Abstract
Δ9-Tetrahydrocannabinol (THC) is a psychoactive phytocannabinoid found in the Cannabis sativa plant. THC is primarily metabolized into 11-hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC) and 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (COOH-THC), which may themselves be psychoactive. There is very little research-based evidence concerning the pharmacokinetics and pharmacodynamics of 11-OH-THC as an individual compound. Male C57BL/6 mice were treated with THC or 11-OH-THC via intraperitoneal injection, tail vein intravenous injection, or oral gavage, and whole-blood compound levels were measured to determine pharmacokinetic parameters [Cmax, time to Cmax (Tmax), elimination half-life, area under the curve, apparent volume of distribution, systemic clearance, terminal rate constant, and absolute bioavailability] while also monitoring changes in catalepsy, body temperature, and nociception. 11-OH-THC achieved a Tmax at 30 minutes for all routes of administration. The maximum concentration at 30 minutes was not different between intravenous and intraperitoneal routes, but the oral gavage Cmax was significantly lower. THC had a 10-minute time to the maximum concentration, which was the first blood collection time point, for intravenous and intraperitoneal and 60 minutes for oral gavage, with a lower Cmax for intraperitoneal and oral gavage compared with intravenous. When accounting for circulating compound levels and ED50 responses, these data suggest that 11-OH-THC was 153% as active as THC in the tail-flick test of nociception and 78% as active as THC for catalepsy. Therefore, 11-OH-THC displayed equal or greater activity than the parent compound THC, even when accounting for pharmacokinetic differences. Thus, the THC metabolite 11-OH-THC likely plays a critical role in the bioactivity of cannabis; understanding its activity when administered directly will aid in the interpretation of future animal and human studies.
SIGNIFICANCE STATEMENT This study establishes that the primary metabolite of THC, 11-OH-THC, displays equal or greater activity than THC in a mouse model of cannabinoid activity when directly administered and even when accounting for route of administration, sex, pharmacokinetic, and pharmacodynamic differences. These data provide critical insight into the bioactivity of THC metabolites that will inform the interpretation of future in vivo cannabinoid research and represent a model for how THC consumption and metabolism may affect cannabis use in humans.
Introduction
The use of cannabis has significantly increased over the last 20 years (Procaccia et al., 2022). The laws governing cannabis use were reformed globally, increasing access to cannabis-based products and decreasing the stigma around cannabis use (Zamarripa et al., 2023). The recreational and medical effects of cannabis have been attributed to more than 140 phytocannabinoids, particularly Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), which are the most studied cannabinoids (Ruiz et al., 2021; Procaccia et al., 2022). THC is the key constituent of cannabis and the main causative agent of its psychoactive effects (Gracia-Lor et al., 2016; Śmiarowska et al., 2022).
The earliest recorded report on the pharmacokinetics (PK) of THC showed that the oxidative metabolism of THC by cytochrome P450 2C9 (CYP2C9) results in two metabolites: the active metabolite, 11-hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC), and the inactive metabolite, 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (COOH-THC) (Huestis et al., 1992; Maurer et al., 2006; Barrus et al., 2016). CYP2C9 forms the pharmacologically active 11-OH-THC, which is further oxidized to the intermediate aldehyde 11-oxo-THC, followed by oxidation to COOH-THC catalyzed by a microsomal aldehyde oxygenase. After glucuronidation of the carboxy group, COOH-THC is excreted in feces and urine. (Ramzy and Priefer, 2021). THC exhibits a rapid onset of effects after smoking, with 11-OH-THC appearing immediately afterward at lower levels. In contrast, the metabolite COOH-THC displays a slower onset and prolonged presence (Huestis et al., 1992). Oral gavage administration of THC results in slow absorption due to first-pass metabolism in the liver. After oral ingestion, 11-OH-THC levels can be significantly higher than inhalation because of this extensive first-pass metabolism (Huestis et al., 1992; Schwilke et al., 2009). THC and 11-OH-THC are both lipophilic compounds and therefore distribute widely in fatty tissues, such as the brain and adipose tissue, with a slower release over time (Huestis et al., 1992; Schwilke et al., 2009). Previous data suggest that THC, 11-OH-THC, and Δ9-tetrahydrocannabinol acetate (Δ9-THC-O-acetate) are responsible for the cannabis-induced psychomimetic effects (https://healthpolicy.usc.edu/wp-content/uploads/2022/07/USC-Schaeffer-Center-white-paper_Federal-Regulation-of-Cannabis-for-Public-Health-in-the-United-States.pdf). Furthermore, 11-OH-THC was shown to be more potent than THC (Lemberger et al., 1972), and its higher ability to cross the blood-brain barrier was demonstrated in an early, albeit limited, study on 12 men (Perez-Reyes et al. 1972). These early studies indicate that 11-OH-THC is a psychoactive cannabinoid; however, the PK and pharmacodynamics (PD) of 11-OH-THC have not previously been directly assessed in a preclinical model system compared with the parent compound, THC.
Many of the effects of cannabis arise from the interaction between phytocannabinoids and the endocannabinoid system. The endocannabinoid system includes the Gαi/o-coupled G protein–coupled receptors cannabinoid 1 (CB1R) and cannabinoid 2 (CB2R). THC is a CB1R and CB2R partial agonist. 11-OH-THC is a partial agonist of CB1R with a higher affinity for CB1R than THC, and 11-OH-THC displays greater potency in tests of catalepsy, nociception, and drug discrimination in mice than THC [Wiley et al., 2021; reviewed in Sideli et al. (2021)]. We have previously reported that 11-OH-THC displays nanomolar binding affinity to CBR, nanomolar potency in the cAMP inhibition assay, and had similar effects to THC in the βarrestin2 recruitment assay (Zagzoog et al., 2022). In male C57BL/6 mice, the effects of intraperitoneal 11-OH-THC are equivalent to or exceed the effects of THC (Wiley et al., 2021; Zagzoog et al., 2022).
In the present study, we hypothesized that 11-OH-THC would produce an intoxicating effect of higher magnitude than THC, even when accounting for potential PK differences. To test this hypothesis, C57BL/6Crl mice were treated with THC and 11-OH-THC intravenously,intraperitoneally,or by oral gavage, and blood samples were acquired to estimate pharmacokinetic parameters [Cmax, time to Cmax (Tmax), elimination half-life (t1/2), area under the curve (AUC), apparent volume of distribution (Vd), clearance (ClS), terminal rate constant (k), and absolute bioavailability (F)]. From these data, we then further assessed THC and 11-OH-THC activity at Tmax via intravenous,intraperitoneal, or oral gavage routes and finally assessed intravenous THC and 11-OH-THC potency and efficacy relative to peak blood drug levels to estimate the intoxication equivalency for 11-OH-THC compared with THC. This study provides valuable data on how 11-OH-THC pharmacology differs from THC and supports the notion that these differences are of medicinal importance and are significant in harm reduction.
