclines and nonanthracycline chemotherapeutics. RNS, reactive nitrogen species.
INa,Late should also be activated by nonanthracycline chemotherapeutics that caused coronary endothelial dysfunction and ischemia, whether silent or heralded by transient arrhythmias. One such mechanism of activation would be potentiated if nonanthracycline chemotherapeutics were combined with anthracyclines that activated INa,Late and caused diastolic dysfunction by their own mechanisms. Ranolazine inhibition of INa,Late might break reciprocal interactions between anthracyclines and nonanthracycline chemotherapeutics in multiagent therapies (Fig. 2B).
Cardiac benefits from ranolazine would be at the cost of minimal discomfort. Dizziness, nausea, and constipation were seen in patients receiving 1000 or 1500 mg BID in registry trials (the maximum recommended dose is 1000 mg BID in the United States or 750 mg BID in Europe). Symptoms may be more frequent in patients weighing <60 kg, but dose reductions relieve the side effects of ranolazine without diminishing its efficacy.
Probing Ranolazine in Diastolic Dysfunction from Antitumor Drugs: Bottlenecks and Requirements.
To focus on diastolic dysfunction from antitumor drugs, and to probe its preventability or curability by ranolazine, one should not recruit patients with preexisting diastolic dysfunction or any other cardiovascular or metabolic disease that causes or aggravates diastolic dysfunction. The LVEF must be ≥50%, and corrected QT interval values must be in the range of normality. In other words, inclusion/exclusion criteria should be tailored to recruit patients who showed “normal” at screening, such that any newly diagnosed diastolic dysfunction could be unambiguously attributed to the effects of antitumor drugs. These requirements introduce a bottleneck in patient recruitment, and call for powering the study with a sufficient number of participating centers.
Cardiac function should be measured by techniques that are easy to perform in the everyday life of a clinical center and cause little discomfort to cancer patients. Transthoracic echocardiography is a rapid, well tolerated, noninvasive technique that measures systolic function (LVEF) and diastolic parameters such as the peak early filling (E-wave) and late diastolic filling (A-wave) velocities, the E/A ratio, and the deceleration time (DT) of early filling velocity. E/A and DT values follow established patterns of alteration in patients with diastolic impaired relaxation or restriction, which can be graded I to III according to deviations from age-adjusted ranges of normality (Nagueh et al., 2009). Operator bias is minimized by having the same cardiologist perform serial measurements in the same patient. With a due tolerance, the E/A ratio and DT can be measured also in patients with a poor acoustic window, as is the case of breast cancer patients carrying expanders or prostheses. Tissue Doppler imaging of early diastolic velocity of mitral annular should be left to the operator’s discretion. This technique proved most informative when operators averaged signals acquired at both septal and lateral sides of the mitral annulus (Nagueh et al., 2009); unfortunately, this may not always be feasible in aforesaid patients with a poor acoustic window.
Early diastolic dysfunction may be subtle enough not to be identified by echocardiography. Biomarkers can help to overcome this problem. When the LV wall tension increases, cardiomyocytes release a pre-prohormone B-type natriuretic peptide (BNP) that is cleaved by circulating endoproteases to release active BNP and an inactive aminoterminal fragment of the prohormone (Nt-proBNP) (Braunwald, 2008). These peptides are good markers of diastolic altered relaxation or stiffness (Mottram and Marwick, 2005). In oncologic settings, transient postinfusional elevations of BNP or Nt-proBNP might denote fluid overload and LV stretch rather than cardiac dysfunction; at a distance from chemotherapy infusions, however, high BNP or Nt-proBNP would denote authentic cardiotoxicity (Sandri et al., 2005). The circulating half-life of Nt-proBNP is appreciably longer than that of BNP (Braunwald, 2008). Persistent elevations of Nt-proBNP should be easier to capture and to correlate with early diastolic dysfunction even before this could be firmly identified by echocardiography.
