Abstract
Patients with sickle cell disease (SCD) display priapism, a prolonged penile erection in the absence of sexual arousal. The current pharmacological treatments for SCD-associated priapism are limited and focused on acute interventions rather than prevention. Thus, there is an urgent need for new drug targets and preventive pharmacological therapies for this condition. This review focuses on the molecular mechanisms linked to the dysfunction of the NO-cyclic guanosine monophosphate (cGMP)-phosphodiesterase type 5 (PDE5) pathway implicated in SCD-associated priapism. In murine models of SCD, reduced nitric oxide (NO)-cGMP bioavailability in the corpus cavernosum is associated with elevated plasma hemoglobin levels, increased reactive oxygen species levels that inactive NO, and testosterone deficiency that leads to endothelial nitric oxide synthase downregulation. We discuss the consequences of the reduced cGMP-dependent PDE5 activity in response to these molecular changes, highlighting it as the primary pathophysiological mechanism leading to excessive corpus cavernosum relaxation, culminating in priapism. We also further discuss the impact of intravascular hemolysis on therapeutic approaches, present current pharmacological strategies targeting the NO-cGMP-PDE5 pathway in the penis, and identify potential pharmacological targets for future priapism therapies. In men with SCD and priapism, PDE5 inhibitor therapy and testosterone replacement have shown promising results. Recent preclinical research reported the beneficial effect of treatment with haptoglobin and NO donors.
SIGNIFICANCE STATEMENT This review discusses the molecular changes that reduce NO-cGMP bioavailability in the penis in SCD and highlights pharmacological targets and therapeutic strategies for the treatment of priapism, including PDE5 inhibitors, hormonal modulators, NO donors, hydroxyurea, soluble guanylate cyclase stimulators, haptoglobin, hemopexin, and antioxidants.
Introduction
Sickle cell disease (SCD) is the most common hereditary hematologic disorder, affecting millions worldwide (Kavanagh et al., 2022). It arises from a mutation in the β-globin gene, producing an abnormal hemoglobin variant called hemoglobin S (HbS) (Telen et al., 2019). Within red blood cells, HbS tends to polymerize, increasing intracellular viscosity (Kim-Shapiro and Gladwin, 2018). This process not only compromises the integrity of the red blood cell membrane, making it less flexible and more fragile, but also triggers a morphologic shift from a biconcave to a sickle shape, a phenomenon directly linked to HbS polymerization. Patients with SCD face a range of complications. Acute complications include acute pain events, acute chest syndrome, meningitis, stroke, sickle hepatopathy, gallstones, papillary necrosis, splenic infarction, splenic sequestration, bone marrow infarction, osteomyelitis, post-hyphema glaucoma, septicemia, and priapism. Chronic complications involve multiple organ damage, such as functional asplenia retinopathy, pulmonary hypertension, cardiomegaly, diastolic heart failure, and chronic kidney disease, contributing to increased morbidity and mortality. (Kavanagh et al., 2022). HbS polymerization also leads to hemolysis, severe hemolytic anemia, vaso-occlusive crises, and leg ulcers (Kato et al., 2018).
Priapism is a prolonged and persistent penile erection that occurs regardless of sexual stimulation or desire (Bivalacqua et al., 2022). There are two main types: ischemic priapism (low-flow) and non-ischemic priapism (high-flow) (Salonia et al., 2014). Ischemic priapism is characterized by reduced or absent intracorporeal blood flow, leading to painful erections (Salonia et al., 2014). This form of priapism is especially prevalent in men with SCD, with up to 48% of those affected experiencing episodes at some point in their lives (Arduini and Trovó de Marqui, 2018). The average age of onset is 15 years. Continuous and untreated episodes can cause irreversible damage to the erectile tissue, resulting in permanent erectile dysfunction (Arduini and Trovó de Marqui, 2018). On the other hand, non-ischemic priapism is typically the result of trauma affecting the arteries (Salonia et al., 2014). In individuals with SCD, ischemic priapism is the most common manifestation, accounting for 95% of cases (Hudnall et al., 2017). Of all priapism episodes, about 74% are of the “stuttering” (recurrent) type, lasting a few hours or less (Idris et al., 2020). These episodes can remit and recur with or without medical intervention (Idris et al., 2020). Notably, nearly half (40%) of patients with SCD who experience recurrent ischemic priapism also develop erectile dysfunction (Anele and Burnett, 2015).
For a long time, it was believed that priapism in SCD was solely due to the obstruction of venous outflow from the corpora cavernosa, a consequence of the vaso-occlusion caused by the interaction of sickled red blood cells with endothelial cells, leukocytes, and platelets (Burnett, 2003). However, experimental studies have revealed that priapism in SCD is also attributed to alterations in the mechanisms regulating erection, leading to excessive and persistent relaxation of the erectile tissue (Champion et al., 2005; Mi et al., 2008; Bivalacqua et al., 2009, 2013; Lagoda et al., 2013, 2014; Ning et al., 2014; Silva et al., 2016a,b; Musicki et al., 2018, 2021).
