Abstract
There is a growing interest in the use of medicinal plants to treat a variety of diseases, and one of the most commonly used medicinal plants globally is Cannabis sativa. The two most abundant cannabinoids (Δ9-tetrahydrocannabinol and cannabidiol) have been governmentally approved to treat selected medical conditions; however, the plant produces over 100 cannabinoids, including cannabichromene (CBC). Although the cannabinoids share a common precursor molecule, cannabigerol, they are structurally and pharmacologically unique. These differences may engender differing therapeutic potentials. In this review, we will examine what is currently known about CBC with regards to pharmacodynamics, pharmacokinetics, and receptor profile. We will also discuss the therapeutic areas that have been examined for this cannabinoid, notably antinociceptive, antibacterial, and anti-seizure activities. Finally, we will discuss areas where new research is needed and potential novel medicinal applications for CBC.
SIGNIFICANCE STATEMENT Cannabichromene (CBC) has been suggested to have disparate therapeutic benefits such as anti-inflammatory, anticonvulsant, antibacterial, and antinociceptive effects. Most of the focus on the medical benefits of cannabinoids has been focused on Δ9-tetrahydrocannabinol and cannabidiol. The preliminary studies on CBC indicate that this phytocannabinoid may have unique therapeutic potential that warrants further investigation. Following easier access to hemp, CBC products are commercially available over-the-counter and are being widely utilized with little or no evidence of their safety or efficacy.
Introduction
More than 300 million people in the world consume herbal plants regularly for their therapeutic benefits, with or without strong scientific evidence (Guo and Mei, 2016). Furthermore, consumers generally have a more positive opinion (whether right or wrong) of natural products compared with prescription medications. Consumers often view natural supplements as safer, more effective, and overall better for some conditions. Although limited side effects have been reported for a number of natural substances, there are also less data available on most of these products compared with prescription medications, as they seldom have been subjected to rigorous clinical testing.
Cannabis sativa L. is one of the mostly widely used medicinal plants around the world and has been used for thousands of years, with recorded use dating back to the second millennium B.C. (Booker et al., 2009; El-Alfy et al., 2010; Blair et al., 2015). Throughout its history, cannabis has been used for spiritual, therapeutic, and recreational purposes (Booker et al., 2009). C. sativa produces a number of compounds of medicinal interest including flavonoids, terpenes, and phytocannabinoids such as Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), cannabigerol (CBG), and cannabichromene (CBC) (Izzo et al., 2012). The medicinal properties of THC and CBD are recognized and approved by government agencies (e.g., Marinol, Syndros, Cesamet, Epidiolex, and Sativex) to treat a variety of conditions, including appetite stimulation, seizure disorders, and nausea (Booker et al., 2009; Blair et al., 2015; Legare et al., 2022). Specifically, synthetically produced THC is present as the active component of both Marinol and Syndros (dronabinol), and a derivative of THC is the key component of Cesamet, all three of these medications are approved to treat nausea and vomiting and to stimulate appetite. Additionally, THC is found in a 1:1 mixture with CBD in Sativex, which is approved to treat symptoms associated with multiple sclerosis including pain, overactive bladder, and spasticity. Purified CBD of botanical origin is the principal component of the anti-seizure medication Epidiolex. It should also be noted that governmental agency approval, although often considered the highest standard for the efficacy of a medication, is not the only measure for the therapeutic utility of a given compound or supplement. Because of this, the therapeutic effects of other cannabinoids, such as CBC and CBG, are being investigated.
Here, we examine what is known about the pharmacokinetics and pharmacodynamics of CBC, as well as the reported interactions between CBC and cellular receptors. We also summarize what is currently known about the therapeutic potential of CBC, and based on this knowledge, suggest areas for future research that may also be of therapeutic potential.
Discussion
Cannabichromene Synthesis.