Troponin (Tn) measurements provide additional information. In blood samples collected immediately after ending chemotherapy infusions, Tn elevations denote that antitumor drugs caused necrosis of a definite number of cardiomyocytes. This was shown to precede LVEF decreases in some patients, particularly if chemotherapy was administered at a high dose and killed more cardiomyocytes than cardiac progenitor cells could replace (Cardinale et al., 2006). At a distance from chemotherapy infusions, however, Tn elevations should more likely denote persistent synergism between subthreshold cellular damage from antitumor drugs and subclinical ischemia from diastolic dysfunction and reduced coronary conductance, with such a synergism causing some cardiomyocytes to die. Looking at persistent Tn elevations is therefore advisable if one suspects that standard-dose chemotherapy is priming the heart to diastolic dysfunction. Regrettably, there is a lack of studies that have prospectively assessed elevations of Nt-proBNP and/or Tn in cancer patients at risk for early diastolic dysfunction. Clinical studies of ranolazine should incorporate these laboratory surrogates and make correlations between them and echocardiographic findings.
Further problems may originate from pharmacokinetic interactions between ranolazine and antitumor drugs. Ranolazine is a substrate and weak inhibitor of CYP3A enzymes; ranolazine is also a substrate for the drug transporter P-glycoprotein, which is common for drugs that are substrates of CYP3A enzymes (Jerling, 2006). Because metabolization and elimination of anthracyclines or nonanthracycline chemotherapeutics also depend on CYP3A enzymes and P-glycoprotein (Wilde et al., 2007), concomitant administration of ranolazine could change patients’ exposure to antitumor drugs. Safety reasons therefore advise not administering ranolazine during chemotherapy. In other words, ranolazine should not be used to prevent diastolic dysfunction; ranolazine should be commenced at the end of chemotherapy to relieve early diastolic dysfunction. In the light of cause-and-effect relations between early diastolic dysfunction and late symptomatic events, this approach still incorporates prevention of delayed cardiotoxicity.
The INTERACT Study
The Ranolazine to Treat Early Cardiotoxicity Induced by Antitumor Drugs (INTERACT) study (EUDRA-CT 2009-016930-29) was designed at the Drug Sciences and Clinical Pharmacology Center of University Campus Bio-Medico of Rome. INTERACT recruits patients with normal cardiovascular function and no comorbidity, it assesses cardiac function by both echocardiography and measurements of Nt-proBNP and TnI, and it introduces ranolazine at an early postchemotherapy assessment if a patient presented with LVEF ≥50% but showed echocardiographic indices of diastolic dysfunction and/or elevations of TnI or Nt-proBNP.
INTERACT was not designed to show that ranolazine could mitigate the lifetime risk of cardiotoxicity. INTERACT was designed as a short term proof-of-concept study that validated the following hypotheses: 1) diastolic dysfunction with preserved LVEF can be detected as early as a patient completed his or her chemotherapy, and 2) ranolazine relieves diastolic dysfunction to the extent that antitumor drugs activated INa,Late or caused processes that were aggravated by INa,Late.
Essential Study Design.
INTERACT is a multicenter, phase IIB, open-label study that plans to recruit 100 patients, aged 18–70 years, scheduled to receive standard-dose anthracycline-containing multiagent chemotherapy for the treatment of non-Hodgkin lymphoma or the adjuvant treatment of breast cancer, or standard-dose nonanthracycline multiagent chemotherapy for the adjuvant treatment of colorectal cancer. The incidence and curability of cardiotoxicity induced by sequential hits (chemotherapy plus targeted therapy or mediastinal irradiation) is not in the scope of INTERACT. Therefore, INTERACT does not recruit either patients who require postchemotherapy mediastinal irradiation or breast cancer patients who require postchemotherapy administration of the antiepidermal growth factor receptor-2 monoclonal antibody, trastuzumab. (To note, radiotherapy for left-sided breast cancer has become much safer than it was in the past; therefore, women scheduled to receive postchemotherapy radiation to the left chest wall or breast are not considered at risk for multiple hits and can be recruited into INTERACT.)
