Abstract
Trace amine-associated receptor 1 (TAAR1) is a G protein-coupled receptor that directly responds to endogenous monoamines as well as amphetamine-related psychostimulants, including methamphetamine. In the present study, we demonstrate TAAR1 mRNA and protein expression in rhesus monkey brain regions associated with monoaminergic systems, variable cellular distribution of TAAR1 in rhesus monkey brain, and TAAR1 coexpression with the dopamine transporter (DAT) in a subset of dopamine neurons in both rhesus monkey and mouse substantia nigra. On this basis, we evaluated rhesus monkey TAAR1 activation by different compounds and its functional relation with monoamine transporters and the dopamine D2 receptor (D2) short isoform (D2s) autoreceptor in vitro using a cAMP response element-luciferase assay. TAAR1 activation by monoamines and amphetamine-related compounds was greatly enhanced by coexpression of dopamine, norepinephrine, or serotonin transporters, and the activation enhancement was blocked by monoamine transporter inhibitors. This enhancement did not occur in control experiments in which the dopamine D1 receptor (D1) was substituted for TAAR1. Furthermore, activation of TAAR1 by dopamine was completely inhibited by D2s when coexpressed with TAAR1, and this inhibition was blocked by the D2 antagonist raclopride. Last, dopamine activation of TAAR1 could induce c-FOS-luciferase expression but only in the presence of DAT, whereas dopamine activation of D1 resulted in equivalent c-FOS expression in the presence or absence of DAT. Together, these data reveal a broad agonist spectrum for TAAR1, a functional relation of TAAR1 with monoamine transporters and D2s, and a mechanism by which D2 receptor drugs can influence brain monoaminergic function and have efficacy through affecting TAAR1 signaling.
Trace amine-associated receptor 1 (TAAR1) is a G protein-coupled receptor that is activated by a broad spectrum of amine compounds as well as psychostimulants, resulting in cAMP accumulation (Borowsky et al., 2001; Bunzow et al., 2001; Miller et al., 2005; Hart et al., 2006; Wainscott et al., 2007). Rat TAAR1 was shown to be a direct target of amphetamine and methylenedioxymethamphetamine (MDMA) (Bunzow et al., 2001), a finding we generalized to rhesus monkey TAAR1 in a previous report (Miller et al., 2005). TAAR1 mRNA has been detected across discrete brain regions in human and mouse, including the major monoaminergic nuclei (Borowsky et al., 2001), and in rhesus monkey substantia nigra (Miller et al., 2005). However, there was reportedly an absence of TAAR1 mRNA expression in mouse brain in a recent report by Liberles and Buck (2006). Nevertheless, the broad agonist spectrum for TAAR1 and reported distribution of TAAR1 in monoaminergic nuclei supports the hypothesis that TAAR1 may be an important modulator of monoaminergic brain function and mediate physiological, pathological, and/or adaptive properties of psychostimulant drugs of abuse.
The study of TAAR1 has been challenging, due to the lack of specific agonists and antagonists, rodent versus primate sequence divergence, as well as the phenomenon of TAAR1 remaining largely intracellular when heterologously expressed in cell lines in vitro (Bunzow et al., 2001; Miller et al., 2005). In this regard, TAAR1 displays a reduced signaling capability in heterologous expression systems, which can be restored by replacing parts of the receptor sequence or the stimulatory G protein with the corresponding rat counterparts, coexpression with rat Gαs, or coexpression with promiscuous Gq,Gα16 (Lindemann and Hoener, 2005; Navarro et al., 2006; Wainscott et al., 2007). We circumvented this obstacle with the use of a highly sensitive CRE-luciferase assay.
In the current study, we demonstrate a wide distribution of TAAR1 mRNA and protein in rhesus monkey brain regions associated with monoaminergic systems and the colocalization of TAAR1 and dopamine transporter (DAT) in a subset of substantia nigra dopamine neurons in both rhesus monkey and mouse. We also survey cellular distribution patterns of TAAR1 in cells in rhesus monkey brain. We expand on our previous report of DAT-mediated enhancement of rhesus monkey TAAR1 signaling (Miller et al., 2005) and include an examination of the norepinephrine transporter (NET) and the serotonin transporter (SERT). We found that all three monoamine transporters have strong enhancement effects on rhesus monkey TAAR1 signaling, which are blocked by monoamine transporter inhibitors. For comparative purposes, we assessed whether signaling by the dopamine D1 receptor (D1) could also be enhanced by coexpression with a monoamine transporter. Unlike TAAR1, D1 is largely expressed on the cell membrane (Trogadis et al., 1995). Although D1 receptors are postsynaptic and are not expressed in dopamine neurons, we reasoned that D1 substitution for TAAR1 would be informative, because, like TAAR1, D1 is a G protein-coupled receptor positively linked to adenylyl cyclase that responds to dopamine. We found that D1 signaling in response to dopamine was unaffected by DAT, and that whereas D1 activation could induce c-FOS, TAAR1 could only do so in the presence of DAT. Last, we found that activation of the dopamine D2 autoreceptor blocked TAAR1 signaling when both receptors were heterologously coexpressed in the same cell and that a D2 antagonist allowed TAAR1 to signal.
Materials and Methods
Materials. β-PEA, tyramine, tryptamine, octopamine, dopamine, norepinephrine, serotonin, (+)-amphetamine, (–)-amphetamine, (+)-MDMA, (–)-MDMA, (+)-methamphetamine, (–)-apomorphine, citalopram, desipramine, methylphenidate, indatraline, GABA, modafinil, (+)-raclopride, radioimmunoprecipitation assay buffer, and protease inhibitor cocktail and chromagen diaminobenzidine were all purchased from Sigma-Aldrich (St. Louis, MO). Salvinorin A was the kind gift of Daniel Seibert (The Salvia divinorum Research and Information Center, Malibu, CA). Dulbecco's modified Eagle's medium (DMEM), 100× nonessential amino acids, fetal bovine serum, 100× penicillin/streptomycin, Geneticin (G418), trypsin/EDTA, Superscript III, Alexa Fluor 488 goat anti-rabbit IgG (H+L), Alexa Fluor 568 goat anti-rat IgG (H+L), Topro-3, and Alexa streptavidin 488 were obtained from Invitrogen (Carlsbad, CA). Human anti-trace amine receptor 1 antibody against the third intracellular loop was originally manufactured by and obtained directly from Lifespan Biosciences (Seattle, WA) and additionally purchased from ABR-Affinity BioReagents (Golden, CO) and from Chemicon International (Temecula, CA). Dopamine transporter antibody and goat anti-rabbit IgG (H+L) were purchased from Chemicon International. Antigen unmasking solution, biotinylated secondary antibody (goat anti-rabbit), streptavidin/biotin block, Vectashield hardset mounting media, Dako protein block, avidin biotin block, and horseradish-peroxidase avidin-biotin complexes Vectastain ABC Elite were purchased from Vector Laboratories (Burlingame, CA). RQ1 RNase-free DNase and dual luciferase reporter assay system kits were purchased from Promega (Madison, WI), and the optimized Renilla luciferase vector pGL4.73 was generously given to us from Promega for evaluation. SuperSignal West Pico Chemiluminescent Substrate was purchased from Pierce Chemical (Rockford, IL). Whatman GF/C glass fiber filter paper was purchased from Whatman (Clifton, NJ). RNAlater was purchased from Ambion (Austin, TX). LightCycler RNA Master SYBR Green I kits were purchased, and human ProbeLibrary probes were generously given to us from Roche Diagnostics (Indianapolis, IN) for beta testing. All reagents and buffers for SDS-PAGE were purchased from Bio-Rad (Hercules, CA). Calcium phosphate transfection reagents were prepared in our laboratory from stock chemicals. [3H]Dopamine (60 Ci/mmol) was purchased from PerkinElmer Life and Analytical Sciences (Boston, MA).