Materials and Methods
Materials
THC (Cat. No. 12068) and 11-OH-THC (Cat. No. 21667) were purchased from Cayman Chemical (Ann Arbor, MI) and stored at –20°C. Analytical standards and deuterated internal standards for THC (Cat. No. T-047), 11-OH-THC (Cat. No. H-027), COOH-THC (Cat. No. T-010), THC-d3 (Cat. No. T-003), 11-OH-THC-d3 (Cat. No. H-041), and COOH-THC-d3 (Cat. No. T-004) were purchased from Cerilliant (Round Rock, TX). Liquid chromatography–mass spectrometry–grade methanol, water, acetonitrile, formic acid, and ammonium formate were purchased from Thermo Scientific (Waltham, MA). All other materials—ethanol, Kolliphor, PBS, DMSO, and HybridSPE-Phospholipid—were from Sigma-Aldrich (Mississauga, ON).
Animals
Male and female C57BL/6Crl mice aged 8–12 weeks were purchased from Charles River Laboratories (Senneville, QC, Canada). Mice of the same sex were group housed [three (males) to five (females) per cage] at the Laboratory Animal Services Unit at the University of Saskatchewan with a standard 12:12 light/dark cycle, ad libitum access to food and water, and environmental enrichment. Animals were acclimatized with no handling for 7 days followed by another 7–10 days with handling prior to experimentation (n = 224; 172 male and 76 female). All protocols complied with the guidelines detailed by the Canadian Council on Animal Care and were approved by the University of Saskatchewan Animal Research Ethics Board (Animal Use Protocol 20200043). The study was conducted per the Canadian Council on Animal Care and the ARRIVE guidelines (Percie Du Sert et al., 2020).
Experimental Design
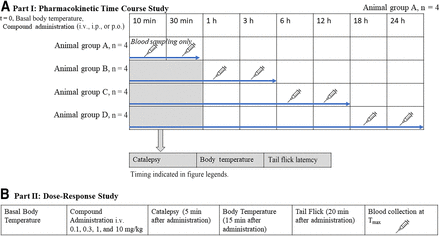
Part I: Pharmacokinetic Time Course Study.
A single dose of 11-OH-THC or THC 10 mg/kg tail vein i.v., i.p., or by oral gavage was administered to male mice for this study. The compound for injection intravenously or intraperitoneally was dissolved in 10% DMSO in PBS and then mixed into a vehicle consisting of 1:1:18 ethanol:Kolliphor EL:1 M PBS. The oral gavage dose was prepared similarly (10% DMSO) and dissolved in olive oil. The vehicle was altered for oral gavage administration because the 1:1:18 vehicle is not palatable to mice. Additionally, oral gavage administration using olive oil was chosen as it closely mimics human use. We acknowledge that this choice might affect absorption, which is why part II (below) was completed using only intravenous administration. This approach overcomes the variability introduced by other routes of administration. Each animal was assigned to provide two blood samples at a pair of time points. Sampling time point pairs were 10 minutes and 30 minutes for group A, 1 hour and 3 hours for group B, 6 hours and 12 hours for group C, and 18 hours and 24 hours for group D (n = 4 per group; i.e., each data point is the mean of four measurements) (Fig. 1). The first sample for each pair was collected via the saphenous vein. At the second time point, animals were killed by deep anesthesia using isoflurane (3%–5%) for approximately 2 to 3 minutes, and cardiac puncture was used to collect the blood sample.
Timeline diagram of experimental design for this study. (A) Pharmacokinetic time course study. (B) Dose-response study.
All animals underwent both PK analysis and physiological assessment (as described below) except for animals assigned time points 10 minutes and 30 minutes due to time constraints (Fig. 1). Pharmacokinetic parameters for THC and 11-OH-THC were determined using GraphPad Prism (v. 9.0). Cmax and Tmax for intraperitoneal and oral gavage administration were obtained directly from the average blood concentration-versus-time profiles. The area under the curve (AUC) for blood concentration-versus-time was calculated using the log-linear trapezoidal method with extrapolation to infinity determined from the ratio of the last measured blood concentration and the terminal rate constant. The terminal phase rate constant was determined by linear regression analysis of the postdistributive natural log-linear terminal concentration-versus-time determinations. The t1/2 was determined as the ratio 0.693/k. ClS was calculated as the Doseintravenous/AUC∞. The Vd was calculated as Doseintravenous/(k × AUC∞). F after intraperitoneal administration was determined as (AUCoral gavage × Doseintravenous)/(AUCintravenous × Doseoral gavage). Note, F could not be determined following oral gavage administration due to the limited number of sampling time points. Additionally, any data point below the lowest limit of quantification (4 ng/mL) was excluded.
Physiological assessments made in part I included tests for catalepsy, body temperature, and nociception.Catalepsy was assessed in the bar-holding assay 5 minutes after compound administration with animals placed so that their forepaw clasped a 0.5-cm ring clamp 5 cm above the surface of the testing space. The trial ended when the mouse removed their forepaws from the bar, turned its head or body, or was immobile for more than 60 seconds [i.e., a percentage of maximum possible effect (MPE) of 60 seconds]. This was performed three times, and the average time spent holding the bar was used for analysis. For animals included in the PK study, body temperature was only measured 15 minutes after compound administration using a rectal thermometer. Antinociceptive effects were measured using the tail-flick test 20 minutes after compound administration. This trial consisted of mice restrained so that the tail was submerged approximately 1 cm into warm water (52 ± 2°C). The trial ended once the mouse removed its tail from the water. Total time before the end of the trial was recorded to a maximum of 20 seconds (i.e., an MPE of 20 seconds) (Zagzoog et al., 2022).
After estimating the Tmax, we assessed another cohort of male and female mice that received either THC or 11-OH-THC via intravenous, intraperitoneal, or oral gavage administration. For animals receiving THC via intravenous or intraperitoneal injection, we followed the same timing as described above (catalepsy at 5 minutes, body temperature at 15 minutes, and tail-flick latency at 20 minutes postinjection). For oral gavage THC, due to its longer Tmax, we measured catalepsy at 50 minutes, body temperature at 55 minutes, and tail-flick latency at 60 minutes postinjection. Additionally, 11-OH-THC was administered intravenously, intraperitoneally, or orally to both male and female mice, with catalepsy data collected at 20 minutes, body temperature at 25 minutes, and tail-flick latency at 30 minutes postinjection. Of note, two female mice that received 11-OH-THC intravenously died during the study, with the cause being unknown; therefore, data from these two mice were not included in analyses.