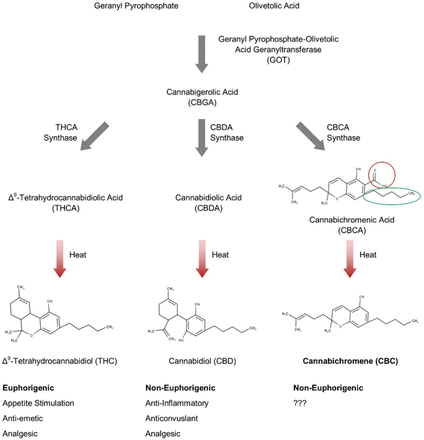
Cannabinoids are a class of terpenophenolic compounds obtained by the alkylation of olivetolic acid with geranyl-pyrophosphate by geranyl pyrophosphate-olivetolic acid geranyltransferase to produce cannabigerolic acid (CBGA), as illustrated in Fig. 1 (Nachnani et al., 2021; Odieka et al., 2022). This is the first step in which variety can be introduced to the cannabinoid structure, because geranyl pyrophosphate-olivetolic acid geranyltransferase can use other phenolic moieties with different alkyl chain lengths, such as divarinolic acid. The use of divarinolic acid leads to cannabinoids that have a three-carbon side chain (i.e., the varniol family of cannabinoids) (highlighted in Fig. 1). CBGA and its derivatives are the substrates for three additional enzymes that are responsible for producing the three other major families of cannabinoids: Δ9-tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), and cannabichromenic acid (CBCA) (Pattnaik et al., 2022). Decarboxylation, via heat and drying, then converts the acidic forms (THCA, CBDA, CBCA, and CBGA) into their neutral forms (THC, CBD, CBC, and CBG) (Odieka et al., 2022). Subsequent spontaneous conversions produce the scores of other cannabinoid molecules.
Biosynthesis of CBC. The enzymatic pathway that leads to CBC production is illustrated. CBC is enzymatically produced from the cannabinoid precursor CBGA, via a CBCA intermediate. Decarboxylation (red circle) of CBCA through heat results in the neutral CBC. The green circle highlights the alkyl-side chain that can vary in length from three to seven carbons in cannabinoids isolated from Cannabis sativa L.
CBC can be produced synthetically from citral and olivetol in several different ways. These synthetic processes are important because most strains of cannabis produce only small amounts of CBC. Yields of CBC from these chemical processes have been reported between 40%–75% depending on the reaction conditions (Lee and Wang, 2005; Pollastro et al., 2018; Quílez del Moral et al., 2021).
Pharmacokinetics of CBC.
A key to assessing the potential therapeutic utility of any compound is to understand its metabolism and bioavailability. There are limited studies involving CBC. When administered at 10 mg/kg via intraperitoneal injection in rats, the maximum concentration in plasma was reached in 30 minutes, whereas brain concentrations did not peak until 120 minutes (Anderson et al., 2021). This study also found that CBC has a relatively significant half-life in both plasma (98 minutes) and brain (193 minutes), and the total exposure of CBC in the brain tissue was close to that of plasma (brain–plasma ratio of 0.83) (Anderson et al., 2021). When comparing the pharmacokinetics of CBC to other cannabinoids, the maximum concentration in plasma for CBG (120 mg/kg) and CBD (120 mg/kg) occurs 120 minutes after injection; however, for cannabidivarin (CBDV) (60 mg/kg) and Δ9-tetrahydrocannabivarin (THCV) (30 mg/kg) peak concentration in the plasma occurs at 30 minutes (Deiana et al., 2012). Although the study by Deiana et al. (2012) did not examine the pharmacokinetics of CBC, the results from the Anderson et al. (2021) study suggest that time-to-peak plasma concentrations of CBC are more similar to THCV and CBDV. In the brain, Deiana et al. (2012) found CBDV and THCV concentrations peak at 30 minutes, CBD concentrations peak at 60 minutes, and CBG concentrations peak at 120 minutes. In contrast, in the brain, CBC pharmacokinetics are closer to those observed for CBG (Deiana et al., 2012; Anderson et al., 2021).