Real-Time Polymerase Chain Reaction. Dissected brain regions from two rhesus monkey brains were collected and placed in RNAlater and stored at 4°C for less than 1 week. The tissue was homogenized with a pellet pestle (Sigma-Aldrich), and total RNA was extracted from tissue homogenate using a Versagene RNA purification system (Gentra Systems, Inc., Minneapolis, MN) according to the manufacturer's protocol. In addition to DNase treatment during processing with the Versagene kit, each 2-μg RNA sample was further treated with 2 U of RQ1 RNase-free DNase for 1 h and then reverse transcribed using Superscript III reverse transcriptase, according to the manufacturer's protocols. TAAR1 was amplified from rhesus monkey genomic DNA as a positive control, and an exon 2-flanking sequence of tryptophan hydroxylase 2 (TPH2) was amplified using intronic primers from rhesus monkey genomic DNA as a positive control and from each cDNA sample for the detection of trace genomic DNA, necessary because TAAR1 is an intronless gene.
Assays were designed using the Roche Universal ProbeLibrary Assay Design Center for the Human ProbeLibrary (www.roche-applied-science.com). Human ProbeLibrary probes were used in assays designed with rhesus monkey sequence data. Two rhesus monkey TAAR1 assays were selected and used: the first used probe 50 and primers 5′-gtccctgctgtttttgcatt-3′ and 5′-tcttcagcgcctttgaagtt-3′; the second used probe 82 and primers 5′-ttcaaaggcgctgaagagat-3′ and 5′-gatattttgctaaagaacacagagca-3′. Both assays gave similar results, with the probe 50 assay being slightly more robust and selected for presentation in this manuscript. A ProbeLibrary assay was designed targeted at rhesus monkey TPH2, and it used intronic primers 5′-tggaaccctaactaacgtttcg-3′ and 5′-caggtttgtaaccaggcaca-3′ that flank exon 2 (Chen et al., 2006), along with probe 38.
For each reaction, a LightCycler Taqman Master kit (Roche Diagnostics) was used with 10 μM each primer, 0.2 μl of probe, and either 2 ng of genomic DNA or 50 ng of cDNA in a total volume of 20 μl. All amplifications were carried out in glass capillary tubes with rapid cycling polymerase chain reaction in a LightCycler 2.0 instrument under the following conditions: preheat for 1 cycle at 95°C for 15 min; amplification for 45 cycles: 95°C for 10 s, 58°C for 30 s, 72°C for 3 s; and final cooling to 40°C.
SDS-PAGE and Western Blotting. Brains were obtained from two rhesus monkeys euthanized for other purposes. The brain was dissected, and different region tissues were collected and weighed. Untransfected and TAAR1-transfected HEK293 cells were grown to more than 90% confluence and collected through treatment with 0.05% trypsin/EDTA. The brain tissues were homogenized, and the cultured cells were lysed in ice-cold radioimmunoprecipitation assay buffer (150 mM NaCl, 1.0% Igepal CA-630, 0.5% sodium deoxycholate, 0.1% SDS, and 50 mM Tris, pH 8.0) at 1:5 (w/v) or 107 cells/ml for 30 min at 4°C, which was supplemented with 1× protease inhibitor cocktail. The homogenates and the cell lysates were centrifuged at 12,000g for 10 min at 4°C. The supernatants were collected, mixed with Bio-Rad Laemmli sample buffer at 1:2 (v/v), heated at 95°C for 5 min, and centrifuged at 12,000g for 5 min at 4°C. The subsequent supernatants were subjected to SDS-PAGE (10% acrylamide separating gel, 4% acrylamide stacking gel), and the proteins were electrotranslocated onto a polyvinylidene difluoride membrane (0.45 μm) presoaked in 100% methanol for 10 min. The membrane was then blocked with blocking buffer (10% nonfat milk, 10 mM Tris-HCl, 150 mM NaCl, and 0.05% Tween 20, pH 7.5) and then incubated with TAAR1 antibody at 1:1000 overnight at 4°C, and goat anti-rabbit IgG (H+L) at 1:5000 for 2 h at room temperature, in blocking buffer. SuperSignal West Pico Chemiluminescent Substrate was used to visualize the blots under a luminescent image analyzer (LAS-1000; Fujifilm, Tokyo, Japan).
Immunohistochemistry and Immunofluorescence. To evaluate regional distribution and cellular expression of TAAR1 in normal brain tissue, adult rhesus brain was collected and examined by immunohistochemistry. Two rhesus monkey brains were dissected and fixed in 10% neutral buffered formalin for 1 day, embedded in paraffin, and sectioned at 5 μm through the substantia nigra. Brain sections were baked, deparaffinized, rehydrated, and blocked with a methanolic block [methanol, H2O2, and phosphate-buffered saline (PBS)] for 5 min. The sections were placed in antigen unmasking solution for epitope retrieval, heated in a microwave for 20 min, allowed to cool, then blocked in 10% normal goat serum for 30 min followed by Dako protein block for 10 min. The blocked sections were incubated with rabbit polyclonal IgG trace amine receptor 1 antibody at 1:250 for 30 min at 25°C followed by an avidin biotin block. Then, they were incubated with a biotinylated secondary antibody (goat anti-rabbit) at 1:200 dilution for 10 min and finally horseradish-peroxidase avidin-biotin complexes. Labeling was detected with the chromagen diaminobenzidine, and slides were counterstained with Mayer's hematoxylin.
Neurons within the substantia nigra in mouse and rhesus brain were examined by immunofluorescence for coexpression of TAAR1 and DAT. A mouse was perfused transcardially with heparinized 0.9% NaCl and 4% paraformaldehyde in 1× PBS buffer and the brain was dissected and embedded in paraffin. Mouse paraffin sections were processed as described above with the following changes. Sections were incubated with rabbit polyclonal IgG trace amine receptor 1 antibody at 1:25 for 30 min at 25°C followed by incubation with Alexa Fluor 488 goat anti-rabbit IgG at 1:1000 for 30 min at 25°C. Sections were then incubated with rat anti-dopamine transporter monoclonal antibody (1:50) for 2 h at 25°C followed by incubation with Alexa Fluor 568 goat anti-rat IgG at 1:1000 for 30 min at 25°C. The sections were mounted in Vectashield hardset mounting media, and the staining was visualized with an IX51 fluorescent microscope (Olympus, Tokyo, Japan).
To amplify the TAAR1 signal, rhesus sections were incubated with the same primary antibody followed by a streptavidin/biotin block, then goat anti-rabbit biotinylated IgG (1:200), and Alexa streptavidin 488 1:1000 for 30 min. Sections were then incubated as described above with rat anti-dopamine transporter monoclonal antibody (1: 50) for 2 h at 25°C followed by incubation with Alexa Fluor 568 goat anti-rat IgG at 1:1000 for 30 min at 25°C. Last, sections were stained with Topro-3 diluted to 1:5000 for 5 min and then mounted in Vectashield hardset mounting media. Slides were examined using a Leica TCS SP laser scanning confocal microscope equipped with three lasers (Leica Microsystems, Exton, PA) using Leica software. Images were collected at 512 × 512 pixel resolution. The images were transferred to a Macintosh G5 computer, and Adobe Photoshop CS (Adobe Systems, Mountain View, CA) was used to assemble the images.
