Abstract
A pathogenic aspect of pulmonary arterial hypertension (PAH) is the aberrant pulmonary arterial smooth muscle cell (PASMC) proliferation. PASMC proliferation is significantly affected by inflammation. A selective α-2 adrenergic receptor agonist called dexmedetomidine (DEX) modulates specific inflammatory reactions. We investigated the hypothesis that anti-inflammatory characteristics of DEX could lessen PAH that monocrotaline (MCT) causes in rats. In vivo, male Sprague-Dawley rats aged 6 weeks were subcutaneously injected with MCT at a dose of 60 mg/kg. Continuous infusions of DEX (2 µg/kg per hour) were started via osmotic pumps in one group (MCT plus DEX group) at day 14 following MCT injection but not in another group (MCT group). Right ventricular systolic pressure (RVSP), right ventricular end-diastolic pressure (RVEDP), and survival rate significantly improved in the MCT plus DEX group compared with the MCT group [RVSP, 34 mmHg ± 4 mmHg versus 70 mmHg ± 10 mmHg; RVEDP, 2.6 mmHg ± 0.1 mmHg versus 4.3 mmHg ± 0.6 mmHg; survival rate, 42% versus 0% at day 29 (P < 0.01)]. In the histologic study, the MCT plus DEX group showed fewer phosphorylated p65-positive PASMCs and less medial hypertrophy of the pulmonary arterioles. In vitro, DEX dose-dependently inhibited human PASMC proliferation. Furthermore, DEX decreased the expression of interleukin-6 mRNA in human PASMCs treated with fibroblast growth factor 2 (FGF2). These consequences suggest that DEX improves PAH by inhibiting PASMC proliferation through its anti-inflammatory properties. Additionally, DEX may exert anti-inflammatory effects via blocking FGF2-induced nuclear factor κ B activation.
SIGNIFICANCE STATEMENT Dexmedetomidine, a selective α-2 adrenergic receptor agonist utilized as a sedative in the clinical setting, improves pulmonary arterial hypertension (PAH) by inhibiting pulmonary arterial smooth muscle cell proliferation through its anti-inflammatory effect. Dexmedetomidine may be a new PAH therapeutic agent with vascular reverse remodeling effect.
Introduction
Pulmonary arterial hypertension (PAH) causes progressive right heart failure (Liles et al., 2015), and the major pathologies of PAH include vasoconstriction, vascular remodeling, and thrombosis of the pulmonary arterioles (Kuhr et al., 2012). Among these factors, improvement of vascular remodeling is a significant issue. The effect of current PAH therapies is focused on vasodilation (Zolty, 2020). Preclinical and clinical trials are underway for new drugs with novel mechanisms targeting vascular remodeling (Humbert et al., 2021; Condon et al., 2022).
Vascular remodeling of the pulmonary arterioles is characterized by medial hypertrophy caused by the aberrant proliferation of pulmonary arterial smooth muscle cells (PASMCs), endothelial hyperproliferation in the intima, and inflammatory cell infiltration and fibrosis in the adventitia (Rabinovitch et al., 2014). Inflammation is critical in the onset and progression of pulmonary vascular remodeling in PAH (Aiello et al., 2017). Inflammatory cells, such as macrophages, and lymphocytes assemble around the pulmonary arterioles (Chan and Loscalzo, 2008). Inflammatory cytokines and chemokines are more prevalent in the blood of individuals with PAH (Humbert et al., 1995; Balabanian et al., 2002). Moreover, patients with inflammatory diseases, such as collagen diseases, are more likely to develop PAH, and anti-inflammatory therapies for the primary disease also improve PAH (Sanchez et al., 2006). Our group previously clarified that a selective nuclear factor κ B (NF-κB) inhibitor suppressed PASMC proliferation induced by fibroblast growth factor 2 (FGF2) (Hosokawa et al., 2013).