Part II: Dose-Response Study.
Physiological assays and blood collections were performed using male and female mice to assess the dose dependency of the effects of THC and 11-OH-THC following intravenous injection only. Doses of 0.1 mg/kg, 0.3 mg/kg, 1 mg/kg, and 10 mg/kg were given via i.v. tail vein injection (n = 4 per dose per sex). Both 11-OH-THC and THC compounds were prepared as described above for intravenous injection. One cohort of mice was assessed for catalepsy, body temperature, and nociception at 5, 15, and 20 minutes, respectively, as described above in part I. A separate cohort of mice from those that underwent physiological assays were used for the determination of blood concentrations of THC and 11-OH-THC at the Tmax as shown in Fig. 1.
All dose-response data were fit to a three-parameter nonlinear regression analysis to ED50 and efficacy. Data were expressed as the mean ± S.E.M. or 95% confidence interval as indicated. Additional calculation of THC equivalency calculated for potency for each measure was done to estimate 11-OH-THC activity relative to THC.
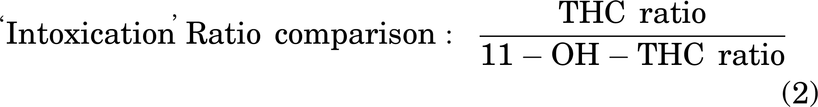
Analytical Methods
Mass Spectrometer Instrument Parameter.
Liquid chromatography tandem mass spectroscopy was performed using a 1290 Agilent high performance liquid chromatography system (Agilent Technologies, Canada, Mississauga, ON) interfaced to a QTRAP 6500 (ABSciex, Concord, ON) triple quadrupole linear ion trap mass spectrometer equipped with a Turbo ion spray interface. Compounds were separated chromatographically by an Agilent ZORBAX RRHD Eclipse Plus C18 column (2.1 × 50 mm, 1.8 μm) and an Agilent ZORBAX RRHD Eclipse Plus C18 column UHPLC guard column (2.1 mm, 1.8 μm) set at 40°C. Applied Biosystems/MDS Sciex Analyst software (version 1.7.0) was used for system control and quantification.
A sample volume of 5 μL was injected using the 1290 Agilent autoinjector set to 4°C. The mobile phase A consisted of water with 1 mM of ammonium formate and 0.1% formic acid, and the phase B consisted of 95:5 methanol:water with 1 mM of ammonium formate and 0.1% formic acid. The mobile phase flow rate was 700 μL/min at a ratio of 30:70 (A:B) up to 0.1 minute, then switched to 100% B in a gradient to 4.5 minutes. The gradient returned to a 30:70 ratio from 4.6 minutes to 6 minutes total run time.
Multiple reaction monitoring was achieved by using electrospray ionization in positive ion mode. The monitored precursor ion and product ion transitions for THC, 11-OH-THC, COOH-THC, and their internal standards are described in Table 1. The source temperature was set to 700°C, ion spray voltage 4000 V, curtain gas 50, nebulizer gas source one 80, heater gas source two 80, and collision gas (CAD) 11 and using exit potential of 10 for all transitions. Dwell time for all transitions was 20 milliseconds at unit resolution. For all cases, nitrogen was used as the gas, and the interface heater was on. ABSciex Analyst 1.7 was used for data acquisition and analysis.
Monitored precursor and product ion transitions for THC, 11-OH-THC, COOH-THC, and internal standards
Method validation followed the Food and Drug Administration and European Medicines Agency Guidance for Bioanalytical Method Validation (https://www.moh.gov.bw/Publications/drug_regulation/Bioanalytical%20Method%20Validation%20FDA%202001.pdf), including matrix effects, selectivity, carryover, linearity, precision, accuracy, recovery, reproducibility, and stability; details are found within the Supplemental analytical methods and validation results for this manuscript (Supplemental Figs. 1 and 2; Supplemental Tables 1–4).
Statistics
All data are presented as a mean ± S.E.M.; n represents individual mice within treatment groups. For time course experiments, blood analyte concentrations are presented as natural logarithms (ln) to better estimate PK parameters (Cmax, Tmax, t1/2, AUC, Vd, ClS, k, and F) (Gabrielsson and Weiner, 2012). Data from the catalepsy assay and antinociceptive assay are reported as MPE such that 60 seconds and 20 seconds were the maximum times in these assays, respectively. Data were analyzed via two-way ANOVA followed by Tukey’s post hoc test (GraphPad Prism v. 9.0), and P < 0.05 was considered statistically significant.
Results
Part I: Pharmacokinetic Time Course Study
Male mice were administered THC or 11-OH-THC at a dose of 10 mg/kg i.v., i.p., or orally, and whole-blood samples were collected 10 minutes, 30 minutes, 1 hour, 3 hours, 6 hours, 12 hours, 18 hours, and 24 hours after compound administration.
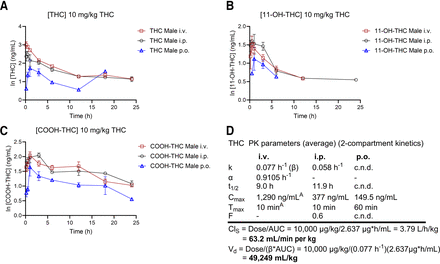
Figure 2 displays data from animals that received THC directly, presented as mean ln of blood concentrations of THC (Fig. 2A), 11-OH-THC (Fig. 2B), and COOH-THC (Fig. 2C). Figure 2D presents the PK parameters (Cmax, Tmax, t1/2, AUC, Vd, ClS, k, and F) for THC for each mode of administration. THC disposition curves demonstrated a slow terminal elimination phase. COOH-THC was the more abundant of the two metabolites for both intravenous and intraperitoneal routes of administration and detectable up to 24 hours (Fig. 2, B and C), whereas oral gavage THC was only detectable to the 18-hour measurement (Fig. 2C). 11-OH-THC (as a metabolite of THC) was quantifiable in blood (>4 ng/mL) up to 12 hours intravenously, 24 hours intraperitoneally, and 6 hours orally (Fig. 2B). The data showed two-compartmental kinetics, where the t1/2 for intravenous administration of THC was 9 hours, whereas intraperitoneal administration showed a t1/2 of 11.9 hours (Fig. 2D). However, t1/2 and F could not be estimated for oral gavage THC administration from the current dataset. For intravenous administration of THC, the Tmax was taken as the first sampling point (i.e., 10 minutes), where the observed THC blood concentration was 1290 ng/mL (Fig. 2, A and D). After intraperitoneal administration, the Cmax of THC reached 377 ng/mL at 10 minutes (Tmax), with F being approximately 0.6 (i.e., 60%) (Fig. 2D). In comparison, oral gavage administration of THC showed a Cmax of 149.5 ng/mL at 60 minutes (Fig. 2D). The k values for THC were 0.077 hours−1 intravenously and 0.058 hours−1 intraperitoneally (Fig. 2D). In addition, the Vd and ClS for THC were calculated at 49,249 mL/kg and 63.2 mL/min per kg, respectively (Fig. 2D).