In humans, the pharmacokinetics of CBC was investigated in the presence of CBD and THC. The study examined daily administration of CBC and saw that in the presence of CBD and THC, CBC was tolerable up to daily doses of 26.4 mg (Peters et al., 2022a). The study on the CBC pharmacokinetics was a follow-up study in which patients were given four different doses of a cannabis extract (Peters et al., 2022b). Initially, the authors were interested in the pharmacokinetics of CBD and THC and administered increasing daily doses of oil based on CBD and THC content. However, subsequent analysis of the oil revealed higher levels of CBC than THC in the extract (oil composition: 20 mg/ml CBD, 0.9 mg/ml THC, and 1.1 mg/ml CBC) (Peters et al., 2022a,b). Study participants were divided into five groups based on daily cannabinoid dose: group A (120 mg CBD, 5.4 mg THC, 6.6 mg CBC), group B (240 mg CBD, 10.8 mg THC, 13.2 mg CBC), group C (360 mg CBD, 16.2 mg THC, 19.8 mg CBC), group D (480 mg CBD, 21.6 mg THC, 26.4 mg CBC), or placebo.
These studies found that THC was quantifiable in fewer plasma samples than CBC even though the doses of THC (21.6 mg) and CBC (24.6 mg) were similar in the treatments (Peters et al., 2022a,b). In the highest treatment group (treatment D), although the dose of CBD was 480 mg (approximately 18-fold higher than CBC), the area under the curve0-t of CBD was 6.6- to 9.8-fold higher than the area under the curve0–t of CBC (Peters et al., 2022a). The Peters and colleagues suggest that that CBC may have preferential absorption over CBD or THC; however, differential metabolism is also a possibility especially in light of the fact that data on the metabolism of CBC is lacking in the literature. The study found an average tmax for CBC of 3.3 hours; this compares to an average tmax for CBD of 4.5 hours, a tmax for THC was not able to be quantified due to levels below detection (Peters et al., 2022a,b). In another human study, looking at the pharmacokinetics of THC and CBD, when given orally at a dose of 20 mg THC or 40 mg CBD maximal plasma concentrations occurred after 60–120 minutes, a much shorter tmax than reported by Peters et al. (2022b) and Grotenhermen (2003).
Additional studies will be needed, particularly with CBC alone to better understand the pharmacokinetics and pharmacodynamics of this molecule. It is interesting to note that despite being administered at similar levels and having similar levels of detectability, the levels of THC were always undetectable (metabolites of THC were detectable), the levels of CBC were always within detectable limits in patients (Peters et al., 2022a,b). Currently, there is a marked absence in the literature on the enzymatic metabolism of CBC, and this is a key area where data are needed. Similarly, additional data are needed to understand the distribution and pharmacokinetics of CBC.
CBC Activity in the Endocannabinoid System.
The activity of CBC at the cannabinoid receptor 1 (CB1) remains unclear, despite a number of studies that have investigated this activity. CBC was found to displace CP 55,940, a potent synthetic CB1 and cannabinoid receptor 2 (CB2) agonist, from CB1-containing cell membranes in cultured cells and act as an agonist by inhibiting forskolin-stimulated cAMP synthesis (Rosenthaler et al., 2014; Zagzoog et al., 2020). However, CBC was not found to induce recruitment of β-arrestin-2 in the latter study (Zagzoog et al., 2020). This study also found that CBC reduced the amount of intracellular glutathione in primary mesencephalic cell culture, although the receptor responsible for this was not identified. In contrast to these two studies, CBC at a concentration up to 10 µM failed to displace [3H]-CP55,940 from the CB1 receptor in whole rat brain membranes (Booker et al., 2009). The activity of CBC on the endocannabinoid system is summarized in Table 1. Importantly, the reported binding affinities of CBC at the CB1 receptor, although controversial, are similar to CBG and lower than those reported for Δ9-THC [reviewed in Nachnani et al. (2021) and Legare et al. (2022)]. In contrast, CBC was not found to induce hyperpolarization of pituitary cells overexpressing CB1 (Udoh et al., 2019). Several other studies have also found that CBC does not activate CB1, does not stimulate [35S]-GTPγS binding, nor does it inhibit adenylate cyclase activity (Howlett, 1987; Romano et al., 2013). Furthermore, the lack of activation of CB1 is consistent with the observation that CBC is non-psychoactive (DeLong et al., 2010; Zagožen et al., 2020). These data suggest that although CBC may bind to CB1, this binding does not stimulate activation of the receptor; importantly, CBC did not inhibit the activation of CB1 by CP 55,940 or THC (Udoh et al., 2019). Alternatively, CBC may exhibit biased signaling via the CB1 receptor. Importantly, it has been reported for morphine receptors that failure to recruit β-arrestins can improve analgesia with reduced side effects in mice and mediates reward; therefore, the non-euphorigenic nature of CBC may be linked to the compounds inability to stimulate recruitment of β-arrestin-2 (Darcq and Kieffer, 2018). This is clearly an area where additional studies are needed. The distribution of CB1 receptors within the central nervous system are illustrated in Fig. 2.