Dexmedetomidine (DEX), a selective α-2 (α2) adrenergic receptor agonist, is a clinically used sedative (Keating, 2015; Weerink et al., 2017). Pulmonary arterial pressure is not significantly changed by DEX in children with PAH (Friesen et al., 2013), and DEX can be safely used for PAH patients (Jiang et al., 2015). DEX’s anti-inflammatory effect through α-2 adrenergic receptor inhibited NF-κB (Kawasaki et al., 2013). Brimonidine, another α-2 adrenergic receptor agonist, also presented an anti-inflammatory effect (Piwnica et al., 2014). In vivo, DEX showed an anti-inflammatory impact on acute lung injury caused by lipopolysaccharide through blocking the TLR4/NF-κB pathway in rats (Meng et al., 2018).
Based on this background, we hypothesized that DEX’s anti-inflammatory effect improved PAH induced by monocrotaline (MCT) in rats.
Materials and Methods
Chemical Compounds
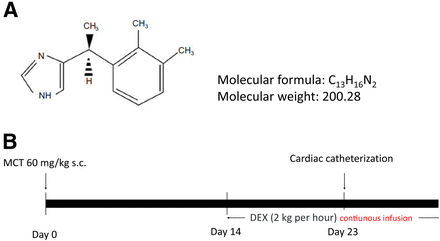
DEX was provided by Maruishi Pharmaceutical Co., Ltd. (Osaka, Japan) (Fig. 1A). This compound was dissolved in pure water. MCT was obtained from Wako (Osaka, Japan).
Molecular structure of DEX and animal experiment protocol. (A) Molecular structure of DEX. (B) Schematic diagram of the animal experimental protocol. Rats were injected with MCT on day 0, continuous infusions of DEX were started on day 14, cardiac catheterization was performed on day 23, and the survival rate was analyzed in the remaining rats.
Animal Experiments
Male Sprague-Dawley rats (CLEA Japan, Inc., Tokyo, Japan) aged 6 weeks and weighing between 228 g and 253 g were subcutaneously injected with MCT (60 mg/kg). Continuous DEX infusions (2 µg/kg per hour) were initiated using osmotic pumps in one group (MCT plus DEX group) but not in another group (MCT group) on day 14 following MCT injection (Fig. 1B). The dose of continuous DEX infusion (2 µg/kg per hour) was used in the previous rat experiments (van Oostrom et al., 2010; Qiu et al., 2018). The dose of DEX might seem to be higher than the dose used in human in clinical settings. However, it is known that the dose of DEX depends on species, and it may be as high as 10 µg/kg per hour in the in vivo experiments in rats (Yang et al., 2008; Tasdogan et al., 2009). Therefore, we believe that the DEX dose in our study is not too much. The reason for starting DEX infusion at day 14 was that a prior study revealed that rat pulmonary arterial pressure has already increased at that point (Jasmin et al., 2006). The Institutional Animal Care and Use Committee of Tokyo Medical and Dental University approved all animal protocols.
Survival Analyses
The condition of the rats was observed each day. The Kaplan-Meier method was employed to examine the survival rate.
Physiologic Analyses
On day 23, a catheterization study was performed on the surviving rats. For anesthesia, the rats received an intraperitoneal injection of inactin hydrate (80 mg/kg; Sigma-Aldrich, St Louis, MO). After checking for unresponsiveness against the stimulus, the rats were intubated by tracheostomy and mechanically ventilated with air. A micromanometer-tipped 3-Fr catheter (SPR-524; Millar Instruments, Houston, TX) was inserted into the right ventricle (RV) to measure RV pressure and heart rate (Supplemental Methods) under thoracotomy. Once the measurements were completed, the hearts and lungs of all rats were collected. Then, the Fulton index, which was defined as the weight ratio between the right ventricle and left ventricle plus septum, was calculated with heart dissections. The hearts and right lungs were preserved in liquid nitrogen, and the left lungs were treated with 10% formalin.
Histologic Analyses
Paraffin-embedded rat lung tissues were sliced into 3-µm sections. To assess the muscularization of the pulmonary arterioles, H&E, Elastica van Gieson, and α-smooth muscle actin (α-SMA) (1:100 dilution, ab5694; Abcam, Cambridge, MA) were used to stain serial slices. Fifty small arterioles (with diameters <100 µm) were counted and classified as fully muscularized (75%–100%), partially muscularized (25%–75%), and nonmuscularized (<25%) based on α-SMA–positive cells surrounding the vessels (Chai et al., 2015).
