Abstract
Recently identified trace amine receptors are potential direct targets for drugs of abuse, including amphetamine and 3,4-methylenedioxymethamphetamine (MDMA). We cloned full-length rhesus monkey trace amine receptor 1 (rhTA1) that was 96% homologous to human TA1. The trace amines tyramine and β-phenylethylamine (PEA) and the monoamine transporter substrates (±)-amphetamine and (±)-MDMA stimulated cAMP accumulation in rhTA1-expressing cell lines, as measured by a cAMP response element-luciferase assay. Cocaine did not stimulate cAMP accumulation in rhTA1 cells, but it blocked [3H]PEA transport mediated by the dopamine transporter. Cotransfection with the human dopamine transporter enhanced PEA-, amphetamine-, and MDMA-mediated rhTA1 receptor activation, but it diminished tyramine activation of rhTA1. Because TA1 (EGFP-rhTA1 chimera) was largely intracellular, conceivably the dopamine transporter can facilitate access of specific agonists to intracellular TA1. rhTA1 mRNA expression was detected in rhesus monkey substantia nigra, implying that TA1 may be colocalized with the dopamine transporter in dopamine neurons. In summary, primate TA1 receptors are direct targets of trace amines, amphetamine, and MDMA. These receptors could also be indirect targets of amphetamine, MDMA, and cocaine through modification of monoamine transporter function. Conceivably, rhTA1 receptors may be located on pre- or postsynaptic membranes. Interference with the carrier function of monoamine transporters with a consequent rise of extracellular levels of trace amines could activate these receptors. The cloning of a highly homologous TA1 from rhesus monkey and demonstration that rhTA1 receptors are activated by drugs of abuse, indicate that nonhuman primates may serve to model physiological and pharmacological TA1-mediated responses in humans.
Trace amines have been known to exist in mammalian brain for more than 25 years (Boulton, 1976) and are implicated in substance abuse, depression, attention deficit hyperactivity disorder, eating disorders, schizophrenia, and other neuropsychiatric diseases (Branchek and Blackburn, 2003). Distinguishable from the well characterized biogenic amines [dopamine or DA, norepinephrine or NE, and 5-hydroxytryptamine (serotonin) or 5-HT], tyramine, tryptamine, β-phenylethylamine (PEA), octopamine, and other endogenous amine compounds are intriguing candidates as neuromodulators (Berry, 2004). With high turnover rates, trace amines are dynamically and spatially regulated by enzymes, released with other monoamines, modulate neurotransmitter release, and elicit electrophysiological responses distinct from those of classical biogenic amines (Juorio, 1976; Durden and Philips, 1980; Jones and Boulton, 1980). Early research identified specific binding sites for [3H]PEA, [3H]tryptamine, and [3H]tyramine in mammalian brain that displayed a unique pharmacology and localization (Ungar et al., 1977; Hauger et al., 1982; Kellar and Cascio, 1982; Altar et al., 1986; Perry, 1986). The functional relevance of these trace amine binding sites was supported by the high correlation of binding site density with levels of endogenous trace amines (Karoum et al., 1981; Hauger et al., 1982).
Octopamine and tyramine function as principal insect neurotransmitters, and cloning of receptors for trace amines was initially accomplished in insects and mollusks (Axelrod and Saavedra, 1977; Arakawa et al., 1990; Saudou et al., 1990; Gerhardt et al., 1997; Han et al., 1998; Blenau et al., 2000). Genes encoding mammalian trace amine receptors, in contrast, were discovered serendipitously in the course of cloning novel serotonin 5-HT1 receptor subtypes (Borowsky et al., 2001). A human trace amine receptor, designated TA1, bound trace amines with high affinity and triggered cAMP accumulation. A systematic cloning strategy revealed the existence of 14 rat TAs (rTA1–rTA4, rTA6–rTA15) and four human TAs (hTA1, hTA3, hTA4, and hTA5). Unexpectedly, a wide range of drugs of abuse, including amphetamine, 3,4-methylenedioxymethamphetamine (MDMA), and d-lysergic acid diethylamide-like hallucinogens, stimulated rat TA1-mediated cAMP accumulation at a 1 μM concentration (Bunzow et al., 2001). Because this newly discovered receptor family presents a novel target for psychoactive drugs, including drugs of abuse, the physiological and pharmacological role of trace amine receptors warrants systematic investigation and development of appropriate animal models.
Rodents present a challenge for modeling human trace amine receptors and responses to drugs because human and rodent TAs diverge significantly in sequence (76–78%) compared with sequence homology of other G protein-coupled receptors expressed in brain, in subtype number (14 in rat, four in human), in brain distribution of TA1 (Borowsky et al., 2001), and possibly in pharmacological specificity (Borowsky et al., 2001). Conceivably, these key differences reflect rapid evolution of trace amine receptors and warrant investigation of the trace amine receptor family in a species with evolutionary proximity to humans. Accordingly, we explored whether the rhesus monkey genome encodes TA1, whether it has a higher degree of homology to human than the rodent TA1, and whether it responds to psychostimulant drugs of abuse, directly or indirectly.
Materials and Methods
Genomic DNA Sample Isolation. Venous blood samples (10 ml) were collected from rhesus monkeys in Vacutainer tubes (BD Biosciences, Franklin Lakes, NJ) containing EDTA and stored at –80°C. Genomic DNA was isolated from 400 μl of whole blood using a Generation Capture Column kit (Gentra Systems, Inc., Minneapolis, MN). Eluted DNA was then ultrapurified using the Wizard DNA Cleanup system (Promega, Madison, WI).