Nitric oxide (NO), recognized as a potent vasodilator, is crucial in regulating vascular tone and erectile function (MacDonald and Burnett, 2021). Over the past decade, numerous studies have suggested that the decrease in NO-cGMP bioavailability is closely linked to the onset of priapism associated with SCD (Champion et al., 2005; Bivalacqua et al., 2009, 2013; Lagoda et al., 2013, 2014; Silva et al., 2016a,b; Musicki et al., 2018, 2021). Moreover, restoring this bioavailability might be an effective therapeutic strategy. Notably, preclinical studies aimed at recovering NO-cGMP bioavailability have shown efficacy in treating priapism in SCD (Bivalacqua et al., 2013; Lagoda et al., 2014; Silva et al., 2016b; Musicki et al., 2018, 2021; Pereira et al., 2022). On the other hand, some preclinical studies have reported that excess plasma hemoglobin generated by intravascular hemolysis might limit the efficacy of specific NO donors, suggesting an NO resistance mechanism in the penis in SCD contexts (Pinheiro et al., 2022; Pereira et al., 2023).
This review focuses on the molecular mechanisms linked to the dysfunction of the NO-cyclic guanosine monophosphate (cGMP)-phosphodiesterase type 5 (PDE5) pathway implicated in SCD-associated priapism. We further discuss the impact of intravascular hemolysis on therapeutic approaches, present current pharmacological strategies targeting the NO-cGMP-PDE5 pathway in the penis, and identify potential pharmacological targets for future priapism therapies.
Molecular Mechanisms of Penile Erection
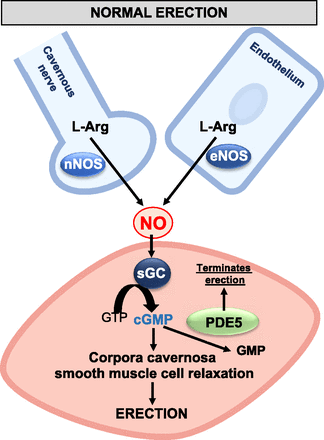
A penile erection results from a complex interplay of vascular, neural, and hormonal factors (Andersson, 2011; MacDonald and Burnett, 2021). At the core of this process, NO acts as the primary mediator, with its release from the endothelium and penile nerve terminals essential for initiating an erection (Fig. 1). Two critical NO synthase (NOS) enzymes exist in the penis: endothelial (eNOS) and neuronal (nNOS) (MacDonald and Burnett, 2021). These enzymes are pivotal in starting and sustaining an erection, catalyzing the transformation of L-arginine into L-citrulline and NO. NO then diffuses into the smooth muscle cell and binds to the ferrous iron in the heme moiety of soluble guanylate cyclase (sGC-Fe2+), which in turn triggers the production of the secondary messenger, cGMP (Andersson, 2011). High concentrations of cGMP trigger the activation of cGMP-dependent protein kinase, which influences various proteins that mediate muscle relaxation, such as myosin light chain phosphatase and potassium channels (Andersson, 2011). This sequence of events reduces intracellular calcium levels, therefore causing smooth muscle relaxation and dilation of penile vessels, which facilitates an erection. cGMP production and its degradation by phosphodiesterase type 5 (PDE5) are essential to maintain balance in this process. PDE5 converts cGMP into GMP, culminating in the termination of an erection (MacDonald and Burnett, 2021).
Mechanism for NO-dependent penile erection.
Molecular Mechanisms of Priapism Pathophysiology
Nitric Oxide Pathway Dysfunction in SCD-Associated Priapism Pathophysiology.
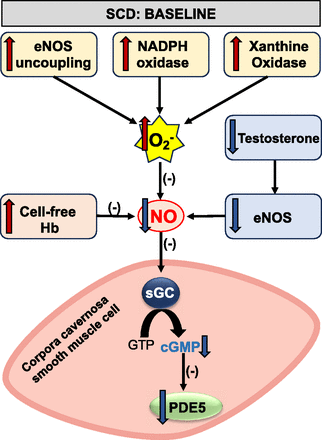
Experimental research has highlighted that priapism related to SCD primarily arises due to decreased endothelial NO bioavailability and the subsequent downregulation of PDE5 function (Champion et al., 2005; Lagoda et al., 2013; Silva et al., 2016b) (Fig. 2). Studies involving eNOS knockout mice, combined eNOS and nNOS knockout mice, as well as transgenic SCD mice models, have demonstrated an amplified erectile response when subjected to electrical stimulation of the cavernous nerve, coupled with penile fibrotic alterations (Burnett et al., 2002; Champion et al., 2005; Bivalacqua et al., 2009; Silva et al., 2016b; Pinheiro et al., 2022). These studies deduced that the PDE5 downregulation originates from lowered basal cGMP levels. The reduced calcium-dependent constitutive activity of NOS is a pivotal factor in this cGMP-dependent PDE5 expression decline (Corbin et al., 2000). In transgenic SCD mice, this diminished eNOS activity is attributed to lessened phosphorylation at Ser-1177, eNOS uncoupling, and a decrease in eNOS binding to the heat shock protein 90 (Musicki et al., 2011, 2012, 2014; Bivalacqua et al., 2013; Sopko et al., 2015; Silva et al., 2016a,b). A similar negative regulation of the eNOS protein was noted in the penile tissues of men with SCD (Lagoda et al., 2013).