Activity of CBC at endocannabinoid receptors and enzymes
Ki and EC50/IC50 values are in nM.
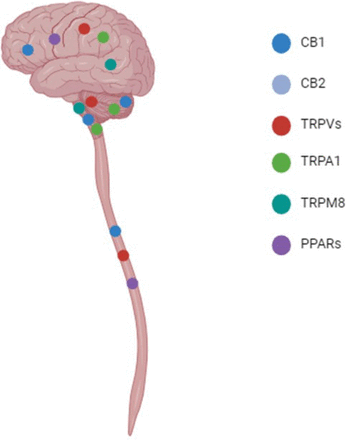
Central nervous system distribution of receptors. The distribution of CBs, TRP channels, and PPAR receptors within the central nervous system are shown. In particular, CB1 expression is high within the cerebral cortex, hypothalamus, and cerebellum; while lower expression has been reported in the brainstem and spinal cord. CB2 is expressed on astrocytes and microglia within the CNS. TRPVs are reported to be expressed in the cerebral cortex, hippocampus, cerebellum, and spinal cord. TRPA1 expression is detected in the olfactory bulb, hippocampus, hypothalamus, cerebral cortex, cerebellum, and brainstem. TRPM8 is expressed at low levels in the hypothalamus and brain steam. Finally, PPARβ/δ is widely distributed within the cerebrum, whereas PPARα is expressed at low levels in astrocytes and PPARγ is expressed by microglia. All three isoforms of PPAR have been detected in the spinal cord (Emir, 2017; Kendall and Yudowski, 2017; Zolezzi et al., 2017; Okine et al., 2019; Haspula and Clark, 2020; Souza Monteiro de Araujo et al., 2020; Ordás et al., 2021).
There is a better agreement of the function of CBC at CB2, where CBC acts as an agonist. CBC has been shown to bind to CB2 and to inhibit forskolin-stimulated cAMP production (Rosenthaler et al., 2014; Zagzoog et al., 2020). The affinity for CBC at the CB2 receptor is similar to THC, and compared with other reported cannabinoids such as CBG and CBD, it is slightly higher [reviewed in Nachnani et al. (2021) and Legare et al. (2022)]. CBC has also been shown to activate CB2 in a hyperpolarization assay, and this activity can be inhibited by pertussis toxin, indicating that the activation is coupled to Gi/o signaling (Udoh et al., 2019). This study also showed that hyperpolarization induced by CBC could be blocked by the CB2-selective antagonist AM630. Importantly, activation of CB2 would be consistent with the reported anti-inflammatory activity of CBC discussed below.
The activity of CBC at other GPCRs that have been shown to bind cannabinoids, such as GPR55, GPR18, and GPR119, has not been reported and it will be interesting to see if any of these receptors can be activated or inhibited by CBC. Cannabinoids can also impact the endocannabinoid system by altering the metabolism of the endocannabinoids anandamide [arachidonoylethanolamine (AEA)] and 2-arachidonoyl glycerol (2-AG). CBC has been reported to inhibit the activity of the 2-AG degrading enzyme monoacylglycerol lipase but not the AEA degradation enzyme fatty acid amide hydrolase (De Petrocellis et al., 2011). Therefore, CBC may skew the endocannabinoid system by increasing levels of 2-AG relative to AEA. However, data on the effects of CBC on endocannabinoid metabolizing enzymes are limited and the compound remains unstudied at a number of other GPCRs that are known to bind cannabinoids making this another area where more data are clearly needed.