PCR Protocol for Cloning rhTA1 Full-Length Coding Sequences. Oligonucleotide primer sets that anneal outside the coding region were designed based on the human TA1 subtype sequence embedded within human DNA sequence data from clone RP11-295F4 on chromosome 6 (GenBank AL513524). One set of primers that resulted in a PCR product of the expected size from genomic DNA (gDNA) samples was selected. All amplifications were carried out in a total volume of 30 μl containing 1 μl of Elongase enzyme mix (Invitrogen, Carlsbad, CA), 6 μlof5× buffer B [300 mM Tris-SO4, pH 9.1, 10 mM MgSO4, 90 mM (NH4)2SO4], 1 μl of 40 μM dNTPs, 10 pmol of each oligonucleotide, and 25–100 ng of gDNA template. Oligonucleotides were designed using Oligo software (Molecular Biology Insights, Inc., Cascade, CO), compared with GenBank sequence libraries to ensure specificity, and custom synthesized (Alph-aDNA, Montreal, QC, Canada). Direct utilization of gDNA as template was possible because the rhTA1 gene is presumed to be intronless (based on human sequence data). Two primers were used (5′ to 3′): TA1-f, GAT TGA CAG CCC TCA GGA ATG ATG and TA1-r, CAA AAC AGT AAA ATA TAA TAA TTC TA. Parameters were 94°C for 1 min, 35 cycles of denaturation at 94°C for 12 s, annealing at 54°C for 30 s, and elongation at 72°C for 3 min, with a final elongation at 72°C for 10 min. PCR products were run on 1% agarose gels that were stained with ethidium bromide. A single band was excised from the gel on a DarkReader transilluminator (Clare Chemical Research, Denver, CO), and the DNA was purified using a QIAquick gel extraction kit (QIAGEN, Valencia, CA). Isolated DNA was cloned into pcDNA3.1/V5/His-TOPO (Invitrogen) according to the manufacturer's protocol. After transformation, bacterial colonies were reserved and screened for insert orientation by PCR using a pcDNA3.1 reverse primer (Invitrogen) and a receptor-specific internal forward primer. Colonies with positive PCR results were selected for plasmid DNA isolation with a miniprep (Promega) or maxiprep (QIAGEN) kit according to the manufacturer's protocols. Eight rhTA1 clones were sequenced from different rhesus monkeys. Occasionally, an individual clone had one or more aberrant nucleotides relative to all other clones. Two of these loci were screened as potential single nucleotide polymorphisms in the rhTA1 coding sequence, but screening results were negative (data not shown). To ensure that the clone of rhTA1 selected for study was accurate, it was chosen on the basis of every nucleotide being represented in the majority of the clones.
DNA Sequencing. Sequencing of both strands was performed on selected clones using a CEQ8000 genetic analysis system and CEQ DTSC quick start kit (Beckman Coulter, Fullerton, CA). The system, housed at The New England Primate Research Center (Harvard Medical School, Southborough, MA), uses four-color dye-labeled terminator cycle sequencing reactions that are detected by laser-induced fluorescence in four spectral channels via capillary electrophoresis.
Prediction of Transmembrane Domains and Potential Sites of Phosphorylation. Determination of potential transmembrane regions was performed de novo on all trace amine receptor (TAR) subtype clones using TMpred-prediction of transmembrane regions and orientation algorithm, which is based on a combination of several weight matrices for scoring. Prediction of potential phosphorylation sites was done using NetPhos 2.0 protein phosphorylation prediction server (http://www.cbs.dtu.dk/services/NetPhos/), a neural network-based method for predicting potential phosphorylation sites at serine, threonine, or tyrosine residues in protein sequences. Prediction of potential kinases that may phosphorylate the selected residues was determined using PhosphoBase version 2.0, a prediction algorithm based on substrate consensus sequence logos of some common protein kinases (Kreegipuu et al., 1999).
CRE-Luc and hDAT Vectors. We used a cAMP-response element (CRE)-driven luciferase reporter construct (CRE-Luc; pTLNC121-3; made by Dr. Graeme Bilbe, Novartis, Basel, Switzerland; obtained from Dr. Walter Borne, University of Zurich, Zurich, Switzerland) to promote firefly luciferase expression in response to cAMP. The construct contains a 21X CRE cassette positioned upstream from a TATA box minimal promoter element. This construct is a highly sensitive and quantitative reporter of total cAMP induction over time and thus results in high baseline levels due to constitutive activation of cAMP during cell growth. A human dopamine transporter (hDAT) expression vector was generated from a full-length hDAT cDNA (generous gift of Dr. B. Hoffman, Eli Lilly, Indianapolis, IN) that was isolated from a human substantia nigra cDNA library and ligated into pcDNA3.1 (Invitrogen), resulting in the human dopamine transporter expression vector pcDNA3.1/hDAT.
Cell Culture and Transient Transfections. HEK-293 cells and stably transfected hDAT cells (American Type Culture Collection, Manassas, VA) were grown in 145-mm untreated tissue culture dishes (Greiner America, Inc., Lake Mary, FL) in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg of streptomycin, and 0.1 mM nonessential amino acids (all reagents from Invitrogen). Cell lines were grown at 5% CO2 in a 37°C water-jacketed incubator. An Effectine reagent kit (QIAGEN) was used for DNA transfection procedures, according to the manufacturer's protocol. Cells were plated in 24-well plates on the day before transfection. Transient transfection with the CRE-Luc reporter construct, or double transfections with the CRE-Luc reporter construct and an rhTA1 receptor expression construct in equal molar ratios were performed. The total amount of DNA transfected was held constant, and stable hDAT cells were always transfected simultaneously with wild-type HEK cells, and with the same reagent preparation. In this regard, Cre-Luc only controls were transfected with twice as much reporter as double transfection cells, and basal relative light units (RLUs) were twice as high in these control experiments as in double transfection cells. The transfection media remained on the cells for 5 h and then was replaced with 1 ml of unsupplemented DMEM with no serum. All drugs and trace amines were dissolved in DMEM and were added to the triplicate wells at each dose within 0.5 h after the replacement of the transfection medium. The cell plates were gently swirled, placed back in the incubator for 18 to 20 h, and then assayed for luciferase.
Luciferase Reporter Assays. Stock solutions of test compounds (10 mM) were made and further diluted to various concentrations in unsupplemented DMEM. Drugs were sequentially diluted and added in volumes ranging from 1 to 10 μl, such that final concentrations ranged from 10 nM to as high as 100 μM and were accurate to within 1%. Approximately 18 to 20 h after drug exposure, the wells were washed with phosphate-buffered saline, pH 7.4 (Ca2+ and Mg2+ free), and reporter lysis buffer (200 μl; Promega) was added to each well. Cell lysates were removed from the wells by vigorous washing. Luciferase assay reagent was made by adding 10 ml of luciferase assay buffer to the lyophilized luciferase assay substrate (Promega). Cell lysates were collected and spun down in Microfuge tubes and lysates (20 μl) from each well were placed in test tubes. Luciferase assay reagent (100 μl) was injected into each sample, and after a 2-s delay, luciferase concentration was measured as RLUs for 10 s.