Immunohistochemistry (IHC) was performed on serial sliced 3-µm paraffin sections. After deparaffinization and antigen activation, they were incubated with phosphorylated p65 antibody (1:100 dilution, ab194726; Abcam) or CD68 antibody (1:50 dilution, sc-20060; Santa Cruz Biotechnology) overnight at 4°C. They were treated for 30 minutes at room temperature with Simple Stain Rat MAX-PO (Nichirei Biosciences, Tokyo, Japan) as the secondary antibody. Simple Stain AEC (Nichirei Biosciences) was used for staining.
Cell Culture
Human PASMCs (hPASMCs) were obtained from Lonza (Basel, Switzerland) and cultured in SmGM-2 (Lonza) at 37°C in 5% CO2/95% air. They were passaged at 70%–80% confluence and used between the fifth and eighth passages.
Cell Proliferation Assay
The cell proliferation experiment was carried out in accordance with a previous report (Hosokawa et al., 2013). Briefly, hPASMCs were seeded at 2000 cells/well on 96-well plates and incubated for 24 hours. They were starved for 24 hours in Dulbecco's modified Eagle’s medium (Sigma-Aldrich) with 0.5% fetal bovine serum and 1% penicillin/streptomycin. Then, they were given DEX (1 ng/mL, 10 ng/mL, and 100 ng/mL) (Kishikawa et al., 2008) for 30 minutes before administration of FGF2 (10 ng/mL) (Miltenyi Biotec, Bergisch Gladbach, Germany). In humans, DEX was used safely at mean plasma concentrations ranging from 0.7 ng/ml to 14.7 ng/ml (Ebert et al., 2000). After 48-hour, Cell Counting Kit-8 solution (Dojindo Laboratories, Kumamoto, Japan) (10 µL/well) was applied to each well, and the cells were incubated for 1.5 hours. A microplate reader (Bio-Rad Laboratories, Hercules, CA) was used to detect absorbance at 450 nm.
Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction
RNeasy Mini Kit (Qiagen, Hilden, Germany) was used to extract total RNA from hPASMCs. Then, High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) was used to synthesize cDNA from 0.2 g of RNA. Power SYBR Green PCR Master Mix was used to quantify the cDNA with StepOnePlus Real-Time PCR system (Applied Biosystems). The PCR primer sequences were as follows: IL-6, 5′-GGTACATCCTCGACGGCATCT-3′ (forward) and 5′-GTGCCTCTTTGCTGCTTTCAC-3′ (reverse) (Chen et al., 2014); monocyte chemotactic protein-1 (MCP-1), 5′-GATCTCAGTGCAGAGGCTCG-3′ (forward) and 5′-TGCTTGTCCAGGTGGTCCAT-3′ (reverse) (Shibakura et al., 2003); GAPDH, 5′-TGCACCACCAACTGCTTAGC-3′ (forward) and 5′-GGCATGGACTGTGGTCATGAG-3′ (reverse) (Kodama et al., 2019). The comparative ΔΔCt method was used to determine relative gene expression.
Statistical Analysis
All data are shown as mean ± standard error of the mean. The one-way analysis of variance and the Tukey test were used to determine statistical significance. The difference was significant at a P value <0.05. All data were analyzed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) (Kanda, 2013), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
DEX Improves Both Survival Rate and RV Systolic Pressure in Rats with PAH
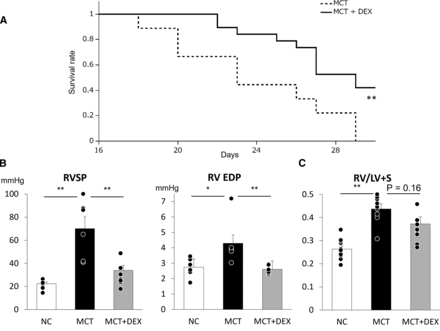
Although no rat in the MCT group survived on day 29, 42% of rats in the MCT plus DEX group survived on the same day (Fig. 2A). Cardiac catheterization measurements showed that RV systolic pressure and RV end-diastolic pressure (RVEDP) were lower in the MCT plus DEX group than the MCT group [RV systolic pressure, 34 mmHg ± 11 mmHg versus 70 mmHg ± 25 mmHg; RVEDP, 2.6 mmHg ± 0.1 mmHg versus 4.3 mmHg ± 0.6 mmHg (P < 0.01)] (Fig. 2B). The Fulton index tended to be lower in the MCT plus DEX group than in the MCT group (0.37 ± 0.03 versus 0.44 ± 0.02), but it was not significantly different (P = 0.16) (Fig. 2C). The life performance of rats in each group had a comparable level.