Mechanisms of nitric oxide resistance in SCD. In SCD corpus cavernosum, baseline levels of endothelial NO and cGMP production are reduced due to decreased eNOS activation, increased superoxide anion production, testosterone deficiency, and the presence of cell-free Hb. Reduced cGMP levels subsequently lead to the downregulation of PDE5 expression and activity.
The uncoupling of eNOS represents an important mechanism leading to endothelial dysfunction (Daiber et al., 2019). The concept of 'functional uncoupling of eNOS' refers to the phenomenon where, instead of oxidizing L-arginine, the enzyme redirects its electron transfer to the oxidation of molecular oxygen (Förstermann and Sessa, 2012). This results in the predominant production of superoxide anion (Daiber et al., 2019). Thus, the uncoupling of eNOS impairs its efficacy in generating NO and enhances its production of superoxide (Daiber et al., 2019). The mechanism of eNOS uncoupling is linked to the oxidation of the essential NOS cofactor, tetrahydrobiopterin (BH4), the depletion of its substrate L-arginine, the accumulation of endogenous methylarginines, and the S-glutathionylation of eNOS itself (Förstermann and Sessa, 2012).
The eNOS activity is modulated by several post-translational modifications, with phosphorylation being especially noteworthy (Fulton, 2016). This modification occurs at several sites, with Ser1177 being the most prominent. Scientific literature has widely agreed that the phosphorylation of eNOS at this site, mediated by the Akt, amplifies and activates its enzymatic function. The heat shock protein 90 protein, when associated with eNOS, plays a vital role in stabilizing the enzyme ensuring its active conformation (Fulton, 2016). However, in SCD mice, there is a significant reduction in the interaction between eNOS and heat shock protein 90 (Musicki et al., 2011). Concurrently, the phosphorylation of eNOS at the Ser1177 site by Akt is also diminished (Musicki et al., 2011, 2018).
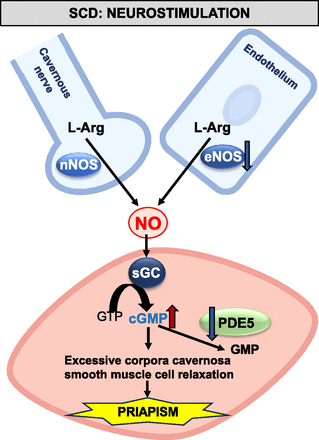
In SCD, reduced endothelial NO bioavailability leads to decreased cGMP production in the penis (Champion et al., 2005; Bivalacqua et al., 2013; Pereira et al., 2022). cGMP is a positive potent regulator of PDE5 gene expression (Corbin et al., 2000; Lin et al., 2002). The reduction in baseline cGMP levels results in lower PDE5 protein expression in the smooth muscle of the corpora cavernosa (Champion et al., 2005; Bivalacqua et al., 2013; Sopko et al., 2015; Silva et al., 2016b). PDE5 protein expression has also decreased in patients with SCD who exhibit priapism (Lagoda et al., 2013). The reduction of baseline cGMP levels may also decrease PDE5 activity by reducing its phosphorylation at the Ser-92 site, a process mediated by cGMP-dependent protein kinase (Corbin et al., 2000; Musicki et al., 2018, 2021). With reduced PDE5 activity, cGMP excessively accumulates in the erectile tissue after sexual stimulation or during nocturnal erections. This accumulation causes an exaggerated relaxation of the smooth muscle in the corpora cavernosa, leading to priapism (Anele et al., 2015) (Fig. 3).
NO signaling alterations in SCD-induced priapism. This illustration highlights the modifications in the NO signaling pathway in the corpus cavernosum during episodes of priapism associated with SCD.
Mechanisms of Nitric Oxide Resistance in SCD
Intravascular Hemolysis.
A hallmark of SCD is intravascular hemolysis (Reiter et al., 2002). This process leads to the rupture of red blood cells within blood vessels, releasing hemoglobin, arginase, and other cellular contents into the circulation (Reiter et al., 2002; Morris et al., 2005). Typically, patients with SCD exhibit a steady concentration of 4 µM cell-free hemoglobin (Hb). However, this concentration can spike to 25 µM during crisis episodes (Ballas and Marcolina, 2006). Notably, computational studies have suggested that cell-free Hb concentrations as low as 1 µM can negatively impact NO bioavailability (Jeffers et al., 2006).
Under conditions where free hemoglobin is prevalent in the plasma or interstitial spaces, this rapidly reacts with NO (Reiter et al., 2002; Schaer et al., 2016). This reaction leads to the production of nitrate and the formation of methemoglobin, the oxidized form of hemoglobin, consequently reducing the bioavailability of NO (Reiter et al., 2002). An additional factor reducing NO bioavailability is the increased activity of plasma arginase, which metabolizes L-arginine, the primary substrate for NO synthesis (Morris et al., 2005). Clinical investigations have highlighted a significant positive correlation between priapism incidents and elevated plasma hemoglobin levels in men diagnosed with SCD (Nolan et al., 2005; Kato et al., 2006; Cita et al., 2016). Moreover, a recent study showcased that inducing intravascular hemolysis in healthy mice resulted in augmented cavernous smooth muscle relaxation governed by the NO-cGMP pathway, similar to that observed in SCD mice (Iacopucci et al., 2022).