CBC Activity at Transient Receptor Potential (TRP) Cation Channels.
In a study that investigated the activation of several TRP channels by cannabinoids, it was found that CBC was the most potent activator of transient receptor potential ankyrin (TRPA1), with an EC50 of 90 nM (De Petrocellis et al., 2008, 2011). These studies also found CBC to be an agonist of transient receptor potential vanilloid (TRPV)1; interestingly the EC50 of CBC at this receptor is closer to the acidic cannabinoids (i.e., CBGA, CBDA), whereas the other neutral cannabinoids (i.e., CBG, THCV) have EC50 values half that found for CBC (De Petrocellis et al., 2011). Furthermore, CBC was found to be an antagonist of transient receptor potential melastatin (TRPM8), although, compared with the other cannabinoids tested CBC was the least potent TRPM8 antagonist (De Petrocellis et al., 2007, 2011). In a follow-up study CBC was also found to activate TRPV3 similar to other cannabinoids and was found, along with CBD, to be a highly potent agonist of TRPV4 (De Petrocellis et al., 2012). The function of CBC at TRP channels is summarized in Table 2. Distribution within the CNS of TRP channels is illustrated in Fig. 2.
Activity of CBC at TRP ion channels
Values are µM.
CBC Activity on Peroxisome Proliferator-Activated Receptor (PPAR) Receptors.
Many cannabinoids have been found to activate the PPAR nuclear receptor family of transcription factors, in particular PPARα and PPARγ. CBC has been found to be an agonist at PPARγ receptors (Table 3); however, this study failed to find an EC50 for CBC because the highest dose tested was 25 µM and it failed to produce a maximal effect (Granja et al., 2012). CBC was the least potent cannabinoid agonist of PPARγ in the study and is slightly less potent than THC (EC50 = 21.2 µM) or CBD (EC50 = 20.1 µM) based on the presented dose–response curves, and much less active than CBG (EC50 = 12.7 µM) (Granja et al., 2012). It has not been determined if CBC can activate PPARα transcription factors. PPAR receptor distribution within the CNS is illustrated in Fig. 2.
Activity of CBC at PPAR receptors
EC50/IC50 values are all in µM.
Potential Therapeutic Effects for CBC
Based on the pharmacological activities and pharmacokinetics of CBC described above, there are a number of reasons to think that CBC will possess diverse therapeutic activities. In this section, we will further examine the research that demonstrates the anti-inflammatory, anti-convulsant, anti-microbial, anti-nociceptive, and anti-anxiety effects of CBC.
Anti-Inflammatory Properties.
The anti-inflammatory properties of CBC are the most characterized and have been documented in both in vitro and in vivo animal models. CBC treatment, of lipopolysaccharide (LPS, a bacterial toxin)-stimulated, peritoneal macrophages, was found to reduce production of nitrite, INF-γ, and interleukin (IL)-10 (Romano et al., 2013). A similar reduction in inflammatory cytokine production was found following CBC treatment of the macrophage cell line, RAW 267.2, after stimulation with LPS. This study found a decrease in nitrite production and decreases in mRNA levels for iNOS, IL-1β, IL-6, and TNF-α (Hong et al., 2023). The decrease in IL-1β and iNOS mRNA levels differs from what was observed with primary macrophages and may be due to a higher concentration of CBC used in the second study (20 µM vs. 1 µM) or a difference between primary macrophages and the response of an immortalized cell line. The production of anti-inflammatory cytokines upon treatment with CBC is not limited to macrophages. A recent study observed a decrease in IL-8 after 24 hours and a decrease in IL-22 after 48 hours in LPS-stimulated keratinocytes following treatment with CBC (Tortolani et al., 2023).