Reporter Constructs and Expression Vectors. The CRE-Luc reporter is a 21xCRE firefly luciferase reporter construct (pTLNC121-3) that was obtained from Dr. Walter Borne (University of Zurich, Zurich, Switzerland). The c-FOS-Luc construct is derived from a human c-FOS gene promoter spanning from –2000 upstream of the messenger RNA cap site to +42, cloned upstream of luciferase in pGL2-basic. Human DAT, human NET, human SERT, rhesus monkey TAAR1, rhesus monkey dopamine D2 receptor short isoform (D2s), and rhesus monkey dopamine D1 cDNAs were constructed in pcDNA3.1-derived vectors in our laboratory as described previously (Goulet et al., 2001; Miller et al., 2001; Yatin et al., 2002; Verrico et al., 2005).
Cell Culture and Transfection. Stable human DAT, human NET, human SERT, rhesus monkey TAAR1, rhesus monkey dopamine D1, and rhesus monkey D2s stable cell lines were generated in our laboratory in HEK293 cells (American Type Culture Collection, Manassas, VA) via transfection with cDNA expression vectors as described previously (Goulet et al., 2001; Miller et al., 2001; Yatin et al., 2002; Verrico et al., 2005). Cells were grown in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.1 mM nonessential amino acids at 5% CO2 in a 37°C water-jacketed incubator as described previously (Miller et al., 2005). G418 (250 μg/ml) was used for maintenance of selection of the stable cell lines. Calcium phosphate reagents [250 mM calcium chloride and 2× HBS buffer (280 mM NaCl, 10 mM KCl, 1.5 mM Na2HPO4, 12 mM d-glucose, and 50 mM HEPES, pH 7.05)] were prepared for transfection according to the procedures described previously (Xie et al., 2005). Cells were placed in 48-well plates 2 days before transfection. Transient cotransfection with luciferase reporter vectors (CRE-Luc or c-FOS-Luc, along with pGL4.73) and the indicated receptor expression constructs at a constant ratio were performed. The total amount of DNA was held constant with pcDNA3.1. The cells were exposed to the transfection media for 12 h and then incubated in serum-free DMEM and subjected to drug challenge.
Drug Treatment and Dual Luciferase Reporter Assay. We used a dual luciferase reporter assay to indirectly detect variations of cAMP levels or induction of c-FOS in transiently transfected cell lines treated with different compounds in a range of concentrations (typically 1 nM to 100 μM). The assay used a highly sensitive CRE-Luc vector and a c-FOS-Luc vector, with an 18-h drug treatment period. Accordingly, the assay results reflect an indirect measure of the aggregate accumulation of cAMP over this period of drug exposure.
Drugs were dissolved in dimethyl sulfoxide or water to make stock solutions and then diluted with DMEM and added to the triplicate wells at each dose within 0.5 h after the replacement of the transfection media. The culture plates were gently swirled and placed back into the incubator. Monoamine transporter inhibitors (indatraline, citalopram, desipramine, and methylphenidate) were added to the cells 15 min before and remained during receptor agonist treatment, and the D2 dopamine receptor inhibitor raclopride was added to cells 5 min before and remained during dopamine treatment. We prepared 1× passive lysis buffer and luciferase assay substrate reagents according to manufacturer's protocol (Promega). Cell lysates were prepared by addition of 100 μl of 1× passive lysis buffer into each well. The samples were incubated on a shaking platform for 30 min at 25°C, and then the lysates (20 μl) were transferred into wells of opaque 96-well microplates (PerkinElmer Life and Analytical Sciences) for measurement of luciferase expression. Luciferase substrate reagents (50 μl) were injected into each well, and after a 2-s delay, luciferase levels were measured as relative light units (RLUs) for 12 s on a Wallac 1420 multilabel counter, Victor 3V (PerkinElmer Life and Analytical Sciences).
Radioligand Binding Assay. A binding assay was performed according to procedures described by Xie et al. (2005). Cells were grown to approximately 90% confluence in 100-mm cell culture dishes, collected using ice-cold trypsin/EDTA, and centrifuged at 1000g for 5 min. The cell pellets were rinsed once with ice-cold PBS, pH 7.4, and centrifuged at 1000g for 5 min again. The cells were resuspended in serum-free DMEM at a density of 1 × 106 cells/ml. The cell suspension (100 μl) was then added into binding buffer (25 mM HEPES, 120 mM NaCl, 1.5 mM CaCl2, 5 mM KCl, and 1.5 mM MgCl2, pH 7.4) to perform a competition binding assay in which whole cells were incubated at 25°C for 2 h with 20 nM [3H]dopamine and six concentrations (10–9–10–4 M) of the compounds in a final volume of 500 μl. Nonspecific binding was determined in the presence of 1 mM raclopride. The experiments were repeated three times in duplicate for each drug dose. Bound radioligand was separated from the free by rapid filtration through a 24-well harvester (Brandel Inc., Gaithersburg, MD) using Whatman GF/C glass fiber filter paper presoaked in a 0.25% solution of polyethylenimine in water. The filter patches with trapped cells were rinsed three times with 4.0 ml of 25 mM HEPES buffer, pH 7.4, precooled to 4°C, and then they were placed in scintillation vials with 4.0 ml of ReadySafe scintillation cocktail (Beckman Coulter, Fullerton, CA) and counted on an LS6000IC scintillation spectrophotometer (Beckman Coulter) for 1 min/sample.
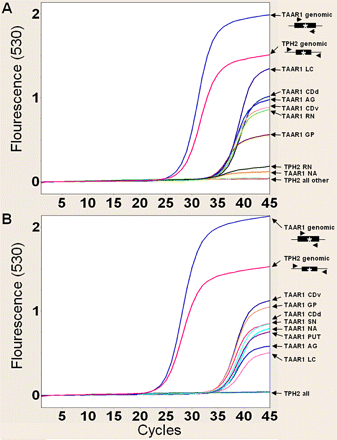
Real-time RT-PCR amplification curves for cDNA derived from selected brain regions of two rhesus monkeys (A and B) indicate that TAAR1 mRNA is widely expressed across rhesus monkey brain nuclei. Total RNA was subjected to DNase treatment and converted to cDNA. Assays using ProbeLibrary technology (Roche Diagnostics) were designed and performed on a LightCycler 2.0. TAAR1 was amplified from rhesus monkey genomic DNA (TAAR1 genomic) as a positive control, and an intron-flanked exon 2 of TPH2 was amplified from rhesus monkey genomic DNA (TPH2 genomic) as a positive control and was used for the detection of trace genomic DNA in each cDNA sample, which was necessary because TAAR1 is an intronless gene. Each cDNA sample derived from brain tissues was assayed, and all regions tested were positive for TAAR1 but negative for TPH2 in both brains with an exception of RN in A, which remained after repeated DNase treatment. LC, locus coeruleus; CDd, caudate nucleus, dorsal part; AG, amygdala; CDv, caudate nucleus, ventral part; RN, raphe nucleus; GP, globus pallidus; NA, nucleus accumbens; SN, substantia nigra; PUT, putamen.