Patients with non-Hodgkin lymphoma will receive a cumulative dose of 300 mg/m2 doxorubicin. Women with breast cancer will receive 240 mg/m2 doxorubicin or 300–600 mg/m2 epirubicin, with the latter being equiactive to 200 or 400 mg/m2 doxorubicin, respectively. Patients will therefore be exposed to cumulative doses of 200 to 400 mg/m2 doxorubicin equivalents, which is equal to or lower than the cumulative dose associated with a 5% risk of systolic dysfunction in patients without risk factors. This should help to identify patients who developed early diastolic dysfunction with preserved LVEF. Recruitment is competitive by center and tumor type. Eleven Italian centers participate in INTERACT.
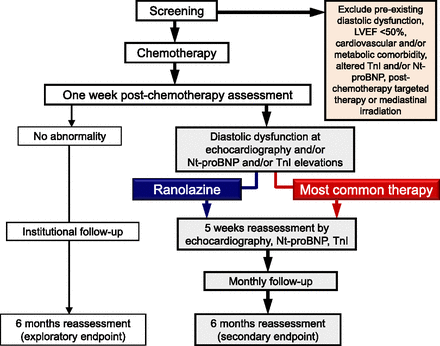
Seven ± three days after completing chemotherapy, patients with echocardiographic indices of diastolic dysfunction, and/or persistent elevations of TnI or Nt-proBNP, are randomized 1:1 to receive ranolazine or most common therapy (i.e., drugs or combination of drugs, like β blockers or others, that the cardiologist feels appropriate according to his or her own experience at his or her own institution). This is the interventional arm of INTERACT. In light of a preliminary experience at the Coordinating Center, it is expected that approximately 40% of the recruited patients should enter the interventional arm. Patients randomized to ranolazine or most common therapy are reassessed at 5 weeks and then enter a monthly follow-up that ends at 6 months with a final assessment by both echocardiography and TnI and Nt-proBNP measurements. Patients who show “normal” at an early postchemotherapy assessment enter an institutional follow-up whose modalities are left to the investigators’ discretion. The institutional follow-up ends at 6 months with a complete reassessment by both echocardiography and TnI and Nt-proBNP measurements (Fig. 3).
Essential flowchart of the INTERACT study.
The Objectives of INTERACT.
The interventional arm of INTERACT is designed to determine the following: efficacy with which 5 weeks of ranolazine treatment relieves echocardiographic and/or biohumoral indices of chemotherapy-induced diastolic dysfunction (primary efficacy endpoint), tolerability of 5 weeks of ranolazine treatment in postchemotherapy cancer patients (primary safety end point), tolerability of 6 months of ranolazine treatment (secondary safety endpoint), and tolerability of 5 weeks to 6 months of ranolazine treatment in comparison with most common therapy (exploratory safety endpoint).
The observational arm of INTERACT aims at approximating the incidence of echocardiographic and/or biohumoral indices of diastolic dysfunction in patients who had proven “normal” at an earlier postchemotherapy assessment. The objectives of this arm are to retrieve information that could prove useful in forthcoming clinical studies. Should a significant incidence of diastolic dysfunction occur in the observational arm, one might consider starting ranolazine in any patient who completed antitumor chemotherapies.
Post Hoc Research Considerations.
Patients' accrual started in December 2010. At the time when this perspective was submitted, 84 patients had been successfully screened and maintained in the study, which corresponds to a net accrual rate of approximately three patients per month in the face of the participation of 11 clinical centers. Although study progression was slowed down by unavoidable delays in the involving of participating centers and by patients’ drop out due to tumor-related clinical events, these low figures confirm that INTERACT had to go through the bottleneck of very rigorous inclusion/exclusion criteria. With that said, the bottleneck was worth the effort. After INTERACT had been designed or the first patient had been recruited in it, several research papers appeared and denoted possible confounding factors in the interpretation of ranolazine effects. In pressure overloaded rats, INa,Late increases correlated with the expression of neuronal isoforms Nav1.1 and Nav1.6 rather than Nav1.5 (Xi et al., 2009). In oxidative stress-prone deoxycorticosterone acetate-salt hypertensive mice, which in principle should express a hyperactive INa,Late, ranolazine relieved diastolic dysfunction by modulating Ca2+ effects on the contractile apparatus rather than by inhibiting INa,Late (Lovelock et al., 2012). In established models of mechanosensitivity, ranolazine bound to Nav1.5 by utilizing sites other than the canonical F1760 (Beyder et al., 2012). Each of these reports alludes to alternative modes of action of ranolazine in patients with comorbidities. Probing ranolazine in cancer patients without comorbidities may therefore be rewarding to the extent it allows for detecting and treating diastolic dysfunction under the cleanest possible conditions (i.e., when diastolic dysfunction built on chemotherapy activation of INa,Late and ranolazine relieved diastolic dysfunction by binding to its known sites in Nav1.5). Once ranolazine efficacy was defined in such settings, clinical studies of more complex patients would be easier to design and to interpret. The experience gained through INTERACT may also help to design studies of the effects of ranolazine on other echocardiographic indices of subclinical cardiotoxicity (e.g., decreases of myocardial strain) (Sawaya et al., 2012). Normal ranges of global or segmental strain, and applicability of reference values to different operational procedures, were defined when INTERACT had already been designed (Marwick et al., 2009; Dalen et al., 2010).