Oxidative Stress.
In SCD, the bioavailability of NO can also be compromised due to its interaction with reactive oxygen species (ROS) (Kim-Shapiro and Gladwin, 2018). The penis of SCD mice exhibits increased ROS production, which has been linked to various sources. High levels of xanthine oxidase activity have been identified in the plasma of SCD patients and the penises of SCD mice (Aslan et al., 2001; Bivalacqua et al., 2013). This enzyme catalyzes the conversion of hypoxanthine and xanthine into uric acid, generating superoxide (Schmidt et al., 2019). This superoxide rapidly combines with NO, leading to the formation of peroxynitrite and reducing the bioavailability of NO (Pacher et al., 2007).
In SCD, NADPH oxidase has also contributed to priapism through the production of superoxide (Musicki et al., 2012; Bivalacqua et al., 2013; Silva et al., 2016b; Pereira et al., 2022). In humans, seven known subtypes of NADPH oxidase are known, labeled NOX1-5 and DUOX1-2, each with distinct tissue distributions and activation mechanisms (Touyz et al., 2019). The NOX-2 protein comprises several subunits, including the membrane-bound gp91phox (also referred to as Nox2) and p22phox, and cytosolic subunits like p47phox, p67phox, and p40phox, and small GTPases (Rac1 and Rac2) (Touyz et al., 2019). The assembly of these subunits culminates in forming an active enzyme complex that catalyzes the transfer of electrons from cytosolic NADPH to molecular oxygen, generating superoxide as the primary reaction output (Zhang et al., 2020). An upregulation in the expression of various NADPH oxidase subunits, such as gp91phox, p47phox, and p67phox, has been observed in the penises of men diagnosed with SCD and SCD mice (Musicki et al., 2012; Bivalacqua et al., 2013; Silva et al., 2016b; Pereira et al., 2022).
As previously highlighted, another factor contributing to the elevated production of superoxide in the SCD penis is uncoupled eNOS (Bivalacqua et al., 2013). Peroxynitrite, produced by its interaction with NO, is a potent oxidizing agent that damages proteins, lipids, and DNA and impairs cellular signaling pathways (Ayala et al., 2014; Pizzino et al., 2017). Peroxynitrite formation can also trigger lipid peroxidation, a chain reaction leading to the breakdown of membrane lipids, thus causing cell membrane damage and dysfunction (Ayala et al., 2014; Pizzino et al., 2017). Peroxynitrite reaction with tyrosine residues in proteins can result in the formation of 3-nitrotyrosine (Radi, 2013). This compound is recognized as a marker of protein damage from nitrosative stress, and its levels are elevated in penile tissue in SCD (Bivalacqua et al., 2013; Lagoda et al., 2014; Silva et al., 2016b; Pereira et al., 2022).
Therapeutic Interventions Based on Normalizing a Pathway NO-cGMP-PDE5
Pharmacological interventions should primarily target the pathophysiologic mechanisms of the disease rather than merely treating the symptoms. This approach enhances therapeutic effectiveness and contributes to minimizing undesired adverse effects. Considering the pivotal role of reduced endothelial NO bioavailability and the subsequent decrease in cGMP levels in the smooth muscle of the corpus cavernosum in the pathogenesis of priapism associated with sickle cell disease, therapeutic strategies that optimize eNOS function, increase the bioavailability of NO and cGMP, or modify subsequent NO targets emerge as promising and emerging treatment options.
PDE5 Inhibitors.
Based on the NO-cGMP-PDE5 signaling pathway, PDE5 inhibitors reduce the intracellular degradation of cGMP in penile smooth muscle cells. Sildenafil citrate emerged as a revolution in sexual medicine, pioneering as a PDE5 inhibitor in treating male erectile dysfunction (Goldstein et al., 1998). Paradoxically, clinical and experimental studies have provided evidence for the pleiotropic use of sildenafil citrate in managing priapism associated with SCD (Burnett et al., 2006a,b, 2014).
Experimental studies have shown that treating with sildenafil for three weeks reversed the effects of oxidative/nitrosative stress in the penises of SCD mice (Bivalacqua et al., 2013; Musicki et al., 2014). This action is primarily due to the reversal of eNOS from its uncoupled state to the functionally coupled state (Bivalacqua et al., 2013; Musicki et al., 2014). This re-coupling of eNOS and the reduction in ROS production promotes the accumulation of cGMP. cGMP, in turn, provides positive feedback on the expression and activity of PDE5, restoring its normative function (Bivalacqua et al., 2013). PDE5 functions to degrade the surplus cGMP produced during neurostimulation efficiently (Bivalacqua et al., 2013). This process prevents excessive relaxation of the cavernous tissue, thereby controlling the occurrence of priapism (Bivalacqua et al., 2013). In clinical studies involving men with SCD, it was observed that prolonged treatment regimens with low doses of sildenafil, not associated with erection-stimulating conditions, reduced recurrent priapism episodes without compromising normal erectile ability (Burnett et al., 2006a,b, 2014).
NO-Donating Compounds.
