Abstract
Abstract ID 94059
Poster Board 350
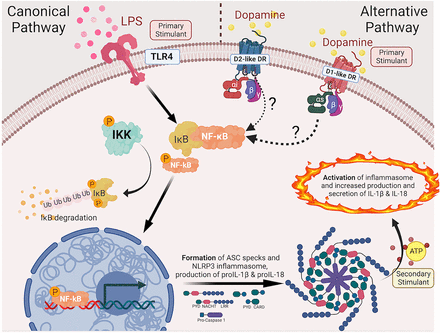
Macrophages play a pivotal role in immune homeostasis and stimuli that alter macrophage functions can have a substantial impact on the innate immune response. Although primarily studied in the context of reward, motor control and learning, the neurotransmitter dopamine has been shown to have substantial immunoregulatory capacity in many types of immune cells, including macrophages. Our previous studies show that dopamine can drive the translocation of NF-kB, priming the NLRP3 inflammasome in increasing the transcription and translation of NLRP3, PYCARD and pro-IL-1β. These effects result in a more robust release of IL-1 cytokines, such as IL-1β, in response to inflammasome activation. There are several different types of inflammasomes, which serve as critical sensors for different types of cellular stress, damage, or pathogen invasion. These multi-protein complexes consist of several proteins, including an effector protein such as NLRP3, NLRC4, or AIM2, procaspase-1 and an adaptor protein called PYCARD or ASC. Based on our prior studies, we hypothesize that the activation of dopamine receptors on myeloid cells alters the formation and activation of inflammasomes in macrophages. To examine this, primary human monocyte-derived macrophages (hMDM) were derived from deidentified human blood, matured in vitro for six days, and treated with dopamine (10-6M). The hMDM were lysed at several different time points (3, 4.5, 6, and 7.5 hours post-treatment) and then evaluated for changes in transcription and expression of inflammasome component, caspase-mediated signaling activation, and changes in cellular morphology. These studies combined high-content imaging with molecular biology and demonstrated distinctive kinetic profiles of inflammasome formation with dopamine compared to the positive control LPS. Moreover, our findings suggest that at least 4.5 hours are required after the activation of dopamine receptors for alterations in the transcription and translation of NLRP3 inflammasome, as well as activation of caspase-mediated signaling. These changes correlated with the emergence of PYCARD positive ‘speck’ formations and with changes in cell morphology. Our findings illuminate the intricate interplay between dopamine signaling and inflammasome activation, highlighting their potential significance in developing immunomodulation therapeutic strategies.
Dopamine’s interaction with dopamine receptors has the potential to trigger the translocation of nuclear factor kappa-light-chain-enhancer of B cells (NF-κB) to the nucleus, leading to an upregulation in the transcription and translation of inflammasome adaptor proteins (ASC or PYCARD) as well as pro-IL-1β. As multiple PYCARDs are recruited, inflammasomes are assembled, and with the presence of a secondary stimulant, they become activated. This activation culminates in the secretion of IL-1β and IL-18 from macrophages.
- Copyright © 2024 by The American Society for Pharmacology and Experimental Therapeutics