Male mice aged 8–12 weeks were treated with 10 mg/kg of i.v., i.p., or oral gavage THC, and blood samples were taken to quantify THC (A), 11-OH-THC (B), and COOH-THC (C) levels at the times indicated. Data are expressed as the ln mean ± S.E.M. for compound concentration-versus-time curves. n = 4 per group. Data were excluded if they fell below the lower limit of quantitation (LLOQ) (4 ng/mL). Note that the y-axes vary between (A), (B), and (C). (D) THC data from (A) were analyzed as described in Methods to estimate the following PK parameters: k (β for intravenous), distribution phase rate constant (α;intravenous only), t1/2, Cmax, Tmax, F, absolute bioavailability; AUC, ClS, and Vd. Ai.v., Cmax, and Tmax are simply the peak measured THC concentration and the first time of measurement, respectively. c.n.d., could not be determined.
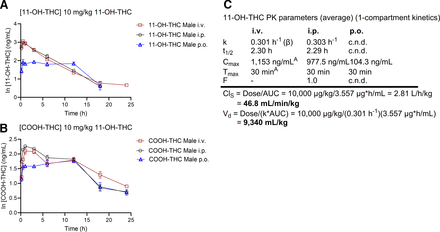
Figure 3 displays data from animals that received 11-OH-THC directly, presented as mean ln of blood concentrations of 11-OH-THC (Fig. 3A) and COOH-THC (Fig. 3B). Figure 3C presents the PK parameters (Cmax, Tmax, t1/2, AUC, Vd, ClS, k, and F) for 11-OH-THC for each mode of administration. The disposition curves of 11-OH-THC demonstrated a sharp terminal elimination phase where intraperitoneal and oral gavage 11-OH-THC were not quantifiable at 24 hours (Fig. 3A). COOH-THC was detectable up to the 24-hour time point in mice that received 11-OH-THC by all routes of administration (Fig. 3B). The data presented are best described by one-compartmental kinetics. For intravenous administration of 11-OH-THC, the Tmax was observed at 30 minutes, where the observed 11-OH-THC blood concentration was 1153 ng/mL (Fig. 3, A and C). Following intraperitoneal administration, 11-OH-THC demonstrated a Cmax of 977.5 ng/mL at a Tmax of 30 minutes (Fig. 3C). Oral gavage administration of 11-OH-THC resulted in the lowest extent of absorption, with a Cmax of 104.3 ng/mL, and Tmax remained similar to intraperitoneal at 30 minutes (Fig. 3C). Administration of 11-OH-THC, either intravenous or intraperitoneal, showed a similar t1/2 of approximately 2.3 hours. However, t1/2 and F could not be determined for oral gavage administration of 11-OH-THC with the current dataset. The k for 11-OH-THC was approximately the same for both intravenous and intraperitoneal (0.303 hours−1). In addition, the Vd and ClS were calculated at 9,340 mL/kg and 43.8 mL/min per kg, respectively. Overall, we noticed poor bioavailability of THC and directly administered 11-OH-THC compounds when administrated orally. However, intraperitoneal administration for 11-OH-THC showed higher bioavailability (1.0) than intraperitoneal administration for THC (0.6).
Male mice aged 8–12 weeks were treated with 10 mg/kg of i.v., i.p., or oral gavage 11-OH-THC, and blood samples were taken to quantify 11-OH-THC (A) and COOH-THC (B) levels at the times indicated. Data are expressed as the ln mean ± S.E.M. for compound concentration-versus-time curves. n = 4 per group. Data were excluded if they fell below the LLOQ (4 ng/mL). Note that the y-axes vary between (A) and (B). (C) 11-OH-THC data from (A) were analyzed as described in Methods to estimate the following PK parameters: k, t1/2, Cmax, Tmax, F, AUC, ClS, and Vd. Ai.v., Cmax, and Tmax are simply the peak measured THC concentration and the time of peak, respectively. c.n.d., could not be determined.
A descriptive comparison of PK parameters presented in Figs. 2D and 3C suggests that the parent drug, THC, has an elimination rate approximately 4 to 5 times slower than 11-OH-THC, a notably longer half-life when administered intravenously or intraperitoneally compared with 11-OH-THC (9.0–11.9 hours vs 2.29–2.30 hours), a similar ClS to 11-OH-THC, a Vd approximately one-fifth that of 11-OH-THC, and a bioavailability only 60% that of 11-OH-THC when administered intraperitoneally. Overall, 11-OH-THC appears to be eliminated from the blood and distributed to other tissues to a greater extent than the parent compound, THC, when 11-OH-THC is administered directly.
Physiological Assessments.