NO donors are compounds that spontaneously release NO or do so following metabolism. The enzymatic and/or non-enzymatic mechanisms that lead to NO release vary according to the specific class of the donor agent. Since the mid-1980s, the growing interest in NO physiology has driven the development of a wide range of new NO donors. Within this category, organic nitrate and nitrite esters are a significant class of NO donor drugs. They have been employed in cardiovascular therapeutics since the 19th century (Steinhorn et al., 2015).
As the reduction in NO bioavailability in the penis contributes to the development of priapism in SCD, pre-clinical studies have assessed the effect of NO donor treatment in SCD mice. Treatment of these animals with 3-(1,3-dioxoisoindolin-2-yl) benzyl nitrate (compound 4C) normalized the NO-cGMP-PDE5 pathway and reduced oxidative stress in the penis, reversing priapism (Silva et al., 2016b). The administration of the nitrosamine-based NO donor, 1,5-Bis-(dihexyl-N-nitrosoamino)-2, 4-dinitrobenzene (C6'), in the penile tissue of SCD mice for a week enhanced eNOS and PDE5 expression and decreased oxidative stress. However, its effect on priapic activity has yet to be determined (Lagoda et al., 2014).
The compound RVT-FxMe was synthesized by hybridizing the resveratrol molecule with the furoxan core, the latter being the structure that endows the compound with its ability to spontaneously donate NO or in the presence of thiol (Sorba et al., 1997; Dos Santos et al., 2012). A recent pre-clinical study assessed the effect of a two-week RVT-FxMe treatment on priapism in murine models exhibiting low NO-cGMP bioavailability, specifically in SCD mice and eNOS−/− mice (Pinheiro et al., 2022). RVT-FxMe successfully normalized the NO-cGMP pathway in the corpora cavernosa and corrected the priapism phenotype in eNOS−/− mice; however, it did not demonstrate the same effect in SCD mice (Pinheiro et al., 2022). A distinct characteristic of SCD mice is the excessive presence of free hemoglobin in the plasma, which inactivates the NO released by RVT-FxMe before it can activate the sGC in the penile tissue (Pinheiro et al., 2022).
Hydroxyurea, a potent ribonucleotide reductase inhibitor, marked a significant milestone in treating SCD. It was the pioneering drug therapy to receive approval from the US Food and Drug Administration (FDA) for managing and treating patients afflicted with this genetic condition (Steinberg et al., 2003). Its efficacy is attributed to its ability to elevate fetal hemoglobin levels, which acts as a deterrent against the polymerization of deoxygenated HbS (Charache et al., 1995). In addition, in patients with SCD, a single dose of hydroxyurea effectively elevated plasma nitrate and nitrite levels, as well as increased the total amount of nitrosylated hemoglobin (HbNO), suggesting enhanced intravascular and intraerythrocytic NO generation (Glover et al., 1999; Gladwin et al., 2002). However, the precise site and mechanism of hydroxyurea conversion into NO in vivo remains unclear. While hydroxyurea is commonly prescribed for male patients with SCD, only a few clinical studies have documented its beneficial impact on priapism in these individuals (Saad et al., 2004; Anele et al., 2014). A recent preclinical study found that hydroxyurea treatment did not modify the priapism phenotype in SCD mice (Pereira et al., 2023). This suggests that the NO generated by hydroxyurea could not effectively reach and activate the sGC in the smooth muscle cells of the corpus cavernosum, potentially due to excessive plasma hemoglobin or ROS in the penile tissue of these mice (Pereira et al., 2023).
NO donors with a nitrate-based molecular structure may hold an advantage over direct NO molecule donors under conditions of intravascular hemolysis (Lundberg et al., 2008; Silva et al., 2016b; Pinheiro et al., 2022). Previous research has indicated that Hb also serves as a nitrite reductase, facilitating the conversion of nitrite into NO during deoxygenation (Huang et al., 2005). Oral nitrate treatment has normalized voiding in sickle mice, with mechanisms likely tied to the upregulation of PDE5 activity (Musicki et al., 2019). The distinct effects of nitrate alone on priapism have not been investigated.
In summary, the challenges presented by the pathophysiology of SCD, such as elevated plasma hemoglobin that can interact with NO, indicate a state of resistance to NO. In general, these studies emphasize the need for therapeutic strategies that are not limited by plasma hemoglobin. The differential efficacy of various NO donors further accentuates the intricate complexity of this subject.
Haptoglobin.
In patients with SCD, chronic intravascular hemolysis releases excess free hemoglobin into the plasma. This hemoglobin overwhelms and depletes haptoglobin, accumulating free hemoglobin in the circulation (Muller-Eberhard et al., 1968; Reiter et al., 2002; Santiago et al., 2018).
Under typical conditions, the plasma protein haptoglobin binds to free hemoglobin, preventing excessive accumulation in the bloodstream (Buehler et al., 2020). The reticuloendothelial system macrophages detect and absorb the hemoglobin-haptoglobin complex via the CD163 receptor, facilitating its breakdown and removal from circulation (Buehler et al., 2020). This process curtails the accumulation of free hemoglobin, thereby minimizing potential tissue damage and oxidative stress (Buehler et al., 2020).