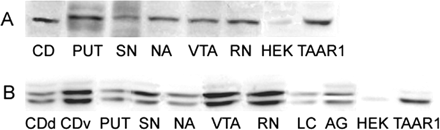
TAAR1 protein was detected in rhesus monkey brain nuclei. Protein derived from brain tissue was isolated from two rhesus monkeys and subjected to SDS-PAGE and Western blotting. The TAAR1 antibody directed against intracellular loop 3 of human TAAR1 was used. Shown are the results for two rhesus monkey brains (A and B), compared with protein derived from untransfected HEK293 and stable rhesus monkey TAAR1-transfected HEK293 cells as control. CD, caudate nucleus; CDd, caudate nucleus, dorsal part; CDv, caudate nucleus; PUT, putamen; SN, substantia nigra; NA, nucleus accumbens; VTA, ventral tegmental area; LC, locus coeruleus; AG, amygdala; HEK, untransfected HEK293 cells; TAAR1, stable TAAR1-transfected HEK293 cells.
Data Calculation and Analysis. The average ratio of firefly luciferase RLU to Renilla luciferase RLU was calculated for each triplicate. The ratio values were then divided by the ratio at baseline (vehicle treatment), and the results were converted into a percentage value. The percentage increase of RLU above baseline, which represents the specific response to the agonist tested at each dose, was derived by subtracting the baseline from the percentage value at each dose. Statistical data are expressed as mean ± S.E. of the indicated number of observations. Student's t test was used to assess significance.
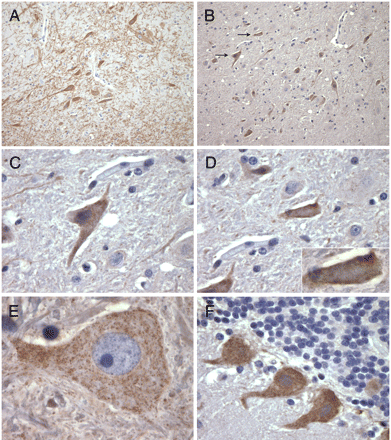
Immunohistochemistry of rhesus monkey substantia nigra, thalamus, and cerebellum. A, DAT-immunopositive neurons in substantia nigra (original magnification, 10×). B, population of TAAR1-immunopositive neurons in the same general region as A (original magnification, 10×). Arrows indicate neurons tracked in C and D. C to F, higher magnification of cellular distribution of TAAR1-positive neurons. C, cytoplasmic distribution of TAAR1 expression in substantia nigra (original magnification, 40×). D, membrane-associated neuronal expression of TAAR1 in substantia nigra (original magnification, 40×; inset, 100×). E and F, diffuse cytoplasmic expression of TAAR1 in a thalamic neuron (E; original magnification, 100×) and in cerebellar Purkinje neurons (F; 40×). All images are 3,3′-diaminobenzidine chromagen with Mayer's hematoxylin counterstain.
Results
TAAR1 mRNA Is Widely Expressed across Rhesus Monkey Brain Nuclei. Real-time RT-PCR detection of TAAR1 mRNA derived from selected brain regions of two adult rhesus monkeys revealed specific expression in all brain areas surveyed. Detection of TAAR1 by real-time RT-PCR presents a challenge, because TAAR1 is an intronless gene. Accordingly, we designed assays to detect TAAR1 mRNA and also to detect genomic DNA contamination. TAAR1 was amplified from rhesus monkey genomic DNA as a positive control (TAAR1 genomic; Fig. 1), and an intron-flanked sequence of TPH2 exon 2 was amplified from rhesus monkey genomic DNA (TPH2 genomic; Fig. 1) as a positive control for the detection of trace genomic DNA in each cDNA sample, which was necessary because TAAR1 is an intronless gene. Data are shown for selected brain regions from two rhesus monkey brains from probe 50 assays in Fig. 1. Each cDNA sample derived from brain tissue was assayed for both TAAR1 and TPH2. All regions tested were positive for TAAR1 mRNA in both brains. TPH2 controls were negative with the exception of raphe nucleus (RN) in A, which remained after repeated DNAsing and may reflect variant TPH2 expression in this key region of expression for this gene.
TAAR1 Protein Detection in Rhesus Monkey Brain. A TAAR1 antibody was used to detect TAAR1 in protein isolated from selected brain regions dissected from two rhesus monkeys by SDS-PAGE and Western blotting. This antibody is directed at a portion of the third intracellular loop of human TAAR1, which has an overall amino acid-positive similarity of 97 and 70% compared with rhesus monkey and mouse, respectively. TAAR1 was widely detected in brain monoaminergic nuclei, including caudate (dorsal and ventral), putamen, substantia nigra, nucleus accumbens, ventral tegmental area, raphe nucleus, locus coeruleus, and amygdala; Fig. 2). Interestingly, we obtained a single band in the regions from one monkey (Fig. 2A) and strong double bands in each region from the other (Fig. 2B). The molecular mass of rhesus monkey TAAR1 is calculated to be 38.8 kDa, and the bands on the gel are located between 30 and 50 kDa, as indicated by a protein standard. Parallel observations revealed that TAAR1 is strongly detected as a single band in protein derived from stable TAAR1-transfected HEK293 cells (TAAR1) but faintly in untransfected HEK293 cells (HEK). We speculate that the extra band, along with a very faint third band, could reflect glycosylation states of TAAR1, a phenomenon we observed previously with the vesicular monoamine transporter 2 (Jassen et al., 2005), but this has not as of yet been explored. Data shown in Fig. 2B are from the same monkey as data shown in Fig. 1B, and there is some agreement apparent in relative expression between RNA and protein.
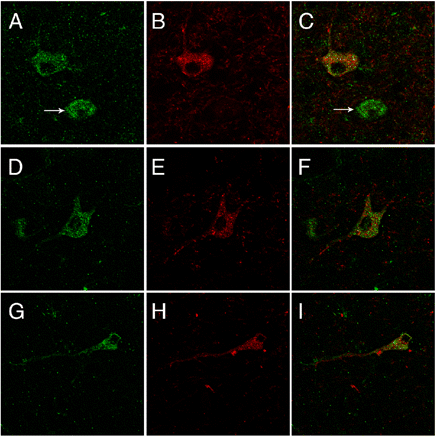
Confocal laser microscopy images from rhesus macaque brain demonstrating coexpression of TAAR1 and DAT with double-label immunofluorescence of TAAR1 (green) and DAT (red) in a subset of dopaminergic neurons in the substantia nigra. A, D, and G demonstrate TAAR1 expression. B, E, and H demonstrate DAT expression. C, F, and I depict coexpression of TAAR1 and DAT represented by superimposition of individual channels. Arrows in A and C indicate a TAAR1-expressing neuron that does not coexpress DAT. All images collected at original magnification, 40×.