The anti-inflammatory effects of CBC have also been reported in a number of animal models. Most notably, several studies have found that treatment with CBC reduces inflammation and edema associated with carrageenan injection into an animal’s hind paw (mice and rats) (Wirth et al., 1980; Turner and Elsohly, 1981; Hong et al., 2023). A similar effect was found in a study using LPS to induce edema in the paw of mice (DeLong et al., 2010). Interestingly, this latter study found that there was an additive effect on edema in mice treated with both THC and CBC. Antagonist experiments performed as part of this study suggest that THC was mediating anti-inflammatory response via CB2, whereas the effects of CBC were not mediated by either CB1 or CB2. This could indicate a potential synergy between the two molecules or other pharmacological interactions between these two molecules. It has also been found that CBC is able to reduce pain during the inflammatory portion of the formalin assay, in which formalin is injected into the hind paw and then animals are monitored for signs of discomfort (Raup-Konsavage et al., 2023). CBC has also been shown to decrease inflammation driven hypermotility of the small intestine in the croton oil model, which was found to occur independently of both CB1 and CB2, as well as TRPA1 (Izzo et al., 2012). Further supporting the anti-inflammatory activity of CBC, it was found that, in the dinitrobenzene sulfonic acid model of colitis, treatment with CBC reduces the severity of inflammation and tissue damage (Romano et al., 2013). Finally, inhaled CBC was shown to reduce cytokine production and inflammation in a mouse model of acute respiratory distress syndrome via TRPA1- and TRPV1-mediated mechanisms (Khodadadi et al., 2021).
Importantly, several of these studies have indicated that the anti-inflammatory mechanism activated by CBC involves the MAPK pathway. However, the receptor mediating this activation remains unclear with evidence suggesting the involvement of CB1, CB2, and/or TRPA1; there are also data that suggest additional, unknown mechanisms for some conditions (Izzo et al., 2012; Romano et al., 2013; Hong et al., 2023; Tortolani et al., 2023). These studies strongly suggest that CBC has anti-inflammatory activities that may be beneficial for diseases such as inflammatory bowel disease and inflammatory pain disorders such as rheumatoid arthritis; however, a better understanding of the mechanism is needed and this appears to vary with inflammatory condition.
Anticonvulsant Properties.
Recent studies have reported that CBC is an abundant phytocannabinoid present in artisanal oils used to treat epilepsy, along with CBD, CBDA, Δ9-THC, and Δ9-THCA. Moreover, these oils were effective at lower doses of CBD than the approved anti-seizure medication Epidiolex (purified CBD), suggesting that other cannabinoids or terpenes in the oils may have anti-seizure activity (Suraev et al., 2018; Anderson et al., 2021; Legare et al., 2022). The anticonvulsant potential of CBC was tested in hypothermia-induced seizures in the Scn1a+/− mouse model of Dravet syndrome and showed that CBC was as effective at reducing seizures as CBD (Anderson et al., 2021). This report also found that CBCA and cannabichromevarinic acid (CBCVA), but not CBCV, were equally effective at reducing seizures. The anti-seizure properties of CBC were confirmed in a zebrafish model, where CBC was found to reduce pentylenetetrazol-induced seizures at a lower dose (1 µM) than either CBD (2 µM) or cannabinol (4 µM) (Kollipara et al., 2023). However, CBC was unable to protect mice from electroshock-induced seizures (Davis and Hatoum, 1983). Taken together, these studies suggest that CBC may have anti-seizure properties similar to CBD; however, additional studies are needed not only to confirm the efficacy of CBC to reduce seizures, but also the mechanisms underlying this activity.
Antinociceptive Properties.