Studies have shown that haptoglobin treatment inhibits vaso-occlusion in SCD mice and preserves vascular NO during hemolysis (Schaer et al., 2016; Shi et al., 2016; Belcher et al., 2018). Preclinical research indicates that haptoglobin reverses priapism in these mice by normalizing the eNOS-NO-PDE5-cGMP pathway in the penis (Pereira et al., 2022). As such, haptoglobin therapy emerges as a promising avenue for addressing priapism linked to SCD. Clinical studies are essential to elucidate the extent of its therapeutic potential further and confirm its efficacy in treating priapism associated with SCD.
Testosterone.
Testosterone, an androgenic steroid hormone, holds fundamental importance in erection physiology. It plays a pivotal role not only in the initial development of the penis but also in maintaining and ensuring the plasticity of the cavernous nerve that innervates it (Dandona and Rosenberg, 2010). Additionally, testosterone provides neuroprotection after potential injuries to the cavernous nerve (Podlasek et al., 2016). Testosterone and dihydrotestosterone play a significant role in relaxing penile arteries and cavernous tissue (Van den Broeck et al., 2020). In the presence of androgen deficiency, there is a noted decrease in the expressions and enzymatic activities of eNOS, nNOS, and PDE5, as well as an increased alpha-adrenergic response in the penis (Podlasek et al., 2016).
In men with SCD, hypogonadism is a common complication, affecting up to 25% of them (Taddesse et al., 2012). Clinical research has shown that primary hypogonadism, characterized by testicular insufficiency, is the main reason for this hormonal irregularity in these patients. Although less common, secondary hypogonadism has also been identified in individuals with SCD, particularly in men who exhibit more severe or advanced forms of the disease (Huang and Muneyyirci-Delale, 2017).
The Berkeley mouse model for SCD exhibits hypogonadism (Musicki et al., 2015). When subjected to testosterone replacement, there is a reduction in priapism, accompanied by the restoration of eNOS activity and the normalization of the expression and activity of PDE5 in the penile tissue (Musicki et al., 2018). The proper function of PDE5 in the corpora cavernosa prevents excessive accumulation of cGMP following neurostimulation, controlling priapic activity, as previously mentioned (Musicki et al., 2018). The enhanced eNOS activity in the penis due to testosterone supplementation is attributed to a non-genomic effect of testosterone on eNOS, involving the increased phosphorylation of eNOS at Ser-1177 (Musicki et al., 2018).
Hypogonadal adult men with SCD who were treated with long-acting testosterone undecanoate injections did not experience an increase in the occurrence of priapism but rather an improvement in sexual function (Morrison et al., 2013). Currently, it is believed that the administration of testosterone at physiologic levels does not induce priapism (Morrison et al., 2013). On the contrary, this treatment seems to reduce priapism due to normalizing molecular mechanisms that promote normal erectile responses (Morrison et al., 2015).
An experimental study in mice with SCD treated with FGIN-1-27, a ligand for the translocator protein (TSPO) that mobilizes cholesterol to the inner mitochondrial membrane, showed that it is possible to achieve eugonadal levels of serum testosterone without affecting intratesticular production (Musicki et al., 2021). As a result, a reduction in episodes of priapism was observed. On a molecular level, the activation of TSPO leads to the restoration of phosphodiesterase 5 activity and a reduction in oxidative stress-mediated by NADPH oxidase in the penis (Musicki et al., 2021). This study suggests that pharmacologic activation of TSPO is a promising therapeutic approach for treating hypogonadal men, especially SCD patients, without compromising fertility.
Promising Therapeutic Directions for the Future Based on Normalization of NO-cGMP Bioavailability
SecondGC Stimulators and Activators.
Approximately two decades ago, the discovery and development of sGC stimulators and activators opened new horizons in the therapeutic approach to several conditions. These agents can directly act on sGC and promote cGMP synthesis (Sandner et al., 2021). These agents are categorized as stimulators (YC-1, BAY 41-2272, BAY 63-2521, BAY 41-8543, and BAY 60-4552) or activators (BAY 58–2667, BAY 60–2770, and HMR 1766) of sGC (Mónica and Antunes, 2018; Sandner et al., 2021). BAY 41-2272, for instance, is a sGC stimulator that acts independently of NO but is heme-dependent. It directly stimulates sGC and amplifies the sensitivity of enzymes to NO, subsequently raising cGMP levels through a NO-independent mechanism (Stasch et al., 2001; Priviero et al., 2005; Gur et al., 2010). BAY 41-2272 induces penile erection in rats (Bischoff et al., 2003) and promotes relaxation of cavernous tissues in vitro from rabbits, rats, and humans. Furthermore, chronic treatment with BAY 41-2272 improves erectile and ejaculatory dysfunctions in rats under conditions of chronic NO deficiency (Claudino et al., 2011; da Silva et al., 2012). Notably, in 2013, the FDA approved riociguat (BAY 63-2521) as a treatment modality for pulmonary hypertension.