Time Course and Transport Assays. Initially, a time course for PEA transport into hDAT and untransfected HEK-293 cells was performed to determine an optimal incubation period for transport assays. hDAT cells were incubated for various times (0, 2, 4, 6, 8, 10, 15, 20, 30, 40, and 60 min) with 0.2 ml of cell buffer (5 mM Tris, 8.5 mM HEPES, 120 mM NaCl, 5.4 mM KCl, 1.2 mM CaCl2, 1.2 mM MgSO4, 10 mM glucose, and 10 mM tropolone, pH 7.4), 0.2 ml of cell suspension, and 0.2 ml of [3H]PEA [50 nM; 4 nM [3H]PEA, 77.0 Ci/mmol, custom-synthesized by Amersham Biosciences, Inc., Piscataway, NJ, and 46 nM unlabeled PEA]. Nonspecific uptake was determined in the presence of 10 μM mazindol. The linear part of the association time study indicated that 6 min was optimal for [3H]PEA transport experiments.
[3H]PEA transport was determined as a function of temperature and presence of hDAT. To measure energy dependence of [3H]PEA transport, a time-course experiment was conducted at 0–4°C. To determine whether transport was hDAT-dependent, corresponding assays were conducted in hDAT and in untransfected HEK-293 cells at 37°C. The affinity of cocaine for blocking [3H]DA and [3H]PEA transport by hDAT was determined in stably transfected hDAT cells. Cells were washed in cell buffer, pH 7.4, centrifuged, and resuspended in cell buffer, and cell concentration was adjusted to 1.25 × 106 cells/ml using a hemacytometer. Cocaine was dissolved in cell buffer to 1 mM, whereas mazindol was dissolved in cell buffer supplemented with 40 μl of lactic acid and brought to 1 mM, and serial dilutions were prepared. Microfuge tubes, in triplicate, containing 0.2 ml of cells, 0.2 ml of diluted test drug or baseline drug, and 0.2 ml of [3H]DA (20 nM) or [3H]PEA (20 nM) were incubated for 10 or 6 min, respectively, at 37°C, as determined by time course experiments. After incubation, cells were harvested by centrifugation at 16,600g for 15 min at 0°C. The cell supernatant was removed, and the pellet was dissolved in 100 μl of 0.1% SDS. The Microfuge tube was clipped and added to 4 ml of Readysafe scintillation fluid, and radioactivity was measured for 5 min on an LS6000IC Beckman scintillation spectrometer. Nonspecific binding was determined in the presence of 10 μM mazindol. Data analyses were performed using GraphPad Prism software (GraphPad Software Inc., San Diego, CA).
N-Terminal EGFP-rhTA1 Chimera. The rhTA1 expression vector was linearized with BamHI and Bsu36I. The BamHI site is native to the MCS of pcDNA3.1-V5/His-TOPO and 5′ to the ATG start codon of the rhTA1 cDNA insert. A Bsu36I site is native to the rhTA1 sequence, lies downstream from the MCS, 5′ to the ATG start codon in the rhTA1 insert, and is unique with regard to the greater construct. The EGFP coding region was amplified by low-cycle, high-fidelity PCR using pIRES2-EGFP (BD Biosciences Clontech, Palo Alto, CA) as template. Primers for this PCR reaction were designed to incorporate BamHI and Bsu36I sites in the 5′ end of the upper and lower primers, respectively, such that the ligated EGFP insert was in frame with the rhTA1 cDNA and lacked only the STOP codon. Primer design left a neutral tether between EGFP and the rhTA1 sequence. The following primers were used to generate PCR-amplified and -modified EGFP: EGFP-BamHI-f (5′ to 3′) TTG AGG ATC CCG ATG ATA ATA TGG CCA CAA CCA and EGFP-Bsu-r (5′-3′) GCG GCC TGA GGA CTT GTA CAG CTC GTC CAT GCC. The amplification product was digested with BamHI and Bsu36I, and cloned into the linearized rhTAR1 vector directionally. Sequencing confirmed the integrity of the selected clone. This clone was transfected into HEK-293 cells for localization studies as well as cotransfected into these cells with CRE-Luc for functional assessment.
Confocal Microscopy. HEK-293 cells plated in eight-well tissue culture glass slides were transiently transfected with an EGFP-rhTA1 chimera for 24 to 48 h. Cells were fixed in 3.7% paraformaldehyde for 5 min. Cells were stained with TO-PRO-3 for 2 min for nuclei staining and/or Vibrant CM-DiI for 1.5 min for membrane staining (Molecular Probes, Eugene, OR). Cells were then washed with phosphate-buffered saline, pH 7.4, coverslipped using Vectashield hard set mounting medium for fluorescence (Vector Laboratories, Burlingame, CA), and painted with clear nail polish to seal the coverslip. Visualization was performed using a Leica TCS SP laser scanning microscope equipped with three lasers (Leica Microsystems, Exton, PA). The fluorescence of individual fluorochromes was captured simultaneously, after optimization to reduce bleedthrough channels (photomultiplier tubes) using the Leica software.
Real-Time RT-PCR of rhTA1 in the Substantia Nigra. Brain tissue was harvested from three rhesus monkeys euthanized for other purposes. The substantia nigra was dissected, placed in RNA-later (Ambion, Austin, TX), and stored at 4°C for less than 1 week. RNA was extracted from homogenized tissue (Dounce tissue grinder, Kontes; VWR Scientific, West Chester, PA) using TRIzol reagent (Invitrogen). RNA was then treated with RQ1 RNase-free DNase (Promega) for 1 h, and 1 μg was reverse transcribed using Superscript II RNase H– reverse transcriptase (Invitrogen), according to the manufacturer's protocols. Oligonucleotides were designed using Oligo software (Molecular Biology Insights, Inc.), compared with GenBank sequence libraries to ensure specificity, and custom synthesized (AlphaDNA). PCR reactions were set up in a reaction volume of 20 μl using components supplied in the LightCycler RNA Master SYBR Green I kit for one-step RT-PCR using the LightCycler 2 instrument (Roche Diagnostics, Indianapolis, IN), and either 1 μlof water or 1 μl (50 ng) of cDNA. In separate reactions, we amplified TA1, DAT, and a portion of the dynorphin promoter as a control for genomice DNA contamination with the following primer sets (5′ to 3′): TA1–862-f, TTG ATT TGG TTT GGC TAC TTG and TA1-r1, CAA AAC AGT AAA ATA TAA TAA TTC TA; DAT7-f, GGC TTC TTC TAC AAC GTC ATC ATC GC and DAT21-r, GCT CTG GTG GAG GTG CAG CAC G; DynProm-15f, AGA GGT TGA AGT TGG CAG CTT ATC; and DynProm-496r, CCA GGC GGT TAG GTA GAG TTG TCA. All amplifications were carried out in glass capillary tubes with rapid cycling with the following conditions: preincubation at 95°C for 7 min; cycling at 95°C for 10 s, 58°C for 15 s, and 72°C for 10 s (12 s for DAT); melting at 95°C for 0 s, 65°C for 15 s, and 95 for 0 s at ramp of 0.1°C/s.