DEX improves survival rate and decreases RV systolic pressure in PAH rats. (A) Survival rates in the MCT group (n = 9) and MCT plus DEX group (n = 14). **P < 0.01 by log-rank test. (B) RV systolic pressure (left) and end-diastolic pressure (right) measured by cardiac catheterization. Negative control (NC), n = 7; MCT, n = 6; MCT plus DEX, n = 7. (C) Fulton index [RV/(LV + S) weight ratio]. NC, n = 10; MCT, n = 8; MCT plus DEX, n = 10. All error bars defined as mean ± S.E.M. **P < 0.01; *P < 0.05 by one-way ANOVA with Tukey test. LV, left ventricle; S, septum.
DEX Suppresses Muscularization of the Pulmonary Arterioles
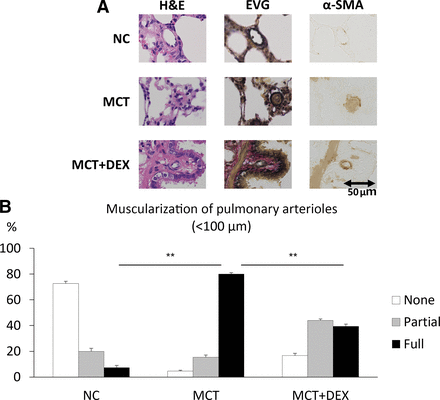
In the histologic analysis, medial hypertrophy of the pulmonary arterioles was significantly increased in the MCT group compared with the negative control group and decreased in the MCT plus DEX group. PASMC proliferation indicated by α-SMA staining was highlighted in the MCT group and reduced in the MCT plus DEX group (Fig. 3A). The MCT plus DEX group had less muscularization of the pulmonary arterioles than the MCT group (Fig. 3B).
Muscularization of the pulmonary arterioles is inhibited by DEX. (A) H&E, Elastica van Gieson (EVG), and α-SMA staining of rat lung tissues. Scale bar, 50 μm. (B) Muscularization of the pulmonary arterioles (each, n = 3). All error bars are defined as mean ± S.E.M. **P < 0.01 by one-way ANOVA with Tukey test. NC, negative control.
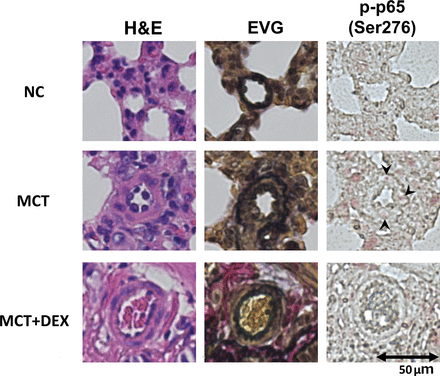
DEX Inhibits Inflammation and NF-κB Activation
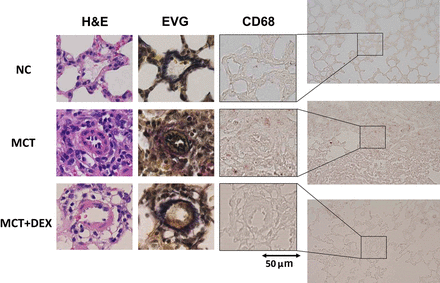
To investigate whether inflammation was related to the effect of DEX to PAH, perivascular macrophage infiltration and the NF-κB activation were examined by IHC. More CD68-positive macrophages were detected around pulmonary arterioles in the MCT group (Fig. 4). Phosphorylated p65-positive cells within the media of the pulmonary arterioles were more highly detected in the MCT group than in the MCT plus DEX group (Fig. 5).
DEX inhibits macrophages infiltration into the perivascular region in PAH rats. H&E, Elastica van Gieson (EVG), and CD68 staining of rat lung tissues. Scale bar, 50 μm. NC, negative control.