Experimental evidence from in vitro studies has shown that sGC can exist in oxidized states and/or heme-free conditions (Stasch et al., 2006; Sandner et al., 2021). secondGC activators stand out as potential therapeutics due to their ability to activate sGC, mainly when the iron of the heme group is in an oxidized state (Fe3+ as opposed to Fe2+) or with the complete absence of this heme group (Stasch et al., 2006). A previous study reported that BAY 60-2770 acts as a heme group mimetic. It is worth noting that the oxidation of the heme portion (Fe3+) of sGC can render the enzyme insensitive to endogenous NO, thereby inhibiting the activation of the sGC-GMPc-cGMP-dependent protein kinase signaling pathway in various tissues (Stasch et al., 2006; Sandner et al., 2021). Experimental studies have shown that BAY 60-2770 displays potent erectile activity in rats and reverses erectile and micturition dysfunctions associated with obesity in mice (Lasker et al., 2013; Alexandre et al., 2014; Leiria et al., 2014; Silva et al., 2014).
In SCD mice, acute treatment with the sGC activator (BAY 54-6544) demonstrated superiority over the sGC stimulator (BAY 41-8543) in improving the endothelial function of pulmonary arteries (Potoka et al., 2018). Chronic treatment with the sGC activator (BAY 54-6544) improves endothelial function and reverses pulmonary hypertension without affecting systemic blood pressure in sickle cell mice (Potoka et al., 2018). Both the sGC activator (BAY 60-2770) and the sGC stimulator (BAY 41-2272) reduces vaso-occlusive events due to decreased leukocyte recruitment to the endothelium in sickle cell mice (Ferreira et al., 2020). BAY 41-2272, but not BAY 60-2770, increases the transcriptional expression of the γ-globin gene and the production of fetal hemoglobin in K562 erythroleukemic cell culture (Ferreira et al., 2020). Olinciguat, another sGC stimulator in focus, is currently under clinical study for use in patients with SCD (NCT03285178), reduces inflammation, vaso-occlusion, and nephropathy in sickle cell mice (Tchernychev et al., 2021).
Based on the presented studies, sGC activators and stimulators emerge as potential therapeutic alternatives for SCD. They can amplify cGMP-dependent signaling and induce the upregulation of PDE5, positioning them as a meaningful therapeutic strategy for preventing or treating priapism. Importantly, sGC activators and stimulators may have an advantage over NO donor compounds since they are not inactivated by plasma hemoglobin and excess ROS.
Hemopexin.
Free hemoglobin in plasma or the interstitial space can rapidly oxidize, promptly releasing the heme group into the plasma (Schaer et al., 2013; Gladwin, 2016). Under physiologic conditions, hemopexin counteracts the accumulation of heme in plasma. Hemopexin binds to the heme group, forming a hemopexin-heme complex that is metabolized in hepatocytes (Smith and Morgan, 1979; Hvidberg et al., 2005). As a protective mechanism, the enzyme heme oxygenase-1 contributes to the enzymatic degradation of heme, thus restricting damage from hemolysis-related molecules. In patients and mice with SCD, elevated heme concentrations are released into the plasma, saturating hemopexin, whose levels decline rapidly and, thus, accumulate heme in plasma (Muller-Eberhard et al., 1968; Reiter et al., 2002; Gbotosho et al., 2021).
Plasma heme is a potent inducer of inflammation, known as a damage-associated molecular pattern (Figueiredo et al., 2007; Bozza and Jeney, 2020). In murine models of SCD, it was shown that heme can activate vascular endothelium through toll-like receptor 4 (TLR4). Activation of this receptor leads to the production of inflammatory mediators (interleukin-1, interleukin-6, interleukin-8), reactive oxygen species, and the release of von Willebrand factor and P-selectin, involved in the activation of blood coagulation and cell adhesion, favoring the occurrence of vascular stasis and vaso-occlusion (Belcher et al., 2014). Therefore, through TLR4 activation, free heme plays a fundamental role in the pathophysiology of SCD (Gbotosho et al., 2021). Experimental studies in corpus cavernosum of animal models of erectile dysfunction have shown that TLR4 activation contributes to alterations in erectile function (Nunes et al., 2017, 2018).
In mouse models with SCD, hemopexin has shown notable beneficial properties. Treatment with hemopexin decreased the release of p-selectin and von Willebrand factor and potentiated heme oxygenase-1 (Belcher et al., 2018). Moreover, studies have shown that hemopexin reduces these animals’ vaso-occlusion and acute renal failure. In the aortas of these mice, hemopexin treatment promotes an increase in NOS activity and a reduction in both ROS and nitrosative stress. Hemopexin improves endothelial dysfunction, corrects cardiac alterations, and decreases mean arterial pressure (Vinchi et al., 2013). Due to its ability to enhance endothelial NO bioavailability and attenuate oxidative stress, hemopexin emerges as a promising therapeutic approach for treating priapism related to SCD.
In summary, hemopexin is vital in modulating the complications associated with SCD, particularly in mitigating the deleterious effects of free heme in plasma. Its beneficial properties, as evidenced in animal models, not only point to an ability to correct endothelial dysfunction but also suggest potential benefits in treating priapism related to SCD. The role of hemopexin in improving the bioavailability of endothelial NO and attenuating oxidative stress highlights it as a promising therapeutic approach. Future preclinical are needed to confirm these findings and validate hemopexin as a therapeutic intervention in patients with SCD who have recurrent priapism.