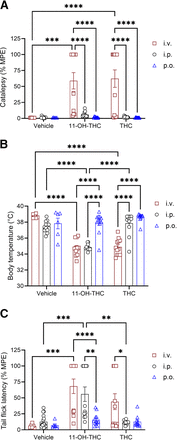
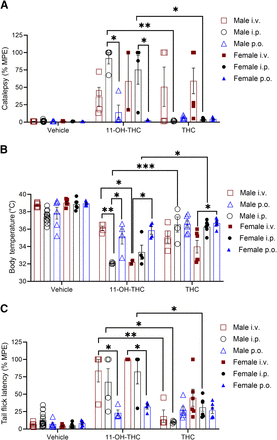
Figure 4 shows results for the bar-holding assay to evaluate catalepsy, body temperature change to assess hypothermia, and tail-flick assay for nociception after administration of vehicle, 10 mg/kg THC, or 10 mg/kg 11-OH-THC at 5 minutes (catalepsy), 15 minutes (body temperature), 20 minutes (nociception) postinjection. THC and 11-OH-THC showed a significant cataleptic response after intravenous administration compared with vehicle and compared with either intraperitoneal or oral gavage administration (Fig. 4A). Hypothermia was significant in intravenous THC and 11-OH-THC compared with the vehicle (Fig. 4B). Intraperitoneal 11-OH-THC (but not intraperitoneal THC) also produced a hypothermic response compared to vehicle; but neither oral gavage 11-OH-THC or THC produced a hypothermic response (Fig. 4B). Although THC produced a greater hypothermic response when administered intravenously, compared with intraperitoneal, this was not the case with 11-OH-THC (Fig. 4B). In addition, the hypothermia observed following intraperitoneal 11-OH-THC administration was greater than the hypothermia following intraperitoneal THC administration (Fig. 4B). The antinociceptive effects of intravenous THC and 11-OH-THC and intraperitoneal 11-OH-THC were greater than vehicle (Fig. 4C). Intravenous or intraperitoneal 11-OH-THC also produced a greater antinociceptive effect than when administered by oral gavage (Fig. 4C), and 11-OH-THC’s intravenous and intraperitoneal antinociceptive effects were greater than THC at the same respective routes of administration (Fig. 4C). Lastly, THC produced a greater antinociceptive effect when administered intravenously than intraperitoneally (Fig. 4C). The Cmax after intraperitoneal injection of 11-OH-THC (977.5 ng/mL) compared with intraperitoneal THC injection (377 ng/mL) aligns with the greater response produced after intraperitoneal 11-OH-THC.
Physiological effects of 10 mg/kg THC and 11-OH-THC in male C57BL/6 mice treated intravenously,intraperitoneally, or by oral gavage (A) Catalepsy 5 minutes postinjection, (B) body temperature 15 minutes postinjection, and (C) nociception in the tail-flick latency test 20 minutes postinjection. Data for catalepsy are represented as MPE during a maximum 60 seconds. Data for the tail-flick latency are represented as MPE during a maximum 20 seconds. n = 6–18 animals per treatment group. Data are expressed as mean ± S.E.M. ****P < 0.0001; ***P < 0.001; **P < 0.01; and *P < 0.05 as determined by two-way ANOVA followed by Tukey’s post hoc test for multiple comparisons.
Figure 5 shows the results of the catalepsy, body temperature, and tail-flick assays according to the Tmax for male and female mice that received either THC or 11-OH-THC via intravenous, intraperitoneal, or oral gavage administration. This meant that for animals receiving intravenous or intraperitoneal THC, testing was: catalepsy at 5 minutes, body temperature at 15 minutes, and tail-flick latency at 20 minutes postinjection. For oral gavage THC, we measured catalepsy at 50 minutes, body temperature at 55 minutes, and tail-flick latency at 60 minutes due to its longer Tmax. Based on a Tmax of 30 minutes, 11-OH-THC was administered intravenously, intraperitoneally, or by oral gavage to both male and female mice, and testing was: catalepsy at 20 minutes, body temperature at 25 minutes, and tail-flick latency at 30 minutes. Vehicle data presented in Fig. 5 for male mice are identical to those presented in Fig. 4, and statistical comparisons to vehicle were not included in the ANOVA. In Fig. 5A, visual comparisons of the data indicate that catalepsy occurred for mice receiving 11-OH-THC or THC, except for females receiving oral gavage 11-OH-THC and males and females receiving intraperitoneal and oral gavage THC. Catalepsy was significantly greater in intraperitoneally injected 11-OH-THC male and female mice compared with oral gavage 11-OH-THC–treated male and female mice (Fig. 5 A). Likewise, intraperitoneal 11-OH-THC–treated male and female mice experience greater catalepsy than intraperitoneal THC–treated male and female mice (Fig. 5 A). In Fig. 5B, visual comparison of the data suggest that hypothermia occurred for all mice receiving 11-OH-THC at Tmax. Decreased body temperature was greater in male intraperitoneal 11-OH-THC–treated mice than either intravenous or oral gavage administration (Fig. 5B). Decreased body temperature was greater in female intravenous 11-OH-THC and THC–treated mice than female oral gavage 11-OH-THC and THC mice, respectively (Fig. 5B). Intraperitoneal injection of 11-OH-THC produced greater hypothermia at Tmax than intraperitoneal THC treatment in both males and females (Fig. 5B). A sex difference was also observed where female intravenous 11-OH-THC–treated mice experienced greater hypothermia than their male counterparts (Fig. 5B). In Fig. 5C, visual comparison of the data indicate that an antinociceptive response occurred for mice receiving 11-OH-THC or THC, except for males receiving intraperitoneal THC, although by comparison a subset of vehicle-treated mice displayed unusually long tail-flick latency times. Both male and female mice that received intravenous 11-OH-THC saw a greater antinociceptive response than mice that received oral gavage 11-OH-THC (Fig. 5C). The antinociceptive response was also greater in male mice receiving intravenous or intraperitoneal 11-OH-THC compared with intravenous or intraperitoneal THC, respectively; this was also the case for female intraperitoneal 11-OH-THC–treated mice compared with female intraperitoneal THC–treated mice (Fig. 5C).
Physiological effect of 10 mg/kg THC and 11-OH-THC in male and female C57BL/6 mice treated intravenously, intraperitoneally,or by oral gavage. (A) Catalepsy time was 5 minutes postinjection for intravenous and intraperitoneal THC, 50 minutes postinjection for oral gavage THC, and 20 minutes postinjection for intravenous, intraperitoneal,and oral gavage 11-OH-THC. (B) body temperature time was 15 minutes postinjection for intravenous and intraperitoneal THC, 55 minutes postinjection for oral gavage THC, and 25 minutes postinjection for intravenous, intraperitoneal,and oral gavage 11-OH-THC. (C) Nociception in the tail-flick latency test was 20 minutes postinjection for intravenous and intraperitoneal THC, 60 minutes postinjection for oral gavage THC, and 30 minutes postinjection for intravenous, intraperitoneal,and oral gavage 11-OH-THC. Data for catalepsy are represented as MPE during a maximum 60 seconds. Data for the tail-flick assay are represented as MPE during a maximum 20 seconds. n = 4–18 animals per treatment group, except female intravenous 11-OH-THC (n = 2). Data are expressed as mean ± S.E.M. Male vehicle data are identical to those presented in Fig. 4. Vehicle data were not included in statistical analyses. ***P < 0.001; **P < 0.01; and *P < 0.05 as determined by two-way ANOVA followed by Tukey’s post hoc test for multiple comparisons.