Materials. Tyramine and β-phenylethylamine were purchased from Sigma-Aldrich (St. Louis MO). (+)-Amphetamine, (–)-amphetamine, (+)-MDMA (hydrochloride), (–)-MDMA (hydrochloride), and (–)-cocaine hydrochloride were obtained from the National Institute on Drug Abuse (Bethesda, MD). [3H]DA (20.5 Ci/mmol) was purchased from PerkinElmer Life and Analytical Sciences (Boston, MA), and [3H]PEA (77.0 Ci/mmol) was custom-synthesized by Amersham Biosciences Inc.
GenBank. The complete coding sequence of Macaca mulatta TAR1 gene is entered in GenBank AY135366.
Results
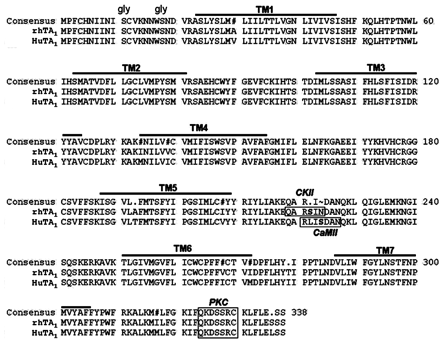
rhTA1 Sequence. Borowsky et al. (2001) identified four human trace amine receptor subtype genes (hTA1, hTA3, hTA4, and hTA5) that are intronless and occur in tandem on chromosome 6. Accordingly, we speculated that it would be feasible to clone rhesus monkey TA1 coding sequences from genomic DNA. Using a PCR strategy based on the human DNA sequence on chromosome 6 (clone RP11-295F4, GenBank AL513524), full-length rhTA1 coding sequences were obtained. Sequence comparisons between rhTA1 and hTA1 revealed that the coding sequences were 96.9% homologous, and the deduced amino acid sequence was 96.4% homologous (Fig. 1). This contrasts with a 78.7% homology with rat and 76.0% homology with mouse amino acid sequences. Hydrophobicity analysis predicted seven transmembrane regions, an extracellular N terminus, and an intracellular C terminus, as expected for GPCRs. Analysis of potential phosphorylation sites using NetPHOS analysis (Blom et al., 1999), revealed a potential casein kinase II phosphorylation site at position 222 unique to rhTA1. hTA1 lacks this site but has a unique potential calmodulin-dependent protein kinase II phosphorylation site at position 224. Additionally, both hTA1 and rhTA1 have a potential protein kinase C phosphorylation site at position 327 in the C-terminal tail.
CRE-Luc Reporter Assays. Rat and human TA1 reportedly are positively coupled to cAMP (Borowsky et al., 2001; Bunzow et al., 2001). Accordingly, we developed a CRE-Luc reporter assay system to detect agonist-induced cAMP stimulation mediated by TA1. HEK-293 cells were transiently transfected with rhTA1 or cotransfected with rhTA1 and CRE-Luc. Normalized comparative data are shown as percentage of increase in RLUs compared with baseline levels (no exposure to compounds). RLUs consistently ranged from 105 to 107, even in cells not transfected with rhTA1. Preliminary studies indicated that basal levels of cAMP in the absence of trace amines resulted in high baseline levels, due to progressive accumulation of luciferase production resulting from cAMP formation over time coupled with the high sensitivity of the CRE-Luc construct. Factors such as plating density, transfection efficiency, drug exposure time, and molar ratios of DNA transfected altered total RLUs robustly in preliminary studies, and great care was taken to control these variables in subsequent experiments. Incubation between 6 and 20 h resulted in optimal luciferase production, and we selected an 18- to 20-h incubation period. Because the β1-adrenergic receptor (BAR-1) is endogenous to the HEK-293 cell line (Friedman et al., 2002), we assessed the possibility that CRE-Luc signaling was mediated by receptors other than rhTA1 in transfected HEK cells. In HEK-293 cells transfected with CRE-Luc only, norepinephrine promoted luciferase production. The BAR-1 antagonist propranolol (300 nM) blocked activation of BAR-1 receptors by norepinephrine or by serum constituents, but alone it had no effect on luciferase production. We surmised that blockade of the BAR-1 receptor is necessary in experiments involving norepinephrine and that the CRE-Luc signal could be augmented by growth medium constituents with β1-adrenergic receptor agonist properties (data not shown).
Comparison of the amino acid sequences of TA1 protein from rhesus monkey and human. Rhesus monkey and human TA1 have 96.4% similarity to human. Seven predicted transmembrane regions are underlined. NetPHOS analysis predicts a protein kinase C site at S327 and a casein kinase II site at S222 in rhesus monkey TA1. There are two N-glycosylation sites (gly).
Trace Amine and Psychostimulant Effects on rhTA1-Transfected HEK-293 Cells. The trace amines tyramine and PEA dose-dependently increased cAMP production in HEK-293 cells transiently transfected with rhTA1 and CRE-Luc (Fig. 2; Table 1). rhTA1 activation was discernible in transfected HEK-293 cells with PEA and tyramine in a range of concentrations (10–1000 nM), with similar activation observed in C6 glioma cells transfected with rhTA1 and CRE-Luc. Drugs of abuse, including amphetamines (amphetamine, methamphetamine, and MDMA), reportedly stimulate rat TA1-mediated cAMP formation at 1 μM concentrations (Bunzow et al., 1999). To determine whether rhTA1 is activated by drugs of abuse, we tested whether (+)-amphetamine, (–)-amphetamine, (+)-MDMA, and (–)-MDMA elicited rhTA1-mediated increases in cAMP formation, as monitored by the CRE-Luc assay. Each drug promoted dose-dependent increases in luciferase expression (Fig. 3) in rhTA1-transfected cells, but failed to increase luciferase activity in CRE-Luc only-transfected HEK-293 cells (data not shown). PEA and tyramine achieved a signal at lower concentrations (10 nM) than amphetamine or MDMA. The stereoisomers of amphetamine apparently were more potent than (+)- or (–)-MDMA, because rhTA1 activity was detected with 100 nM of each isomer of amphetamine, whereas rhTA1 activation by (+)-MDMA required 300 nM and (–)-MDMA required 1000 nM. None of the agonists (tyramine, PEA, amphetamine, mescaline, or MDMA) promoted luciferase production in the absence of rhTA1. All data with these compounds were obtained from experiments that did not include propranolol pretreatments but were performed in the absence of serum.