Antioxidants.
Oxidative and nitrosative stress is pivotal in the pathogenesis of priapism associated with SCD (Musicki et al., 2012, 2014; Silva et al., 2016a; Pereira et al., 2022). Notably, L-glutamine, a precursor to the antioxidant glutathione, received FDA approval in 2017 for patients with SCD (Mullard, 2020). However, its efficacy in addressing priapism still needs to be determined. Other antioxidants, such as N-acetylcysteine and omega-3 fatty acids, studied in the context of SCD complications, have yet to establish their specific effects on priapism (Nur et al., 2012; Daak et al., 2013).
Apocynin, an inhibitor of NADPH oxidases, demonstrated promising effects when administered for four weeks in SCD mice by reducing ROS production and normalizing eNOS expression in the penis (Musicki et al., 2012). Similarly, the xanthine oxidase inhibitor attenuates ROS production in isolated penile tissues of these mice (Bivalacqua et al., 2013). However, the direct efficacy of these inhibitors on priapic activity requires further evaluation, underscoring the need for more comprehensive preclinical studies.
In summary, antioxidants are fascinating in treating SCD and its associated priapism. Given the intricate interplay between oxidative and nitrosative stress and the pathogenesis of priapism, antioxidants emerge as promising candidates for clinical exploration (Musicki et al., 2012, 2021; Bivalacqua et al., 2013; Lagoda et al., 2013, 2014; Silva et al., 2016b; Pereira et al., 2022). While findings from mouse studies are encouraging, it is paramount to conduct additional preclinical research to ensure the safety and effectiveness of these agents before their application in SCD patients. The FDA approval of L-glutamine and ongoing research on other antioxidants accentuate the importance of intensifying investigations into these compounds as potential therapeutic strategies for priapism in SCD.
Integrated Strategies to Elucidate the Pathobiology of Priapism in Sickle Cell Disease
Understanding the pathobiology of priapism in patients with SCD remains a significant challenge, limiting the development of effective treatments. An integrated approach, combining longitudinal clinical studies, translational research with animal models, and detailed molecular analyses, is essential to advance the understanding of this complex condition.
Longitudinal clinical studies following patients with SCD can provide valuable results on the progression of priapism, identifying risk factors and clinical correlations. Including a wide range of demographic and clinical data can help discern specific patterns and triggers of priapism. Concurrently, the use of transgenic mice for SCD is useful for investigating relevant molecular mechanisms, contributing to clinical applications. New molecular studies in human penile tissues may also contribute to a deeper understanding of changes in penile physiology, potentially revealing new therapeutic targets and intervention strategies.
Further expanding on this idea, it is becoming increasingly clear that there are likely multiple molecular derangements in the pathogenesis of priapism rather than a singular molecular mechanism. Our current understanding implicates irregularities in NO signaling, as well as various hematologic factors (Kato et al., 2018; Musicki and Burnett, 2020). Thus, recognizing a range of mechanisms in priapism is purposeful and exciting to research, and we may evolve toward personalized interventions based on biomarkers, but with selective cocktails of therapy in the future, not just a monotherapy.
Better understanding the pathobiology of priapism in SCD has direct implications for developing more effective therapies. Research in this area could lead to personalized interventions, potentially improving the quality of life of patients with SCD and reducing the risk of long-term complications. Thus, by expanding our understanding of the pathobiology of priapism in humans with SCD, we can expect significant advances in the management and treatment of priapism in patients with SCD, aligning scientific research with the urgent clinical needs of this population.
Conclusion
Priapism, associated with SCD, represents a significant clinical challenge as no treatment is available. Consistent experimental evidence indicates that the reduction in the bioavailability of NO-cGMP in the penis in SCD is the central change in the pathophysiology of priapism, with plasma hemoglobin, ROS, and endothelial dysfunction playing a fundamental role in contributing to the decrease in NO bioavailability. The emergence of new therapeutic agents focused on normalizing NO/cGMP signaling offers hope and direction for future interventions. Although PDE5 inhibitor therapy and testosterone replacement have shown promising results, recent preclinical research broadens the therapeutic horizon, suggesting opportunities for NO donors and haptoglobin treatment. However, it is essential to recognize potential limitations and the need for additional studies to validate these therapies.
Data Availability
This article contains no datasets generated or analyzed during the current study.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Pereira, Calmasini, Costa, Burnett, Silva.
Footnotes
- Received October 20, 2023.
- Accepted January 5, 2024.
This work was supported by the São Paulo Research Foundation [Grant 2017/08122-9].
No author has an actual or perceived conflict of interest with the contents of this article.
Abbreviations
- cGMP
- cyclic guanosine monophosphate
- eNOS
- endothelial nitric oxide synthase
- FDA
- US Food and Drug Administration
- Hb
- hemoglobin
- HbS
- hemoglobin S
- nNOS
- neuronal nitric oxide synthase
- NO
- nitric oxide
- PDE5
- phosphodiesterase type 5
- ROS
- reactive oxygen species
- SCD
- sickle cell disease
- sGC
- soluble guanylyl cyclase
- TLR4
- toll-like receptor 4
- Copyright © 2024 by The American Society for Pharmacology and Experimental Therapeutics