Part II: Dose-Response Study
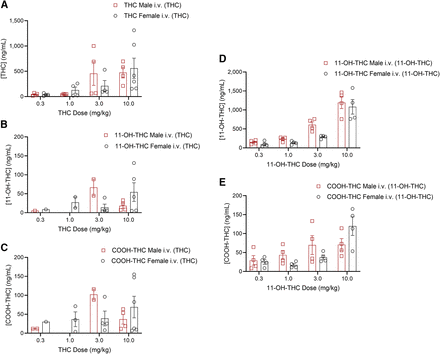
Figure 6 displays blood levels of compounds in male and female mice that received THC or 11-OH-THC at doses of 0.1 mg/kg, 0.3 mg/kg, 1 mg/kg, and 10 mg/kg i.v. Blood concentrations of each compound were determined at 10 minutes for THC and 30 minutes for 11-OH-THC. Figure 7 displays physiological responses to intravenous THC or 11-OH-THC at the same doses recorded at 5 minutes (catalepsy; Fig. 7, A and B), 15 minutes (body temperature; Fig. 7, C and D), and 20 minutes (tail-flick latency; Fig. 7, E and F). After injection of the 3-mg/kg and 10-mg/kg doses of THC, a cataleptic response was observed in male mice, which correlated with the elevated blood concentrations of THC at these doses (Figs. 6A and 7A). A cataleptic response was only present in female mice at the 10-mg/kg dose of THC (Fig. 7, A and B). A dose-dependent decrease in body temperature following THC administration was observed in both sexes (Fig. 7, C and D). Likewise, a dose-dependent antinociceptive response was observed following THC administration in both sexes (Fig. 7, E and F). Overall, blood concentrations of THC and its metabolites were their highest in both males and females at 3 mg/kg and 10 mg/kg, except for 11-OH-THC (a metabolite of THC) at the 3-mg/kg dose for females (Fig. 6, A–C). These dose-dependent elevations in THC and THC metabolite blood levels appear to correlate with the greater observed effects in catalepsy, body temperature, and nociception outcomes as expected.
Blood concentrations (ng/mL) of THC, 11-OH-THC, and its metabolites in male and female C57BL/6 mice that were treated intravenously with different doses of THC (A–C) or 11-OH-THC (D and E) (A) Concentration of THC in blood (ng/mL) following i.v. administration of 0.3, 1, 3, or 10 mg/kg THC. (B) Concentration of 11-OH-THC in blood (ng/mL) following i.v. administration of 0.3, 1, 3, or 10 mg/kg THC. (C) Concentration of COOH-THC in blood (ng/mL) following i.v. administration of 0.3, 1, 3, or 10 mg/kg THC. (D) Concentration of 11-OH-THC in blood (ng/mL) following i.v. administration of 0.3, 1, 3, or 10 mg/kg 11-OH-THC. (E) Concentration of THC in blood (ng/mL) following i.v. administration of 0.3, 1, 3, or 10 mg/kg 11-OH-THC. n = 4–6 per dose per sex, except where data were excluded when values were below the LLOQ (4 ng/mL). Data are expressed as mean ± S.E.M. Note that the scale of the y-axes varies from panel to panel.
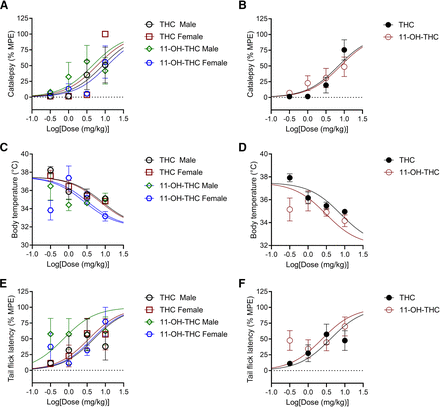
Physiological effect of THC and 11-OH-THC in male and female C57BL/6 mice treated i.v. with 0.3, 1, 3, or 10 mg/kg of THC or 11-OH-THC. (A) Catalepsy 5 minutes postinjection for each sex separately and (B) catalepsy 5 minutes postinjection for both sexes combined. (C) Body temperature 15 minutes postinjection for each sex separately and (D) body temperature 15 minutes postinjection for both sexes combined. (E) Nociception in the tail-flick latency test 20 minutes postinjection for each sex separately and (F) nociception in the tail-flick latency test 20 minutes postinjection for both sexes combined. Data for catalepsy are represented as MPE during a maximum 60-second trial. Data for the tail-flick assay are represented as MPE during a maximum 20-second trial. All data were analyzed in GraphPad (v. 9). n = 4 animals per treatment group per sex. Data are expressed as mean ± S.E.M. Corresponding estimates of ED50 are presented in Table 2.
After administration of 11-OH-THC, a dose-dependent increase in plasma concentration of 11-OH-THC and COOH-THC was seen in both sexes (Fig. 6, D and E). Figure 7, A and B shows that the cataleptic response in males followed an upward trend; however, the 3-mg/kg dose produced a greater response than the 10-mg/kg dose, which did not follow the same trend as blood concentrations (Fig. 6, D and E). A low cataleptic response was seen in females until the 10-mg/kg dose, in which the cataleptic response was the most pronounced (Fig. 7, A and B). Body temperature decreased in a dose-dependent manner in both sexes following 11-OH-THC administration, although some females displayed a greater-than-expected hypothermic response at the 0.3 mg/kg dose (Fig. 7, C and D). Antinociception in males was consistent regardless of the 11-OH-THC dose, whereas females showed a marked dose-dependent antinociceptive response (Fig. 7, E and F). It is important to consider that plasma concentrations and tetrad analysis were conducted in two separate cohorts of animals as necessitated by the mouse model, thus contributing to a degree of variability in results. Direct administration of 11-OH-THC yielded 11-OH-THC and COOH-THC levels that followed a dose-dependent relationship; these dose-dependent elevations in 11-OH-THC blood levels correlated with the greater observed effects in catalepsy, body temperature, and nociception outcomes as expected (Fig. 6, D and E).
Supplemental Fig. 3 shows the data presented in Fig. 7 as individual datapoints for male and female physiological responses.