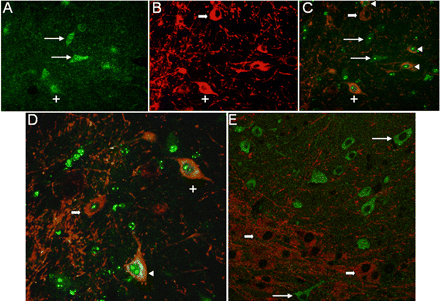
TAAR1 Is Expressed in a Subpopulation of Neurons in the Substantia Nigra, Thalamus, and Cerebellum. Immunohistochemical analysis revealed that the cellular distribution of TAAR1 is diffusely cytoplasmic within the perikaryon extending into the axon (Fig. 3, B and C) with occasional discernible membrane-associated expression (Fig. 3D, inset). In addition, TAAR1 is present in a subset of dopamine neurons in the substantia nigra of rhesus macaque and mouse. To determine whether TAAR1 is expressed in dopamine neurons in the brain, we performed immunofluorescent staining for both TAAR1 and DAT on rhesus and mouse brain sections (Figs. 4 and 5, respectively) cut through the substantia nigra. In both species, we identified neurons in the substantia nigra that expressed TAAR1, or DAT, or coexpressed TAAR1 and DAT. There were also frequent TAAR1-expressing neurons in the midst of DAT-positive neurons and processes that did not coexpress DAT but that were in approximation to DAT neurons (Fig. 5E).
Evidence supporting coexpression of TAAR1 and DAT in substantia nigra neurons in mouse brain. Immunocytochemistry for TAAR1 (green) and DAT (red) on 10-μm sections of mouse brain. A to C, detection of TAAR1 (A), DAT (B), or both signals simultaneously (C) by fluorescence microscopy. Long arrows, TAAR1-positive cells that are DAT-negative. Short thick arrow, DAT-positive/TAAR1-negative cell. +, a dopamine neuron coexpressing TAAR1 and DAT. Arrowheads, TAAR1 and DAT coexpressed in three other neurons in this field. D, another composite image from the same section. +, same neuron followed in A, B, and C. Arrowhead, TAAR1 and DAT coexpressed in another neuron. Short thick arrow, another DAT-positive cell that is TAAR1-negative. E, composite image of a different section shows TAAR1-positive or DAT-positive cells, but none are double-labeled. Long arrows, TAAR1-positive/DAT-negative. Short arrows, DAT-positive/TAAR1-negative.
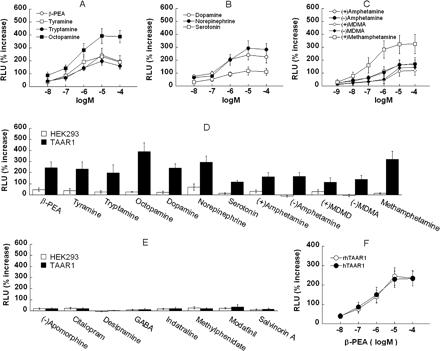
TAAR1 Is Activated by Monoamines and Amphetamines. Trace amines (β-PEA, tyramine, tryptamine, and octopamine), classic biogenic amines (dopamine, norepinephrine, and serotonin), and amphetamine-related compounds [(+)-amphetamine, (–)-amphetamine, (+)-MDMA, (–)-MDMA, and (+)-methamphetamine] dose-dependently induced CRE-Luc expression (Fig. 6, A–C). Notably, octopamine and (+)-methamphetamine were the most efficacious inducers of CRE-Luc expression. Serotonin was the least efficacious biogenic amine tested. A comparison of HEK293 cells transfected with the reporter constructs and empty pcDNA3.1 vector (white bars) or TAAR1 (black bars) for each 10 μM dose is shown in Fig. 6, D and E. In contrast to the TAAR1-dependent CRE-Luc expression in response to the above-mentioned compounds, other compounds [(–)-apomorphine, citalopram, desipramine, GABA, indatraline, methylphenidate, modafinil, and salvinorin A] had very weak or no effect on CRE-Luc expression. Accordingly, TAAR1 is activated by trace amines, biogenic amines, and amphetamine-related compounds.
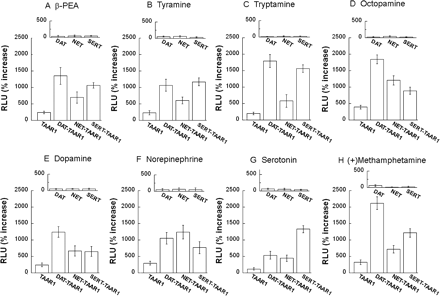
Coexpression of Monoamine Transporters Results in Greatly Enhanced Activation of TAAR1. We compared TAAR1 activation by compounds in TAAR1 cells, DAT-TAAR1, NET-TAAR1, and SERT-TAAR1 cells (HEK293 and stable DAT-, NET- or SERT-HEK293 cell lines transiently transfected with TAAR1, CRE-Luc, pGL4.73). Data for each 10 μM dose of β-PEA tyramine, tryptamine, octopamine, dopamine, norepinephrine, serotonin, and (+)-methamphetamine are shown in Fig. 7, and the full dose-response curves for these compounds as well as (+)-amphetamine, (–)-amphetamine, (+)-MDMA, (–)-MDMA modafinil, GABA, and salvinorin A are shown in Supplemental Fig. S1. Monoamine transporter coexpression in DAT-TAAR1, NET-TAAR1, or SERT-TAAR1 cells resulted in very large enhancements of CRE-Luc expression when TAAR1 was activated by agonist compounds (Fig. 7; also see Supplemental Fig. S1). Enhanced CRE-Luc activation occurred at 100 nM and higher doses of β-PEA in all cell lines, but it was generally evident for other agonist compounds at concentrations above 1 μM, which reached more than 20-fold above baseline in some conditions. In control cells without TAAR1 (stable DAT, stable NET, or stable SERT cells transfected with pcDNA3.1(+), CRE-Luc, and pGL4.73), there was no dose-dependent response to any agonist.
TAAR1 is activated by trace amines, classic biogenic amines, and psychostimulants. HEK293 cells were transiently transfected with TAAR1, CRE-Luc, and pGL4.73 for 12 h and then treated with different compounds for 18 h. Dual luciferase assay was used to monitor CRE-Luc expression as an indicator of total cAMP accumulation induced by drug treatments. Dose-response effects of trace amines (A), classic biogenic amines (B), and psychostimulants (C) on TAAR1-transfected cells are shown. Each compound was tested at a range of concentrations (A and B, 10 nM to 100 μM; C, 1 nM to 100 μM). Data are presented as percentage of increase of RLUs above baseline (vehicle treatment). A comparison of effects of these agonists (D) and other compounds that are not agonists (E) at 10 μM on HEK293 cells transfected with the reporter constructs and either pcDNA3.1 empty vector (open bars) or TAAR1 (black bars) indicates a wide agonist spectrum and demonstrates that the responses are TAAR1-dependent. F, dose response of β-PEA for TAAR1 (open circles) compared with human TAAR1 (filled circles). Data shown are for three independent experiments performed in triplicate (means ± S.E.).