Previous studies suggested that ranolazine could be used to treat diastolic dysfunction. Ranolazine improved diastolic function in patients with previous transmural MI (Hayashida et al., 1994), or in patients with long QT syndrome 3 due to SCN5A gene mutations and slow inactivation of INa,Late (Moss et al., 2008); however, these were limited studies of the effects of acute intravenous ranolazine. At the dosages approved in the United States (500 mg BID for a week and 1000 mg BID in maintenance therapy), oral ranolazine improved echocardiographic indices of myocardial performance in patients with stable angina. This latter study did not focus primarily on diastolic dysfunction and did not assess ranolazine effects at prespecified checkpoints that could tell how quickly and effectively oral ranolazine exerted and maintained its effects; in fact, patients were assessed anytime from 30 to more than 200 days after ranolazine was commenced (Figueredo et al., 2011). INTERACT is entirely focused on diastolic dysfunction, titrates oral ranolazine through the dosages approved in the European Union (375 mg BID for 2 weeks, followed by 500 mg BID for 10 days and 750 mg BID until the end of the study), and sets its primary and secondary endpoints at prespecified times that should tell more about how quickly and persistently ranolazine exerted its effects.
Perspectives
Neither oncology nor cardiology would embrace the rationale of INTERACT. In everyday clinical practice, both oncologists and cardiologists tend to underestimate the importance of early diastolic dysfunction as a culprit of the lifetime risk of HF or ischemic disease. Cardiovascular assessment of cancer patients remains almost invariably bound to measuring LVEF. INTERACT is a good example of how pharmacology can work for cardio-oncology in improving cardiac surveillance and management of patients exposed to cardiotoxic chemotherapeutics. INTERACT combines appreciation of the mode of action of ranolazine with the need for intercepting and treating early diastolic dysfunction before it slowly progressed toward late sequelae.
Results from INTERACT will pave the road to further studies of ranolazine in cancer patients, which is a steadily growing population at risk for cardiotoxicity. INTERACT should also help to define the working philosophy of cardio-oncology teams and to dignify the role of pharmacologists in such teams.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Minotti.
Footnotes
- Received April 5, 2013.
- Accepted June 27, 2013.
This work was supported by University Campus Bio-Medico [Cardio-Oncology Special Project]; and TCI-Telecomunicazioni Italia [unrestricted charity fund]. INTERACT is promoted by Menarini Group.
Abbreviations
- ACEI
- angiotensin-converting enzyme inhibitor
- ARB
- angiotensin II receptor blocker
- BID
- twice daily
- BNP
- B-type natriuretic peptide
- HF
- heart failure
- INa,Late
- late inward sodium current
- INTERACT
- The Ranolazine to Treat Early Cardiotoxicity Induced by Antitumor Drugs study
- LV
- left ventricle
- LVEF
- left ventricle ejection fraction
- MI
- myocardial infarction
- Nt-proBNP
- inactive aminoterminal fragment of B-type natriuretic peptide
- ROS
- reactive oxygen species
- Tn
- troponin
- Copyright © 2013 by The American Society for Pharmacology and Experimental Therapeutics