Percentage of increase in RLUs for trace amines tyramine and PEA in rhTA1-expressing HEK-293 cells (ANOVA followed by Tukey's post hoc comparisons)
Effect of tyramine and PEA (0 nM, 10 nM, 100 nM, and 1 μM) on luciferase activity in HEK-293 cells transiently transfected with rhTA1 and CRE-Luc or in C6 glioma cells transiently transfected with rhTA1 and CRE-Luc. In HEK-293 cells transfected with CRE-Luc only, trace amines elicited no response. Data shown are percentage of increase in RLUs compared with 0 nM drug treatment.
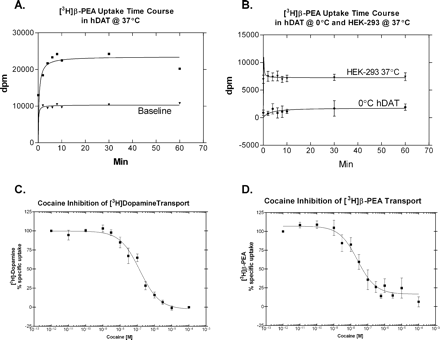
Cocaine Effects on rhTA1 and [3H]PEA Transport by hDAT. To determine whether another potent stimulant cocaine was a direct agonist at rhTA1, we transfected HEK-293 cells with rhTA1 and CRE-Luc and stable hDAT cells with CRE-Luc only, and tested a wide range of doses. In neither cell preparation did cocaine affect CRE-Luc expression (Fig. 4). We reasoned that trace amine levels in brain may be altered by blockade of monoamine transporters, since tyramine has been demonstrated to be a substrate for the dopamine transporter. We were, however, unable to find strong evidence in the literature that PEA serves as a substrate for the dopamine transporter. Accordingly, we tested hDAT cells to determine whether [3H]PEA is a substrate and whether cocaine is an effective blocker of PEA transport by the dopamine transporter. A time course of [3H]PEA transport into hDAT cells was assessed to provide an appropriate incubation period for subsequent studies. [3H]PEA (50 nM) was quickly sequestered by hDAT cells with a peak transport occurring at 8 min and stabilizing thereafter (Fig. 5A; n = 4). Nonspecific binding was measured using 10 μM mazindol to inhibit the hDAT. In subsequent studies, an incubation period of 6 min was used as this time period fell within the linear part of the curve. To ensure the uptake observed was due to the presence of hDAT, we conducted similar experiments in native HEK-293 cells. [3H]PEA was bound by untransfected HEK-293 cells, but unlike the hDAT cells, association was not time-dependent and was less than 50% compared with hDAT cells (Fig. 5B; n = 4). Presumably, this represents nonspecific binding of [3H]PEA seen in hDAT cells in the presence of excess mazindol (Fig. 5A). To ascertain whether transport of [3H]PEA was an energy-dependent process, we compared transport at 0 and at 37°C. Transport of [3H]PEA at 0°C was minimal, suggesting that [3H]PEA was a temperature-dependent process (Fig. 5B; n = 2). Cocaine blocked [3H]dopamine transport in a concentration-dependent manner with moderately high affinity (Fig. 5C; IC50 = 167.3 ± 81.8 nM; n = 4), confirming previous data (Yatin et al., 2002). Intriguingly, cocaine was a more potent inhibitor of [3H]PEA transport, mediated by the dopamine transporter (Fig. 5D; IC50 = 19.8 ± 3.5 nM; n = 4).
[3H]PEA Binding to TA1. To determine whether binding sites for rhTA1 were detectable in rhTA1 cell lines, we incubated whole or homogenized cells with [3H]PEA at 0–4°C. To improve signal-to-noise ratio, we compared Tris and glycine buffers and found improved binding with a glycine buffer. [3H]PEA uptake was inhibited in a concentration-dependent manner, with an affinity in the low nanomolar range. Nonetheless, specific binding was low and represented 20% or less of total binding. Binding increased if Na+ was omitted from the buffer and decreased with its addition, characteristic of Na+-sensitive G protein-coupled receptors (data not shown). We concluded that expression levels of rhTA1 were low, nonspecific binding of [3H]PEA was high, and future studies should be optimized with a high-affinity antagonist, if available.
Dose-response effects of (+)-amphetamine (A), (–)-amphetamine (B), (+)-MDMA (C), and (–)-MDMA (D) on luciferase activity in HEK-293 cells transiently transfected with rhTA1 and CRE-Luc. Data shown are percentage of increase in RLUs compared with 0 nM drug treatment. EC50 values using a nonlinear single site competition binding analysis equation for these data are estimated to be 682 nM for (+)amphetamine and 1700 nM for (–)-amphetamine. Comparable data for the isomeric forms of MDMA were not estimated because at the highest concentrations of MDMA (30 μM), the dose-response relationship did not asymptote.
Effect of cocaine on luciferase activity in HEK-293 cells transiently transfected with rhTA and CRE-Luc (A) and hDAT cells transiently transfected with CRE-Luc only (B). Data shown are percentage of increase in RLUs compared with 0 nM cocaine treatment.
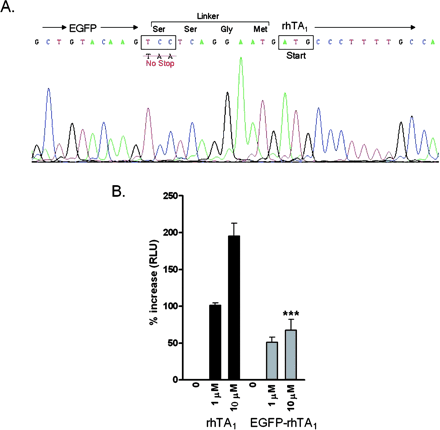
Cellular Location of an EGFP-rhTAR1 Chimera. In view of the low expression of TA1 binding sites and previous reports demonstrating that a FLAG-tagged rat TA1 chimera was localized intracellularly in transfected HEK-293 cells (Bunzow et al., 2001), we investigated the cellular localization of TA1 in transfected HEK-293 cells. To this end, we constructed an N-terminal EGFP-rhTA1 chimera containing a SER-SER-GLY-MET linker (Fig. 6A). This linker was selected for methodological convenience, but the additional SER residues in this construct did not erroneously create novel potential phosphorylation sites that could be active if intracellular, according to NetPHOS analysis. Cyclase generating activity of the chimera was tested by cotransfecting it with CRE-Luc and exposing the cells to 1 and 10 μM PEA. Although not as robust as the wild-type receptor, the EGFP-rhTA1 chimera retained the capacity for PEA activation to increase cAMP production (Fig. 6B). The reduced response of the chimera-expressing cells to 10 μM PEA (p < 0.001 by ANOVA/Tukey's) was a result of either diminished function or fewer moles of chimeric molecules than control rhTA1 molecules being transfected, because we kept constant the amount of DNA transfected. When the transiently transfected chimera-expressing cells were observed on an inverted fluorescence microscope, EGFP-rhTA1 expression was visible at 24 h and was more intense at 48 h. At 24 h, EGFP-rhTA1 distribution seemed largely intracellular in normal-looking cells, although in rare instances, EGFP-rhTA1 was clearly visible in membranes (Fig. 7A). By 48 h, cells with high EGFP-rhTA1 expression looked vacuolized and round, the green fluorescent signal being predominant in misshaped cells. By confocal microscopy, the EGFP-rhTA1 chimeric protein showed robust fluorescence intracellularly in normal-looking HEK-293 cells at 24 h (Fig. 7, B–D).