Relative Activity for 11-OH-THC
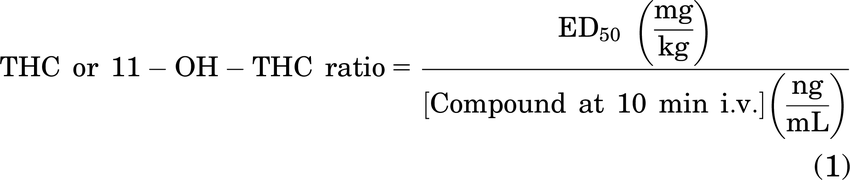
Table 2 presents the estimated ED50 values from nonlinear regression of our data presented in Fig. 7. In the ring-holding test for catalepsy, 11-OH-THC was more potent than THC in males but appeared less potent than THC in females and when both sexes were combined; however, none of these differences were statistically significant (Table 2). For both body temperature and nociception, 11-OH-THC appeared to be 2–7 times more potent than THC in males, females, and when both sexes were combined; but again, these differences were not statistically significant (Table 2). One exception to this was that THC was equally or slightly more potent in females in the tail-flick latency test (Table 2). To account for PK differences, the relative activity of 11-OH-THC versus THC was estimated by dividing each ED50 value by that compound’s intravenous blood concentration at 10 minutes (Figs. 2 and 3) and then comparing relative activity between the two compounds. These estimates are limited because time-course data used only male mice. Using this approach for catalepsy, 11-OH-THC was 78% as “intoxicating” as THC (Table 2). For change in body temperature, 11-OH-THC was 224% as active as THC (Table 2). Finally, 11-OH-THC’s antinociceptive activity was 153% that of THC (Table 2).
Estimated ED50 values and “intoxication” comparisons for data presented in Fig. 7
ED50 values for each physiological outcome were estimated by fitting data to a three-parameter nonlinear regression for both male and female C57BL/6 mice that were treated intravenously with THC or 11-OH-THC (0.3 mg/kg, 1 mg/kg, 3 mg/kg, and 10 mg/kg). Data relate to Fig. 7 and Supplemental Fig. 3. ED50 Data are reported to two significant digits. Intoxication ratios were estimates as described in Methods.
When putting the differences between sexes into perspective, males showed relatively higher ratios that were not compared statistically: 171% for catalepsy, 275% for body temperature, and 608% for antinociception (Table 2). In contrast, females showed about 54% for catalepsy, 198% for body temperature, and 73% for antinociception (Table 2).
Discussion
The goal of this study was to compare the effects of direct 11-OH-THC administration to that of THC administration in mice and compare their PD effects when accounting for the PK of these two compounds. The wide availability of cannabis necessitates warning against its misuse (Carvalho and Evans-Gilbert, 2019) as there have been numerous case reports of acute cannabis intoxication resulting in seizures, ataxia, lethargy, tremors, and even comas among children (Emoto et al., 2020). Cannabis or THC is becoming increasingly important as a therapeutic agent (Hassenberg et al., 2020). Although THC is considered the principal psychoactive agent of the cannabis plant (Dumbraveanu et al., 2023), and 11-OH-THC is the primary metabolite of THC, there is limited data on the pharmacology of 11-OH-THC. Therefore, in-depth assessment of the PK and PD of 11-OH-THC will help to improve therapeutic use of, and reduce harms associated with, cannabis.
We observed that the metabolism and absorption of THC and its metabolites differed depending on the route of administration, with oral gavage administration having the lowest Cmax. Additionally, none of the oral gavage data could be used to calculate PK parameters other than Cmax and Tmax due to the limited number of data points in the curve for both THC and 11-OH-THC. Future studies will work to address this limitation by 1) increasing the number of sampling times, 2) sampling at equally spaced time intervals, and 3) collecting from additional animals if necessary to reduce stress for each animal. THC exhibited two-compartment kinetics, indicating uneven distribution throughout the body. In contrast, 11-OH-THC displayed one-compartment kinetics based on the available data. However, with additional data points beyond 24 hours, 11-OH-THC might also demonstrate two-compartment kinetics. In general, our findings are congruent with previous albeit limited work in humans and rodents suggesting that 11-OH-THC is psychoactive (e.g., Wiley et al., 2021; https://healthpolicy.usc.edu/wp-content/uploads/2022/07/USC-Schaeffer-Center-white-paper_Federal-Regulation-of-Cannabis-for-Public-Health-in-the-United-States.pdf).
Although data assessing the PK of direct 11-OH-THC administration are limited, some preclinical and clinical data are helpful in interpreting our present findings. An earlier preclinical study showed that the concentrations of serum THC were lower, and the onset of locomotor inhibition in the open field was delayed by 30–130 minutes, when cannabis or cannabinoids were administered via subcutaneous injection or oral gavage to male Wistar rats compared with intravenous injection (Hložek et al., 2017). The same study also reported that 11-OH-THC reached its highest concentration (approximately 200 ng/g) 2 hours after oral gavage THC administration (Hložek et al., 2017), reflecting our own observations here. Another study by Sallam et al. (2023) reported delayed time-to-peak levels of 11-OH-THC in C57BL/6 mice following oral gavage administration of 5 mg/kg THC. That study also reported sex-specific partitioning of THC and 11-OH-THC into brain and adipose in male C57BL/6 mice, whereas COOH-THC levels did not differ between sexes across plasma, brain, and adipose. Importantly, we did not conduct our PK time course experiment in female mice, which represents a limitation of the present study. Future work should directly test for PK differences between sexes and in multiple tissues (e.g., brain and adipose), building from the present data.
In 1973, a clinical study examining the effects of intravenously administered THC and 11-OH-THC in nine individuals showed that all subjects experienced a significant increase in heart rate and the psychoactive “high” within 3–5 minutes of receiving 1 mg of 11-OH-THC intravenously (Lemberger et al., 1973). The peak “high” was delayed by 10–20 minutes in the intravenous route. The psychomimetic effects closely correlated with plasma levels of unchanged 11-OH-THC. Perez-Reyes et al. (1972) injected 12 male patients with THC and 11-OH-THC to see if the intravenous route would yield different results and found both compounds to be equipotent. Overall, PK studies have reported that 11-OH-THC is found in higher concentrations when cannabis is administrated orally (https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/information-medical-practitioners/information-health-care-professionals-cannabis-cannabinoids).
In the present study, we observed that THC and 11-OH-THC both produced significant cataleptic, hypothermic, and antinociceptive responses after intravenous administration compared with vehicle, regardless of Tmax, in both male and female mice. In contrast, intraperitoneal injection of 11-OH-THC produced significant cataleptic, hypothermic, and antinociceptive effects at Tmax, whereas intraperitoneal THC injection did not produce catalepsy in either sex or reduce nociception in males, contrary to expectations. Oral gavage administration of 11-OH-THC generally elicited minor cataleptic, hypothermic, and antinociceptive responses at Tmax that were less dramatic than in mice receiving injections of 11-OH-THC and in accordance with lower 11-OH-THC blood levels; this trend was also true of mice that received oral gavage THC. Our results indicate that the route of administration and the specific compound (THC or 11-OH-THC) influenced the magnitude of physiological effects observed, which correlate to amount of compound present in blood.