DEX suppresses NF-κB activation in PAH rats. HE, Elastica van Gieson (EVG), and phosphorylated p65 staining of rat lung tissues. Scale bar, 50 μm. NC, negative control.
DEX Suppresses FGF2-Induced hPASMC Proliferation
Previous studies revealed that FGF2 level was higher in the blood of patients with PAH (Benisty et al., 2004) and MCT-induced PAH rats (Arcot et al., 1995) and PASMCs proliferated with FGF2 stimulation in vitro (Izikki et al., 2009).
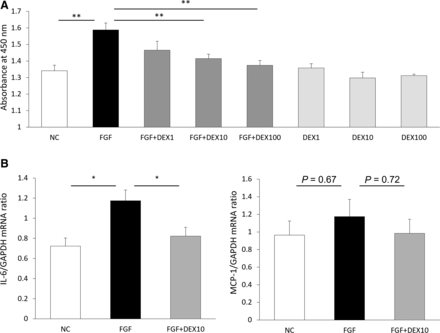
In vitro, we examined hPASMC proliferation with FGF2 stimulation. HPASMCs proliferated with FGF2 stimulation, and DEX significantly suppressed FGF2-induced proliferation dose dependently. DEX did not show any toxicities to hPASMCs or direct affect to cell proliferation. (Fig. 6A).
DEX decreases FGF2-induced hPASMC proliferation by inhibiting IL-6 synthesis. (A) Human PASMC proliferation assay (each, n = 4). (B) mRNA expression in human PASMCs (each, n = 5). Negative control (NC); FGF, FGF2 10 ng/mL; DEX1, DEX 1 ng/mL; DEX10, DEX 10 ng/mL; DEX100, DEX 100 ng/mL. All error bars are defined as mean ± S.E.M. **P < 0.01; *P < 0.05 by one-way ANOVA with Tukey test.
DEX Decreases IL-6 mRNA Expression in hPASMCs after FGF2 Stimulation
Previous studies showed that IL-6 level was higher in the blood serum and lungs of patients with PAH and was negatively correlated with the prognosis of patients with PAH (Soon et al., 2010), and mice with IL-6 overexpression developed PAH (Steiner et al., 2009). In this study, IL-6 mRNA expression by FGF2 stimulation was significantly inhibited by DEX treatment in hPASMCs (Fig. 6B). MCP-1 expression tended to show a similar change, but it was not significantly different (Fig. 6B).
Discussion
Our study clarifies that DEX improves PAH in vivo and suppresses PASMC proliferation through its anti-inflammatory effect. Although the effects of DEX on the systemic circulation are previously reported (Giovannitti et al., 2015), to the best of our knowledge the effects on the pulmonary arteries in the PAH model remains unknown.
Our results demonstrate that DEX improves the survival rates of MCT-induced PAH rats with decreasing RV pressure overload and improvement of RV function indicated by RVEDP reduction (Yoshida et al., 2018). Because DEX is a sedative and pulmonary arterial pressure can be decreased by rest, it may be a concern that the effect of DEX as a sedative contributes to the improvement of survival rates. However, similar to rats without DEX infusion, those with DEX infusion were active, and heart rates during the catheterization studies were not significantly different among the groups (Supplemental Fig. 1). Thus, we consider that the DEX used in this study exerts primary effects to the pulmonary arterioles, not secondary effects to PAH as a sedative.
Consistent with this consideration, DEX attenuated muscularization of the pulmonary arterioles and PASMC proliferation in IHC. DEX suppressed hPASMC proliferation in vitro under FGF2 stimulation. The action of DEX on cell proliferation depends on the cell type. DEX inhibits proliferation of human esophageal cancer cells (Zhang et al., 2022) and human liver cancer cells (Lv et al., 2018) but promotes proliferation of human neuroglioma, human lung carcinoma (Wang et al., 2018), mouse breast cancer (Bruzzone et al., 2008), and rat adrenal pheochromocytoma (Xue et al., 2020) cells. Regarding the effect on vascular smooth muscle cells (VSMCs), DEX promotes rat aortic VSMC proliferation in vitro (Huhtinen et al., 2017). The opposite reaction to DEX treatment between aortic VSMCs and PASMCs can be explained by heterogeneous phenotypes of SMCs (Hao et al., 2003). Because the phenotype can vary due to the type of SMCs in vitro, it is important to confirm the effects in vivo. Our results suggested that PASMC proliferation was suppressed by DEX both in vivo and in vitro.