Phytocannabinoids such as THC, CBD, and CBG have been found to demonstrate antinociceptive effects in animal models (Ward et al., 2011, 2014; Henderson-Redmond et al., 2021; Stauch et al., 2021; Sepulveda et al., 2022a,b; Nachnani et al., 2023). Additionally, pain is a major issue for which patients report using medical cannabis and cannabis extracts (Alexander, 2016; Aviram and Samuelly-Leichtag, 2017; Legare et al., 2022). CBC, when applied directly into the periaqueductal gray (PAG) region of the brain in rats, was found to increase tail-flick latency, indicating an increased pain threshold (Maione et al., 2011). Similarly, in mice, CBC was found to have analgesic effects in the tail-flick assay and, when combined with THC, there was a synergistic effect on anti-nociceptive activity (Davis and Hatoum, 1983). In contrast, another study found only an additive effect of THC and CBC in the mouse tail-flick assay (DeLong et al., 2010). This contrast may be due to a higher dose of THC used in the earlier study (20–50 mg/kg). The later study used a dose of THC (0.3 mg/kg) that alone did not produce any effect (Davis and Hatoum, 1983; DeLong et al., 2010). In a recent study from our laboratory, we report that CBC, when administered at 20 mg/kg, demonstrated anti-nociceptive properties not only in the tail-flick assay but also formalin-induced inflammatory pain and cisplatin-induced peripheral neuropathy models, suggesting a broad range of anti-nociceptive uses for this cannabinoid (Raup-Konsavage et al., 2023).
Evidence suggests that CBC mediates its antinociceptive properties through CB1, TRPA1, and the adenosine A1 receptor (A1R) as antagonists of CB1, TRPA1, and A1R could block the antinociceptive effects of CBC (Maione et al., 2011). These data support the findings that CBC is an agonist of TRPA1, corroborate CBC binding at CB1 (discussed above), and also support previous work that found an interaction between the CB1 receptor and A1R (Ferré et al., 2010; De Petrocellis et al., 2011; Zagzoog et al., 2020). In contrast, others have reported no role for the CB1 receptor in mediating the anti-nociceptive properties of CBC, as the CB1 antagonist rimonabant failed to block the pain reducing activity of CBC (DeLong et al., 2010). This finding is in line with other studies that have found that CBC does not have activity at the CB1 receptor (Howlett, 1987; Romano et al., 2013; Udoh et al., 2019). Based on these findings, more work is needed to understand the mechanism by which CBC reduces pain, but also the activity of CBC at the CB1 receptor.
Antimicrobial.
Several studies have examined the impact of cannabinoids as antibacterial and antifungal agents, especially in light of the need for the development of novel antimicrobial agents due to increasing drug resistance. Of the cannabinoids tested, CBC has been found to be one of the most effective. One study found CBC to have a similar antibacterial activity to the antibiotic streptomycin in Gram-positive bacteria (Bacillus subtilis and Staphylococcus aureus) and mycobacteria (Mycobacterium smegmatis) (minimum inhibitory concentration 0.39–25 vs. 1.56–25 µg/ml) (Eisohly et al., 1982). In contrast, CBC was found to be ineffective against Gram negative bacteria (Escherichia coli) in this study. Importantly, additional studies have found that CBC and CBCA have antibacterial activity against methicillin-resistant S. aureus [minimum inhibitory concentration (MIC) 1–2 µg/ml for CBC and MIC 3.9–7.9 µM for CBCA] (Appendino et al., 2008; Farha et al., 2020; Galletta et al., 2020). The antifungal activities of CBC have also been reported and, although CBC does have some antifungal activity (MIC 25–50 µg/ml), it is much less efficacious than amphotericin B (MIC 3.12 µg/ml) (Eisohly et al., 1982). CBC (12.5%) has also been reported to be highly effective, second only to cannabinol, at reducing the growth of oral bacteria isolated from dental plaque of patients (Stahl and Vasudevan, 2020). Interestingly, this study found that all cannabinoids (12.5%) tested were markedly more effective than commercial toothpastes (undiluted) at reducing colony formation on agar plates. These studies, although limited, suggest that CBC has potential as a novel antibacterial agent particularly against gram-positive bacteria, with notable activity against current antibiotic-resistant strains. However, additional studies will be needed to confirm and broaden these initial studies, particularly as it pertains to understanding their activity spectrum and potential off target effects against commensal members of the microbiota.