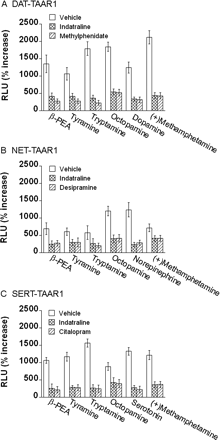
Blockade of Monoamine Transporters Reduces Their Effect on TAAR1 Activation. To clarify whether the enhancement of TAAR1 activation is dependent on transport of monoamine agonists by the monoamine transporter, we assessed the effects of monoamine transporter inhibitors on TAAR1 activation enhancements. Cells were exposed to monoamine transporter inhibitors for 15 min before and during exposure to compounds. In DAT-TAAR1, NET-TAAR1 or SERT-TAAR1 cells, indatraline, or methylphenidate, desipramine, and citalopram (each at 10 μM), respectively, largely reduced enhancement of TAAR1 activation by β-PEA, tyramine, tryptamine, octopamine, dopamine, or (+)-methamphetamine. Data for each 10 μM dose is shown in Fig. 8, and the full dose-response curves for these compounds are shown in Supplemental Fig. S2. Accordingly, blockade of monoamine transporters largely reduced the transporter-mediated enhancements of TAAR1 activation by these compounds, which are substrates for the transporters.
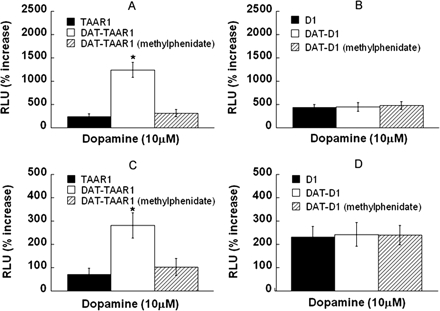
The Dopamine Transporter Does Not Change the Activation of Rhesus Monkey D1 Dopamine Receptor by Dopamine. To determine whether monoamine transporter-mediated enhancement of TAAR1 activation generalizes to another G protein-coupled receptor positively coupled to adenylyl cyclase, we compared dopamine activation of TAAR1 to D1. HEK293 cells and stable DAT cells were transiently transfected with TAAR1 or D1 along with CRE-Luc and pGL4.73, resulting in four cell preparations: TAAR1, D1, DAT-TAAR1, and DAT-D1. Whereas DAT enhanced the TAAR1 response to dopamine, DAT had no effect on the D1 response to dopamine. Data for each 10 μM dose are shown in Fig. 9, A and B, and the full dose-response curves are shown in Supplemental Fig. S3. Methylphenidate (10 μM) blocked enhancement of CRE-Luc signaling in DAT-TAAR1 cells, but it had no effect on DAT-D1 cells. An analogous experiment was performed substituting a c-FOS-Luc vector for the CRE-Luc vector, as an indicator of c-FOS induction. Likewise, DAT enhanced the TAAR1 response to dopamine, but it had no effect on the D1 response to dopamine. Data for each 10 μM dose is shown in Fig. 9, C and D, and the full dose-response curves are shown in Supplemental Fig. S3. Methylphenidate (10 μM) blocked enhancement of c-FOS-Luc in DAT-TAAR1 cells, but it had no effect on DAT-D1 cells. Notably, c-FOS-Luc expression in response to 10 μM dopamine in D1 cells was higher than in TAAR1 cells, and no further enhancement of c-FOS-Luc expression occurred in the DAT-D1 cells. Methylphenidate pretreatment did not affect the c-FOS-Luc expression in response to dopamine in DAT-D1 cells.
Monoamine transporter coexpression enhances agonist activation of TAAR1. HEK293, stable DAT, NET, and SERT cells were transiently transfected with TAAR1, CRE-Luc, and pGL4.73, resulting in four cell preparations: TAAR1, DAT-TAAR1, NET-TAAR1, and SERT-TAAR1. After 12-h transfection, each cell preparation was treated with different compounds for 18 h. Dual luciferase assay was used to monitor CRE-Luc expression as an indicator of cAMP accumulation induced by drug treatments. Data shown are for each compound tested at 10 μM and are presented as percentage of increase of RLUs above baseline (vehicle). TAAR1-dependent CRE-Luc expression in DAT-TAAR1, NET-TAAR1, or SERT-TAAR1 cells by β-PEA (A), tyramine (B), tryptamine (C), octopamine (D), dopamine (E), norepinephrine (F), serotonin (G), and methamphetamine (H) was enhanced compared with TAAR1-dependent CRE-Luc expression in TAAR1 cells. The effects of each drug on stable DAT, NET, or SERT cells transiently transfected with CRE-Luc and pGL4.73 only (A–H, insets) indicates that agonist responses were TAAR1-dependent. Data shown are for three independent experiments performed in triplicate (means ± S.E.). Full dose-response curves are included in Supplemental Fig. S1.
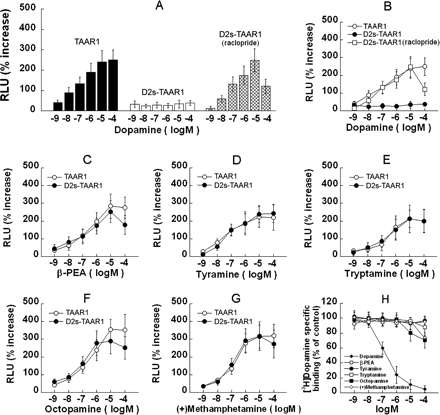
The Dopamine D2s Autoreceptor Modulates TAAR1 Signaling in Response to Dopamine. To assess the effects of D2s on TAAR1 activation, we compared the dopamine-induced CRE-Luc expression in TAAR1 and D2s-TAAR1 cells (HEK293 and stable D2s cells that were transiently transfected with TAAR1, CRE-Luc, and pGL4.73). In TAAR1 cells, dopamine dose-dependently induced CRE-Luc expression, whereas in D2s-TAAR1 cells, no such effect was observed (Fig. 10). The D2 antagonist raclopride (10 μM) did not change the CRE-Luc expression in TAAR1 cells, but it blocked the suppression effect of D2s on TAAR1 activation in D2s-TAAR1 cells (Fig. 10B). In the presence of raclopride, TAAR1 activation was still partially suppressed by D2s at the 100 μM dopamine concentration, which is presumably due to the surpassing of dopamine over raclopride to bind to D2s. β-PEA, tyramine, tryptamine, octopamine, and (+)-methamphetamine activated TAAR1 similarly in the presence or absence of D2s (Fig. 10, C–G). The binding affinity of the drugs at D2s revealed high affinity for dopamine (180 nM) and low affinity (>10 μM) for the other compounds (Fig. 10H).
Discussion
In the present study, we demonstrate TAAR1 mRNA and protein expression in rhesus monkey brain regions associated with monoaminergic systems, variable cellular distribution of TAAR1 in rhesus monkey brain, and TAAR1 coexpression with the dopamine transporter in a subset of dopamine neurons in both rhesus monkey and mouse substantia nigra. Our data are in agreement with Borowsky et al. (2001), who reported receptor mRNA expression in a variety of brain regions in the mouse and human, including the major areas of monoaminergic cell groups. Interestingly, a recent study by Liberles and Buck (2006) reports the absence of TAAR1 mRNA in mouse brain by quantitative PCR and in situ hybridization. Our results indicate a widespread distribution of TAAR1 in primate brain. Our real-time RT-PCR results are in general agreement with Borowsky et al. (2001) and provide further support for TAAR1 mRNA expression in primate brain monoaminergic areas.