Past work from others has found that THC-induced catalepsy occurred when male albino CD-1 strain mice were force-fed 0.625–25 mg/kg of THC (Formukong et al., 1988); this effect was later shown to be mediated in mice through CB1R in the amygdala, nucleus accumbens, and hypothalamus (Fujiwara and Egashira, 2004; Egashira, 2017). Our own earlier work hinted that 11-OH-THC displayed greater CB1R affinity and similar potency to THC in vitro and could reproduce or exceed the effects of THC in vivo (Zagzoog et al., 2022). Like our findings, Wiley et al. (2021) observed that 11-OH-THC displayed greater affinity than THC for CB1R and was an equipotent partial agonist in the [35S]GTPγS assay. Moreover, Wiley et al. (2021) reported 11-OH-THC had greater potency in catalepsy and body temperature assays (7–31-fold) than THC when administered intraperitoneally to albino ICR strain mice. In general, our findings appear to be concordant with these data; although our observed potencies for intravenous 11-OH-THC were not statistically greater than intravenous THC, 11-OH-THC was two- to sevenfold more potent than THC (Table 2). By comparison, human observations made by Perez-Reyes et al. (1972) do not account for the PK differences between 11-OH-THC and THC that we observed, in particular where 11-OH-THC had a higher Cmax than THC. Additional research is required to determine whether the effects of THC are attributed more to THC itself and the parent compound or instead to 11-OH-THC as the primary metabolite when THC is consumed.
Reports show that the metabolism of THC into its psychoactive metabolite 11-OH-THC may differ between sexes, ages, and species. A study in humans revealed that both THC and 11-OH-THC undergo first-pass metabolism, like oral THC in rodents (Lucas et al., 2018). In mice, sex differences were not observed for drug discrimination, catalepsy, or hypothermia following THC or 11-OH-THC treatment, nor were age differences observed (Wiley et al., 2021). These results parallel our own, where only one sex difference was noted in hypothermia for these compounds. However, intraperitoneal THC has been shown to be significantly more potent in Sprague-Dawley rats in drug discrimination tests for females compared with males (Wiley et al., 2021), although previous studies have not observed sex differences for hypothermia, locomotion, or catalepsy despite observing accumulation of 11-OH-THC in female rat brains more than male rat brains (Wiley and Burston, 2014). Tseng et al. (2004) reported that the levels of THC and 11-OH-THC were higher among female mice and were attributed to the increased behavioral THC-induced effects in females compared with male mice. Furthermore, 11-OH-THC was twice as potent among female rats (Tseng et al., 2004). Variation in the metabolism of THC was noticed among male and female rats where the liver microsomes of male rats broke THC into 8-hydroxy-Δ9-tetrahydrocannabinol, 8,11-dihydroxytetrahydrocannabinol, 3′-hydroxy-THC, and 11-OH-THC, whereas in females selective metabolism of THC to 11-OH-THC was noted (Narimatsu et al., 1991). This might explain the variation of 11-OH-THC levels with similar doses of THC and might cause elevated 11-OH-THC levels in the brains of female rats compared with male rats (Wiley and Burston, 2014). The authors of these studies note that considerations of species differences are critical to interpreting cannabinoid data in animal models.
In humans, the differences between cannabis effects in men and women may be related to the differences between their metabolism and percentage of body fat such that THC may be retained by fat cells and less concentration would be available in the blood, thus producing weaker effects (Fattore and Fratta, 2010). There are conflicting reports on the difference in CB1R density between males and females (Castelli et al., 2014; Liu et al., 2020). However, whether these differences contribute to the different effects of cannabis between men and women is uncertain (Cooper and Craft, 2018).
Conclusion
Our findings demonstrate that the THC metabolite 11-OH-THC produces in vivo effects to an equal or greater degree than the parent drug, THC, even when accounting for potential PK differences. Intriguingly, 11-OH-THC’s activity was not uniform across the tetrad, with more activity in the antinociceptive test that warrants further study to determine receptor target(s) and kinetics. These findings support the hypothesis that 11-OH-THC is active in producing cannabinoid-evoked physiological responses.
Acknowledgments
The authors thank to Deborah Michel for LC-MS troubleshooting and consultation and thanks to Jane Alcorn for critical review and appraisal of all pharmacokinetic data and analyses.
Data Availability
The original contributions presented in the study are included in the article/Supplemental Material; further inquiries can be directed to the corresponding author.
Authorship Contributions
Participated in research design: Zagzoog, Laprairie.
Conducted experiments: Zagzoog, Halter, Jones, Bannatyne, Cline, Wilcox, Smolyakova, Laprairie.
Contributed new reagents or analytic tools: Zagzoog, Laprairie.
Performed data analysis: Zagzoog, Halter, Laprairie.
Wrote or contributed to the writing of the manuscript: Zagzoog, Laprairie.
Footnotes
- Received October 30, 2023.
- Accepted June 3, 2024.
This work was supported by funding from the College of Pharmacy and Nutrition, University of Saskatchewan (to A.Z.) and the Saskatchewan Endowed Research Chair fund at the University of Saskatchewan (to R.B.L.).
Robert B. Laprairie has received compensation within the last 3 years on legal cases involving cannabis and currently serves as a consultant on the scientific advisory board for Shackleford Pharma Inc. The remaining authors declare no competing interests.
↵
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
Abbreviations
- 11-OH-THC
- 11-hydroxy-Δ9-tetrahydrocannabinol
- AUC
- area under the curve
- CB1R
- type 1 cannabinoid receptor
- CBD
- cannabidiol
- Cls
- clearance
- COOH-THC
- 11-nor-9-carboxy-Δ9-tetrahydrocannabinol
- F
- absolute bioavailability
- k
- terminal rate constant
- ln
- natural logarithm
- MPE
- percentage of maximum possible effect
- PD
- pharmacodynamic
- PK
- pharmacokinetic
- t1/2
- elimination half-life
- t1/2
- elimination half-life
- THC
- Δ9-tetrahydrocannabinol
- Tmax
- time to Cmax
- Vd
- apparent volume of distribution
- Copyright © 2024 by The American Society for Pharmacology and Experimental Therapeutics