Characterization of HEK-293 cells stably transfected with the hDAT. A, time course of [3H]PEA transport in HEK-293 cells stably transfected with hDAT. hDAT or native HEK cells were incubated at 37°C with 50 nM [3H]PEA for various times (the curves are representative; n = 4). Nonspecific binding was measured using 10 μM mazindol to inhibit the hDAT (Baseline). B, untransfected HEK-293 cells were incubated at 37°C. The curve is the mean of four independent experiments ± S.E.M.; n = 4. The data are compared with hDAT cells incubated at 0°C (this curve is the mean of two independent experiments, ± S.E.M.) with [3H]PEA for various times. C, representative example of cocaine blockade of [3H]DA transport (IC50 = 167.3 ± 81.8 nM; n = 4). D, representative example of cocaine blockade of [3H]PEA transport (IC50 = 19.8 ± 3.5 nM; n = 4). All experiments were conducted in triplicate.
Modulation of rhTA1 Activity by hDAT. Our observations with EGFP-rhTA1 and those reported with a FLAG-tagged rat TA1 chimera (Bunzow et al., 2001), suggested that a high proportion of TA1 may be localized intracellularly. Conceivably, the access of TA1 agonists tyramine and PEA to intracellular rhTA1 could be compromised in the transfected HEK-293 cells. To improve trace amine transport to the intracellular milieu, we simultaneously transfected HEK-293 cells and stably expressing hDAT cells with rhTA1 and CRE-Luc and conducted side-by-side luciferase assays. In HEK-293 cells transiently transfected with rhTA1 and CRE-Luc, once again PEA dose-dependently increased cAMP production (Fig. 8A, left; n = 3). In simultaneously and identically treated stable hDAT cells, PEA dose-dependently increased cAMP production, but luciferase response was strikingly potentiated at 1 μM PEA (Fig. 8A, right; p < 0.001 versus rhTA1 at 1 μM). In sharp contrast, the presence of the hDAT significantly diminished tyramine activation of rhTA1-mediated cAMP production (Fig. 8B; p < 0.01 at 100 nM, p < 0.001 at 1 μM).
A, schematic illustrating the artificial linker region of EGFP-rhTA1 chimera and sequence verification. B, response to PEA treatments in rhTA1 and CRE-Luc-cotransfected HEK-293 cells (black columns) and in EGFP-rhTA1 and CRE-Luc-cotransfected HEK-293 cells (gray columns). Data shown are percentage of increase in RLUs compared with 0 nM drug treatment. ***, treatment effects of 10 μM were significantly different from each other (p < 0.001; ANOVA/Tukey's).
Photomicrographs of EGFP-rhTA1 chimera transiently expressed in HEK-293 cells. A, photomicrograph obtained on an inverted fluorescence microscope (Olympus IX70). A rare instance of EGFP-rhTA1 expression observed in the cell membrane at 24 h. B and C, confocal micrographs that indicate intracellular distribution of EFP-rhTA1 expression at 24 h. Green, EGFP-rhTA1; red, nuclei. D, confocal micrographs that indicate intracellular distribution and minimal membrane expression of EFP-rhTA1 expression at 24 h. Green, EGFP-rhTA1; red, membrane staining.
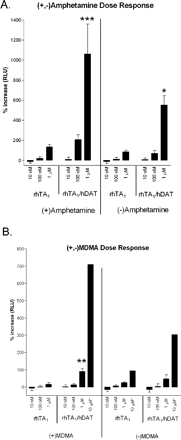
To determine whether hDAT influence on TA1 activity extended to amphetamines, parallel experiments were conducted with (±)-amphetamine and (±)-MDMA in simultaneously and identically treated HEK-293 cells and hDAT cells transiently transfected with rhTA1 and CRE-Luc. As shown previously, both isomeric forms of amphetamine and MDMA activated TA1 activity, but the presence of the hDAT significantly potentiated rhTA1 activity (Fig. 9, A and B).
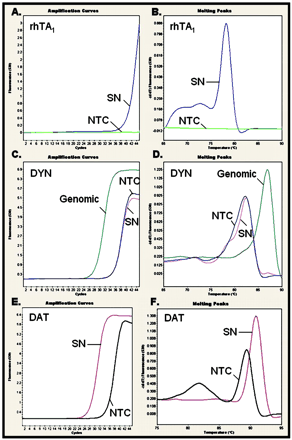
Coexpression of TA1 and the Dopamine Transporter in Substantia Nigra. Based on the intriguing observation that rhTA1 and hDAT may have agonist/substrate-dependent intersecting functions in cell lines, we investigated whether rhTA1 was coexpressed in substantia nigra, a brain region with substantial levels of DAT in monkey brain. With primers designed to amplify rhTA1, we conducted real-time PCR on cDNA generated from RNA extracts of rhesus monkey substantia nigra (n = 3). TA receptors are intronless and at low expression (Borowsky et al., 2001), so it was necessary to develop a strategy to ensure that a detectable signal originated from mRNA and not trace genomic DNA in the sample. As a negative control, we chose to amplify the promoter region of the dynorphin gene for this purpose. To confirm the presence of DAT, a multiexonic segment of the rhesus monkey DAT coding region was also amplified. Real-time PCR confirmed the presence of rhTA1 in the substantia nigra (Fig. 10). The primer set designed to detect genomic DNA failed to amplify a product, indicating that the amplification product obtained with the rhTA1 primer set targeted cDNA converted from RNA extracted from tissue and not trace genomic DNA contamination. The detection of DAT was also confirmed. A melting curve conducted on the same samples confirmed the identity of the product and distinguished it from the melting curve derived from a water (no template) control sample containing no tissue cDNA, an essential control for SYBR Green-based real-time PCR.