Monoamine transporter inhibitors reduce the TAAR1-dependent CRE-Luc expression enhancements in DAT-TAAR1, NET-TAAR1, and SERT-TAAR1 cells. Stable DAT, NET, and SERT cells were transiently transfected with TAAR1, CRE-Luc, and pGL4.73 for 12 h and then treated with the indicated TAAR1 compounds for 18 h. Dual luciferase assay was used to monitor CRE-Luc expression as an indicator of cAMP accumulation induced by drug treatments. Data shown are for each compound tested at 10 μM and are presented as percentage of increase of RLUs above baseline (vehicle). DAT-TAAR1 (A), NET-TAAR1 (B), and SERT-TAAR1 (C) cells were pretreated with vehicle (white bars), 10 μM indatraline (crossed bars), or with a specific monoamine transporter blocker: 10 μM methylphenidate, 10 μM desipramine, or 10 μM citalopram, respectively (striped bars). Monoamine transporter blockers were added 15 min before TAAR1 agonist testing and remained present thereafter. Data shown are for three independent experiments performed in triplicate (means ± S.E.). Full dose-response curves are included in Supplemental Fig. S2.
TAAR1 was identified as a direct target of a wide range of amine compounds as well as drugs of abuse ranging from amphetamine, methamphetamine, MDMA, d-lysergic acid diethylamide, and other ergolines (Borowsky et al., 2001; Bunzow et al., 2001). We cloned rhesus monkey TAAR1 and characterized this receptor in vitro using a CRE-luciferase reporter assay (Miller et al., 2005). In the present study, we expand on the characterization of this receptor. Both endogenous monoamines and psychostimulants that are monoamine transporter substrates stimulated CRE-Luc expression, an indicator of cAMP accumulation, in TAAR1-transfected HEK293 cells. It is notable that octopamine, which is a weak agonist at human TAAR1 (about 7 μM; Wainscott et al., 2007), was highly efficacious at stimulating CRE-Luc expression. Cotransfection with DAT, NET, or SERT greatly enhanced TAAR1 receptor signaling as measured by CRE-Luc expression, and this enhancement was blocked by the monoamine transporter inhibitor indatraline, and either methylphenidate, desipramine or citalopram, respectively. Previously, we showed that DAT inhibited tyramine-induced TAAR1 activation at 100 nM and 1 μM concentrations (Miller et al., 2005). Presently, we demonstrate consistent enhancement of TAAR1 activation by tyramine as well as other amines at 1 μM. We are unsure as to why our two studies differ specifically with regard to tyramine effects. It is possible that the use of the dual luciferase assay with the optimized control vector pGL4.73, transfection methodology, or other methodological changes in the present study are a factor.
Monoamine transporters enhanced the response of TAAR1 to all compounds tested. These data confirm a broad agonist spectrum for TAAR1 and substrate promiscuity for the monoamine transporters. The most parsimonious explanation for the enhancement phenomenon is that a pool of TAAR1 inside the cell can respond to monoamines delivered by the monoamine transporters. Although the cellular location of human and rhesus TAAR1 in transfected cells seems to be intracellular, it is still unclear whether TAAR1 is also largely intracellular in neurons. In this regard, we examined TAAR1 distribution in brain cells using chromogenic immunocytochemistry with a TAAR1 antibody. Distribution commonly occurred in cells as punctate foci but widely diffuse throughout the cytoplasm, whereas in other cells TAAR1 staining seemed to be aggregated at the cell membrane. This raises the issue of the topology of TAAR1, which apparently has a standard GPCR transmembrane structure. If the receptor is retained in the endoplasmic reticulum or in some other cellular compartment, the ligand binding site could be inside the organelle. The concept that intracellular GPCRs are capable of signaling is supported by experiments demonstrating that constitutively internalized GPCRs can retain signaling capability (Whistler et al., 2002; Head et al., 2005; Scearce-Levie et al., 2005). Alternatively, the receptor may dynamically relocalize in response to transporter-mediated agonist uptake and translocate to the membrane. In this regard, our study measures cAMP elevations via CRE-Luc expression over a long period of drug exposure, during which redistribution of TAAR1 may occur.
For comparative purposes, we cloned and expressed the rhesus monkey D1 dopamine receptor and performed analogous experiments to those with TAAR1. Although D1 receptors are postsynaptic and are not expressed in dopamine neurons, we reasoned that D1 substitution for TAAR1 would be an important control experiment, because, like TAAR1, D1 is a G protein-coupled receptor that is positively linked to adenylyl cyclase and responds to dopamine. D1, however, is largely expressed on the cell membrane (Trogadis et al., 1995), has higher affinity for dopamine than TAAR1 (Niznik et al., 1986; Borowsky et al., 2001), and rapidly internalizes in response to dopamine (Ng et al., 1995). In contrast to TAAR1, D1 was stimulated by dopamine in a dose-dependent manner in D1-transfected HEK293 or DAT cells equivalently, and pretreatment with the DAT blocker methylphenidate (10 μM) did not affect D1-mediated CRE-Luc expression. HEK293 cells lacking either receptor showed no dose response to dopamine. These data demonstrate that the activation profile and cellular itinerary of TAAR1 are distinctly different from the D1 receptor. Accordingly, a detailed examination of the cellular localization of TAAR1 in identified neurons, the cellular trafficking of the receptor in response to ligand, and the cellular location where TAAR1 is activated are warranted, for which separate studies are in progress.
Dopamine activation of TAAR1 is enhanced by DAT, but dopamine activation of D1 is not. A and B, HEK293 cells and stable DAT cells were transiently transfected with TAAR1 (A) or D1 (B) along with CRE-Luc and pGL4.73, resulting in four cell preparations: TAAR1, D1, DAT-TAAR1, and DAT-D1. After 12-h transfection, each cell preparation was treated with dopamine for 18 h. Dual luciferase assay was used to monitor CRE-Luc expression as an indicator of cAMP accumulation induced by drug treatments. Data shown are for each compound tested at 10 μM and are presented as percentage of increase of RLUs above baseline (vehicle). For DAT blockade, 10 μM methylphenidate was added 15 min before and remained during dopamine treatment. A, CRE-Luc expression in response to 10 μM dopamine in TAAR1 cells (black bar), DAT-TAAR1 cells (white bar), and DAT-TAAR1 cells pretreated with methylphenidate (shaded bar). B, CRE-Luc expression in response to 10 μM dopamine in D1 cells (black bar), DAT-D1 cells (white bar), and DAT-D1 cells pretreated with methylphenidate (shaded bar). C and D, an analogous experiment was performed substituting a c-FOS-Luc vector for the CRE-Luc vector, as an indicator of c-FOS induction. C, c-FOS-Luc expression in response to 10 μM dopamine in TAAR1 cells was low (black bar); c-FOS-Luc expression in response to 10 μM dopamine in DAT-TAAR1 cells was enhanced (white bar), and reduction of the enhancement in DAT-TAAR1 cells pretreated with methylphenidate occurred (shaded bar). D, c-FOS-Luc expression in response to 10 μM dopamine in D1 cells was higher that in TAAR1 cells (black bar), and no further enhancement of c-FOS-Luc expression occurred in DAT-D1 cells (white bar). Methylphenidate pretreatment did not affect the c-FOS-Luc expression in response to 10 μM dopamine in DAT-D1 cells (shaded bar). Each graph shows data for three independent experiments performed in triplicate (means ± S.E.). *, p < 0.01 compared with the level in the absence of DAT or in the presence of methylphenidate. Full dose-response curves are included in Supplemental Fig. S3.