Other Potential Therapeutic Areas.
One study has examined the potential antidepressant activity of CBC. This study found that CBC reduced depression-like behaviors in the forced swim test in mice at a commonly used dose (20 mg/kg) (El-Alfy et al., 2010). This dose of CBC was ineffective in a second model of depression, the tail-suspension test; however, higher doses of CBC were effective in this assay (El-Alfy et al., 2010).
Many cannabinoids, particularly THC and CBD, have been reported to reduce the viability of cancer cells, and one study has examined the effects of CBC on cancer cell growth (Gaweł-Bęben et al., 2023). The study by Gaweł-Bęben et al. (2023) found that CBC (IC50 = 23.0 µM) had an activity against melanoma cell lines similar to the anticancer medication, 5-fluorouracil (IC50 = 30.0 µM).
Although single studies are certainly not enough to warrant the indication of CBC to treat these conditions, they do suggest areas where additional research is needed to validate the effectiveness of CBC to treat depression and cancer. Additionally, these studies highlight that there may be many other conditions where CBC may have medical utility. A summary of the in vitro work on the potential therapeutic activity of CBC is summarized in Table 4.
Summary of in vivo experiments on the therapeutic benefits of CBC
Shown is the animal used, route of CBC delivery and dose, along with the experimental model used and the major outcome of the study. Dose is provided as mg/kg unless otherwise noted.
Conclusion
The growing interest in cannabis and cannabinoids for medicinal purposes has led to an increase in studies examining the effects of minor cannabinoids such as CBC. Although the studies on CBC remain limited, the current data do indicate a promising therapeutic potential for CBC, particularly as an anti-inflammatory, anti-microbial, anti-convulsant, and antidepressant agent. Further studies are needed to expand on what is currently known regarding the use of CBC to treat disease, as well as the safety and tolerability of this medication in humans.
Acknowledgments
This work was supported by National Institutes of Health National Center for Complementary and Integrative Health (R01AT012053-01 to K.E.V.). Additionally, K.E.V. and the Penn State College of Medicine are the recipients of research support from PA Options for Wellness (a state-approved medical marijuana clinical registrant). The funding sources were not involved in study design, providing any experimental materials, data collection, analysis and interpretation, writing of the report, or the decision to submit the article for publication.
Data Availability
This article contains no datasets generated or analyzed during the current study.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Sepulveda, Vrana, Kellogg, Bisanz, Desai, Graziane, Raup-Konsavage.
Footnotes
- Received February 6, 2024.
- Accepted April 24, 2024.
This work was supported in part by a grant from the National Institutes of Health National Center for Complementary and Integrative Health [Grant R01AT012053-01] (to K.E.V.). Additionally, KEV and the Penn State College of Medicine are the recipient of research support from PA Options for Wellness (a state-approved medical marijuana clinical registrant). The funding sources were not involved in study design, providing any experimental materials, data collection, analysis and interpretation, writing of the report, or the decision to submit the article for publication.
No author has an actual or perceived conflict of interest with the contents of this article.
Abbreviations
- 2-AG
- 2-arachidonoylglycerol
- AEA
- anandamide (arachidonoylethanolamine)
- CB1
- cannabinoid receptor 1
- CB2
- cannabinoid receptor 2
- CBC
- cannabichromene
- CBCA
- cannabichromenic acid
- CBD
- cannabidiol
- CBG
- cannabigerol
- CBGA
- cannabigerolic acid
- FAAH
- fatty acid amide hydrolase
- IL
- interleukin
- LPS
- lipopolysaccharide
- PPAR
- peroxisome proliferator-activated receptor
- THC
- Δ9-tetrahydrocannabinol
- TRP
- transient receptor potential
- TRPA1
- transient receptor potential ankyrin
- TRPV
- transient receptor potential vanilloid
- TRPM8
- transient receptor potential melastatin
- Copyright © 2024 by The Author(s)
This is an open access article distributed under the CC BY-NC Attribution 4.0 International license.