A, effect of PEA (0 nM, 10 nM, 100 nM, and 1 μM) on luciferase activity in HEK-293 cells and in stable hDAT cells transiently transfected with rhTA1 and CRE-Luc (rhTA1 and rhTA1/hDAT, respectively). ***, p < 0.001 versus 1 μM rhTA1. B, effect of tyramine (0 nM, 10 nM, 100 nM, and 1 μM) on luciferase activity in HEK-293 cells and in stable hDAT cells transiently transfected with rhTA1 and CRE-Luc (rhTA1 and rhTA1/hDAT, respectively). **, p < 0.01 versus 100 nM rhTA1; ***, p < 0.001 versus 1 μM rhTA1. Data shown are percentage of increase in RLUs compared with 0 nM drug treatment.
A, effect of (+)-amphetamine (left) and (–)-amphetamine (0 nM, 10 nM, 100 nM, and 1 μM) on luciferase activity in HEK-293 cells and in stable hDAT cells transiently transfected with rhTA1 and CRE-Luc (rhTA1 and rhTA1/hDAT, respectively). ***, p < 0.001 versus 1 μM rhTA1; *, p < 0.05 versus 1 μM rhTA1. B, effect of (+)-MDMA and (–)-MDMA (0 nM, 10 nM, 100 nM, 1 μM, and 10 μM) on luciferase activity in HEK-293 cells and in stable hDAT cells transiently transfected with rhTA1 and CRE-Luc (rhTA1 and rhTA1/hDAT, respectively). **, p < 0.01 versus 1 μM rhTA1. All data are n = 3, with the exception of 10 μM MDMA (n = 1), which was excluded from statistical analysis (ANOVA/Tukey's). Data shown are percentage of increase in RLUs compared with 0 nM drug treatment.
rhTA1 expression in the rhesus monkey substantia nigra. Real-time PCR followed by melting analysis was performed on a LightCycler 2.0 instrument with SYBR Green. A, rhTA1 amplification curve from substantia nigra (SN) compared with no template control (NTC). B, melting analysis of rhTA1 amplification product. C, region of the dynorphin promoter was amplified to assay for trace genomic contamination. D, melting analysis of reaction products to indicate a higher melting temperature for the genomic product and a near complete absence of specific signal generated from SN cDNA. E, DAT amplification curve from SN compared with NTC. F, melting analysis of DAT amplification product.
Discussion
The recent discovery of a family of mammalian trace amine receptors with high affinity for trace amines and activation of rat TA1 by psychostimulant and hallucinogenic drugs of abuse (Borowsky et al., 2001; Bunzow et al., 2001) presents a significant new resource for exploring the physiological, pathological, and pharmacological relevance of trace amines and their receptors in brain. We postulated that nonhuman primates would offer a more effective preclinical model system for exploring trace amines and their receptors in primate species. In support of this premise, rhTA1 displayed high structural similarity to hTA1 and was activated by PEA and tyramine, analogous to their activation of hTA1. We furthermore demonstrated that primate trace amine receptors may contribute to the stimulant and reinforcing properties of psychostimulant drugs, because amphetamine, MDMA, but not cocaine, activated rhTA1 at pharmacologically relevant concentrations. Even though we ruled out a direct role of cocaine at TA1, cocaine may potentially function as an indirect agonist at postsynaptic TA1 receptors because it effectively blocked PEA transport by the dopamine transporter. We also provide evidence that coexpression of hDAT with rhTA1 amplified TA1 activity in an agonist-specific manner, indicative of a potential mechanism for trace amine regulation of monoamine release (Berry, 2004).
The rhTA1 coding sequence is 96.9% homologous to hTA1. Previous comparisons of hTA1 and rodent TA1 receptors revealed striking differences between coding sequences that are reportedly less than 79% homologous with human. The deduced amino acid sequence of rhTA1 is 96.4% homologous to human, 78.7% homologous to rat, and 76.0% homologous to mouse sequences. The extracellular N-terminal arm of rhTA1 and hTA1 are identical, whereas only 10 of 22 amino acids in mouse TA1 matched hTA1. We particularly note this divergence because we have previously found that a single amino acid difference in the N-terminal arm of the μ-opiate receptor, another GPCR, alters affinity of an endogenous ligand in rhesus monkey (Miller et al., 2004), as well as human (Bond et al., 1998). Notable is the absence of a dileucine motif in hTA1 and rhTA1, which is present only in mouse TA1. Accordingly, internalization and processing of the mouse TA1 may be unique.
The report that amphetamine, methamphetamine, and MDMA activated rodent TA1 activity introduced a potentially novel and additional mechanism for their psychostimulant and therapeutic properties (Bunzow et al., 2001). Amphetamines are established substrates for monoamine transporters and the vesicular amine transporter-2 as well as other targets (Sulzer et al., 1993; Uhl et al., 2000; Krasnova et al., 2001; Yatin et al., 2002; Green et al., 2003) and their capacity to increase extracellular levels of monoamines via transporter exchange is a widely accepted mechanism of action (Rothman et al., 2001). We found that amphetamine and MDMA activated rhTA1, with amphetamine stereoisomers being more potent than the MDMA isomeric forms. Significantly, the concentration range of amphetamine (100 nM–>10 μM) and MDMA (1–30 μM) used fell within the plasma concentration levels achievable by administration of amphetamine to humans (p.o. approximately 300 nM; Asghar et al., 2003) or MDMA to primates (i.m., approximately 6–12 μM; Bowyer et al., 2003).
The discovery that cocaine is a more potent inhibitor of [3H]PEA transport than [3H]dopamine transport by hDAT (Table 2) supports the view that cocaine may indirectly activate rhTA1. Accordingly, by blocking trace amine transport and increasing extracellular trace amine levels, cocaine could indirectly activate membrane-bound pre- or postsynaptic trace amine receptors. Alternately, if rhTA1 is localized intracellularly in dopamine neurons, cocaine may block trace amine efflux through the DAT, thereby increasing the availability of intracellular trace amines. In view of these findings, psychostimulant drugs may directly or indirectly activate rhTA1. Conceivably, the higher potency of cocaine for inhibiting [3H]PEA compared with [3H]dopamine transport may be attributable to high membrane absorption by [3H]PEA, resulting in significant ligand depletion. Alternately, [3H]PEA transport may promote a conformational change different from that of [3H]dopamine and give rise to increased cocaine accessibility to binding site on the DAT.