Methamphetamine is a toxin that induces production of free radicals and dopaminergic denervation. When transported into dopamine neurons by DAT, methamphetamine induces dopamine efflux via transport reversal (Azzaro and Rutledge, 1973; Fischer and Cho, 1979; Liang and Rutledge, 1982), which is a phosphorylation-dependent event (Doolen and Zahniser, 2002; Vaughan, 2004). In the present study, we demonstrated that TAAR1 is activated by methamphetamine and that DAT robustly enhanced TAAR1 signaling. Methamphetamine activation of TAAR1 was also enhanced by NET or SERT coexpression, but to a lesser extent. These data suggest a mechanism by which methamphetamine may cause large cAMP increases in a subset of monoamine or other cells that express TAAR1. Accordingly, TAAR1 may play a contributory role in the large extracellular dopamine levels induced by methamphetamine, particularly via direct or indirect alteration of cellular phosphorylation cascades, which would be predicted to modify DAT and vesicular monoamine transporter 2 uptake.
The expression of c-fos in brain occurs in a variety of conditions and serves as a widely used marker of neuronal activation (Morgan and Curran, 1991; Herrera and Robertson, 1996). We demonstrated that direct activation of TAAR1 by methamphetamine or dopamine induced c-FOS-Luc expression in a DAT-dependent manner in vitro. Considering the DAT-independent c-FOS-Luc induction by D1 activation in the same context and that both TAAR1 and D1 are G protein-coupled receptors linked to adenylyl cyclase, we infer that the c-FOS-Luc expression, which also parallels CRE-Luc expression, results from an increase of intracellular cAMP in the cells. An implication of these data is that TAAR1 could promote early immediate gene expression cascades in cells that coexpress a monoamine transporter but perhaps not in cells that do not, whereas D1 may promote these gene expression cascades regardless. It is likely that D1 is the major driver of c-fos in response to methamphetamine in mouse brain, because amphetamine can no longer stimulate neurons to express c-fos in D1 knockout mice (Moratalla et al., 1996).
The dopamine D2s autoreceptor modulates TAAR1 signaling in response to dopamine. A to G, HEK293 and stable D2s cells were transiently transfected with TAAR1, CRE-Luc, and pGL4.73, resulting in two cell preparations: TAAR1 and D2S-TAAR1. After 12-h transfection, each cell preparation was treated with different compounds for 18 h. Dual luciferase assay was used to monitor CRE-Luc expression as an indicator of cAMP accumulation induced by drug treatments. A, dose response for dopamine in TAAR1 cells (black bars). In D2S-TAAR1 cells, there was no dose-dependent induction of CRE-Luc expression by dopamine (white bars), but the response was restored in D2S-TAAR1 cells pretreated with the D2 antagonist raclopride (10 μM; crossed bars). Raclopride was added 5 min before addition of dopamine and remained present thereafter. When dopamine levels were 10-fold higher than raclopride (100 μM DA; 10 μM raclopride), signaling was reduced. B, same data as in A, shown for comparison with C to G. C to G, dose-response curves for β-PEA (C), tyramine (D), tryptamine (E), octopamine (F), and (+)-methamphetamine (G) in TAAR1 cells and D2S-TAAR1 cells were comparable. H, binding affinity of the drugs at D2s revealed high affinity for dopamine (180 nM) and low affinity for the other compounds (>10 μM). Octopamine apparently could activate D2s in D2S-TAAR1 cells at 10 μM (F), in agreement with binding data shown in H. Data shown are for three independent experiments performed in triplicate (means ± S.E.).
If TAAR1 is expressed in a subset of dopamine neurons and its activation can produce large intracellular increases in cAMP, why then is there scant evidence that c-Fos is induced in any monoaminergic brain nuclei following methamphetamine (Cole et al., 1995; Umino et al., 1995; Namima et al., 1998; Thiriet et al., 2001; MacGibbon et al., 2002; Zeng et al., 2004)? An intriguing explanation may be that DA neurons express D2 autoreceptors, which may be activated by large increases of extracellular DA following methamphetamine administration, and that D2 signaling may compete against intracellular cAMP increases occurring as a consequence of TAAR1 activation. This hypothesis is supported by the finding that haloperidol induced widespread increases in c-FOS-positive neurons in the rat brain, including the substantia nigra pars compacta (Suzuki et al., 1998).
We found that TAAR1 activation by dopamine was blocked by concurrent D2 activation and that the D2 effects were reversed by the D2 antagonist raclopride. Other compounds tested had low affinity for D2, and they did not suppress TAAR1 signaling through D2 activation. Accordingly, the data suggest that TAAR1 activation can be regulated by the D2 dopamine receptor when both receptors are coexpressed in the same cell. In other monoaminergic neurons, similar regulation of TAAR1 function by autoreceptor activation also may occur. The implication is that drugs that act via blockade of autoreceptors may have therapeutic efficacy, in part, via liberation of TAAR1-mediated signaling cascades within monoaminergic neurons. Accordingly, pharmacological agents that activate TAAR1, such as methamphetamine, may cause augmented signaling cascades in cells that under physiological conditions do not occur. In this regard, TAAR1 may play a role in neuroadaptive processes that are related to addiction as well as therapeutic responses to drugs. Our laboratory has recently demonstrated that D1 activation can elicit expression of axon guidance molecules in vitro and has proposed this as a mechanism by which neuroadaptations that underlie changes in the addicted brain can occur in response to psychostimulant drugs of abuse (Jassen et al., 2006). The present data indicate the possibility that TAAR1 activation by psychostimulants may facilitate neuroadaptive responses, particularly in monoaminergic neurons.
Acknowledgments
We thank Jennifer Carter for administrative support and Karen Boisvert for confocal services.
Footnotes
-
This work was supported by National Institutes of Health Grants DA016606, DA06303, DA021180, and RR00168. This work was presented as follows: Xie Z, Madras BK, and Miller GM (2005) Brain distribution of rhesus monkey trace amine receptors ta1 and ta4, in 2005 Abstract Viewer/Itinerary Planner; 2005 Nov 14; Washington, DC. Program 451.14, Society for Neuroscience, Washington, DC; and Xie Z, Chen G, Yao W, Bahn M, and Miller GM (2006) Functional interactions of rhesus monkey trace amine receptor 1 (rhTA1) with monamine transporters and receptors, in 2006 Abstract Viewer/Itinerary Planner; 2006 Oct 17; Atlanta, GA. Program no. 451.14, Society for Neuroscience, Washington, DC.
-
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
-
doi:10.1124/jpet.106.116863.
-
ABBREVIATIONS: TAAR1, trace amine-associated receptor 1; MDMA, methylenedioxymethamphetamine; CRE, cAMP-response element; DAT, dopamine transporter; NET, norepinephrine transporter; SERT, serotonin transporter; D1, dopamine D1 receptor; D2, dopamine D2 receptor; D2s, dopamine D2 receptor short isoform autoreceptor; β-PEA, β-phenylethylamine; DMEM, Dulbecco's modified Eagle's medium; PAGE, polyacrylamide gel electrophoresis; TPH2, tryptophan hydroxylase 2; RT-PCR, reverse transcription-polymerase chain reaction; HEK, human embryonic kidney; PBS, phosphate-buffered saline; Luc, luciferase; RUL, relative light unit; RN, raphe nucleus; GPCR, G protein-coupled receptor.
-
↵
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material. - Received November 9, 2006.
- Accepted December 15, 2006.
- The American Society for Pharmacology and Experimental Therapeutics