We investigated PASMC proliferation in vitro under FGF2 stimulation. FGF2 is increased in the blood of patients with PAH (Benisty et al., 2004), and PASMCs are proliferated with FGF2 stimulation in vitro (Izikki et al., 2009). Furthermore, FGF2 was upregulated in the MCT rat lungs, and rat PASMCs were proliferated with FGF2 stimulation (Hosokawa et al., 2013). Similarly, our results indicated that FGF2 was a stimulator of hPASMC proliferation, and DEX inhibited the signal.
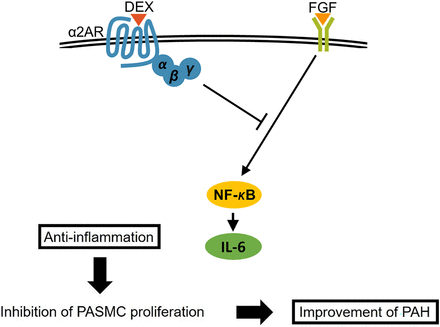
Previous studies reported that DEX suppressed inflammation through NF-κB (Kawasaki et al., 2013) and that FGF2 regulated PASMC proliferation through NF-κB and Erk 1/2 (Hosokawa et al., 2013). Therefore, we examined the effect of DEX on NF-κB related to PASMC proliferation. The result of IHC showed that DEX inhibited PASMC proliferation in rat lungs by reducing perivascular macrophage infiltration and NF-κB p-65 phosphorylation in rats. Furthermore, DEX decreased the level of IL-6, which is an inflammatory cytokine lying downstream of NF-κB in vitro. From these results, we speculated that DEX suppressed PASMC proliferation through its anti-inflammatory effect resulting from the inhibition of NF-κB activation by FGF2 (Fig. 7). One limitation of this study is that the direct effects of DEX on pulmonary arterioles were not assessed. It is not completely ruled out that the effects other than those on pulmonary arterioles of DEX contribute to improved rat survival rate. We wish to verify the vascular effects of DEX in future investigations.
Speculated signaling pathway in our study. DEX improved PAH by suppressing PASMC proliferation through its anti-inflammatory effect, which probably results from inhibition of NF-κB activation.
In conclusion, DEX improves MCT-induced PAH in rats. Furthermore, the discovery that DEX inhibits hPASMC proliferation while being nontoxic to them raises the possibility that DEX could be used as a therapeutic agent with further investigations. DEX improved PAH by reducing RV pressure by inhibiting PASMC proliferation, which resulted from its anti-inflammatory effect by inhibiting the activation of the NF-κB signaling pathway (Fig. 7).
Acknowledgments
The authors would like to thank Maruishi Pharmaceutical Co., Ltd. for providing dexmedetomidine.
Authorship Contributions
Participated in research design: Yamaguchi, Hosokawa, Haraguchi, Morio, Doi, Furukawa.
Conducted experiments: Yamaguchi, Hosokawa, Kajikawa, Sakurai, Ishii, Ando.
Performed data analysis: Yamaguchi, Kajikawa.
Wrote or contributed to the writing of the manuscript: Yamaguchi, Hosokawa, Furukawa.
Footnotes
- Received August 3, 2022.
- Accepted February 7, 2023.
This research was supported by Japanese Ministry of Education, Culture, Sports, Science, and Technology [Grant 16K10061-0].
No author has an actual or perceived conflict of interest with the contents of this article.
↵
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
Abbreviations
- DEX
- dexmedetomidine
- FGF2
- fibroblast growth factor 2
- hPASMC
- human PASMC
- IHC
- immunohistochemistry
- MCT
- monocrotaline
- NF-κB
- nuclear factor κ B
- PAH
- pulmonary arterial hypertension
- PASMC
- pulmonary arterial smooth muscle cell
- RV
- right ventricle
- RVEDP
- RV end-diastolic pressure
- VSMC
- vascular smooth muscle cell
- α-SMA
- α-smooth muscle actin
- Copyright © 2023 by The American Society for Pharmacology and Experimental Therapeutics