Potencies of cocaine for blocking [3H]dopamine or [3H]PEA transport by the dopamine transporter in cloned human DAT expressed in HEK-293 cells or rodent DAT derived from striatal synaptosomes
The potential interaction between the cloned rhTA1 receptor and monoamine transporters extended beyond overlapping substrate specificity and influence of transport inhibitors. Converging observations led us to investigate a potential role of the DAT in modulating rhTA1 activity. A previous report that FLAG-tagged rat TA1 chimera was localized almost exclusively in the intracellular compartment of transfected HEK-293 cells (Bunzow et al., 2001), suggested that access of agonists to the receptor may require translocation to the cell interior. Accordingly, we directly assessed cellular distribution and activation of an EGFP-rhTA1 chimera transiently expressed in HEK-293 cells. In conformity with the previous report using a different construct, the majority of fluorescently labeled TA1 sites were located intracellularly. Detectable rhTA1 activation by trace amines could be mediated by low levels of extracellular membrane-bound TA1 interacting with extracellular trace amines or by intracellular TA1, activated by trace amines that diffuse into the cell in the course of the 20 h used for the CRE-Luc assay. Conceivably, TA1 activity is enhanced by DAT-driven delivery of tyramine and PEA to intracellular TA1 receptors. In support of this, we demonstrated dopamine transporter-mediated translocation of [3H]PEA as have others with [3H]tyramine (Sitte et al., 1998). Paradoxically, PEA activation of rhTA1 was strikingly enhanced by coexpression of the hDAT, whereas tyramine activation of cAMP production was significantly attenuated. Several mechanisms may account for the divergent influence of DAT on PEA or tyramine activation of rhTA1 receptors. In contrast to tyramine, PEA 1) may be sequestered efficiently by hDAT to intracellular compartments accessible to rhTA1; 2) may facilitate formation of an active hDAT-rhTA1 dimer; 3) is more stable in this cell line, although less likely as both amines are of similar potencies in the absence of the hDAT; 4) may promote formation of a rhTA1-hDAT complex that is relatively ineffective in extruding PEA but effective is releasing tyramine; and 5) may promote rhTA1 trafficking differently from tyramine, thereby enhancing PEA access to rhTA1 to a greater extent than tyramine. Intriguingly, rhTA1 activation was also enhanced by amphetamine and MDMA, furnishing evidence that the rhTA1-hDAT interaction generalized to exogenous substrates. Thus far, the depression of rhTA1 activity observed in the presence of hDAT is unique to tyramine and permits speculation on whether the physiological effects of the endogenous trace amines PEA and tyramine are distinguishable in brain and whether different transporters will display different influences on rhTA1 activity in their presence. Clearly, there are notable differences in the relative concentrations of PEA and tyramine in different brain regions and in concentrations required to promote release or inhibit monoamine transport (Berry, 2004), but a side-by-side comparison of their electrophysiological effects in midbrain dopamine neurons failed to reveal distinct differences (Geracitano et al., 2004). The relevance of these findings to DAT-TA1 interaction in brain and to brain function in general is unknown. Only trace amounts of TA1 mRNA were detected by RT-PCR in discrete regions of human brain (Borowsky et al., 2001), but these low levels of expression may be attributable to methodologies, tissue quality, or subject variations. Relevant to our findings, mouse brain TA1 mRNA is detectable within several monoaminergic cell groups, including the dorsal raphe, locus coeruleus, ventral tegmental area, and substantia nigra (Bunzow et al., 2001). Equally germane to the physiological relevance of rhTA1-hDAT interaction in HEK-293 cells, is our detection of rhTA1 expression in the dopamine transporter-rich rhesus monkey substantia nigra by real-time RT-PCR.
In primates, a detailed analysis of the pharmacological specificity of TA1 and other TA subtypes is a necessary next step in deciphering the physiological and pharmacological relevance of this intriguing class of receptors. Notwithstanding this void, the present study provides evidence that TA1 is a potential contributor to the effects of drugs of abuse in the primate via at least four putative mechanisms. Psychostimulant drugs of abuse (e.g., amphetamine) may 1) directly activate trace amine receptor subtypes; 2) increase endogenous trace amine levels by modulating monoamine transporter function, thereby indirectly activating trace amine receptors and/or possibly other biogenic amine receptors; 3) increase release or block transport of endogenous biogenic amines that activate trace amine receptors; and 4) alter trace amine levels, thereby modulating release of other neurotransmitters (Juorio and Danielson, 1978). These postulates are predicated on the production and release of trace amines at relevant concentrations. Because amphetamines but not cocaine activate rat TA1 (Bunzow et al., 2001), this receptor system may differentiate the neurochemical targets and adaptive processes mediated by these two classes of psychostimulants. rhTA1 functional responses to psychostimulants may have profound relevance to mechanisms of psychostimulant drug action and to physiological and behavioral parameters of human drug addiction. The recent discovery of polymorphisms in human trace amine receptor 4 associated with schizophrenia and a null mutation in the human TA3 (Vanti et al., 2003; Duan et al., 2004) highlights the need to explore the relationship between molecular and functional properties of the trace amine receptors and neuropsychiatric disorders. Accordingly, we postulate that nonhuman primates will provide an appropriate resource for clarifying the physiological, pharmacological, and pathological relevance of TA subtypes relevant to humans.
Acknowledgments
We thank Teresa Branchek for helpful insights.
Footnotes
-
This study was supported by Grants DA06303 (to B.K.M.), DA016606 (to G.M.M.), DA15305 (to B.K.M.), and RR00168. The research was presented in abstract form at Society for Neuroscience (2002, 2004), the annual meeting of the College on Problems of Drug Dependence (2004), and the American College of Neuropsychopharmacology (2004).
-
doi:10.1124/jpet.105.084459.
-
ABBREVIATIONS: DA, dopamine; NE, norepinephrine; 5-HT, 5-hydroxytryptamine (serotonin); PEA, β-phenylethylamine; TA, trace amine; rTA, rat trace amine; hTA, human trace amine; MDMA 3,4-methylenedioxymethamphetamine; TAR, trace amine receptor; PCR, polymerase chain reaction; gDNA, genomic DNA; hDAT, human dopamine transporter; CRE, cAMP response element; Luc, luciferase; HEK, human embryonic kidney; DMEM, Dulbecco's modified Eagle's medium; RLU, relative light unit; EGFP, enhanced green fluorescent protein; RT-PCR, reverse transcriptase-polymerase chain reaction; ANOVA, analysis of variance.
- Received February 3, 2005.
- Accepted March 8, 2005.
- The American Society for Pharmacology and Experimental Therapeutics