Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) that results in demyelinating lesions and accumulating neurologic disability. Although the etiology of MS is incompletely understood, both genetic and environmental factors are implicated. The disease is characterized by T and B cells autoreactive to myelin-related epitopes that enter the CNS and orchestrate immune attacks. This results in injury to the oligodendrocytes, their myelin sheaths, and ultimately the axons they insulate (Baskaran et al., 2023). MS most commonly presents as a relapsing-remitting disease characterized by recurring inflammatory activity followed by full or partial recovery. Remission occurs in part by a process of remyelination that enhances nerve impulse conduction and provides axons with vital metabolic support (Nave and Werner, 2021). When neurologic disability progressively worsens and accumulates, the disease is termed secondary progressive MS. In a minority of cases, progressive disease is apparent from the outset and is termed primary progressive MS. The permanent disability that characterizes progressive stages reflects damage to axons and a microenvironment inhibitory to myelin regeneration (Absinta et al., 2020). Currently, there are 24 disease-modifying therapies available for MS (Baskaran et al., 2023). Notably, many of these have high efficacy for relapsing-remitting disease but show limited to no efficacy for progressive stages. Altogether, these data emphasize the complex pathogenesis of MS and that consideration of immune, neuronal, and glial mechanisms will be important in the design of therapies needed to halt disease and promote regenerative repair.
In this issue of the Journal of Pharmacology and Experimental Therapeutics, Eftekhari et al. (2023) demonstrate the ability of P2pal-18S, a cell penetrant lipopeptide (pepducin) targeting protease-activated receptor 2 (PAR2), to prevent the development of neuroinflammatory disease in a murine model of MS, experimental autoimmune encephalomyelitis (EAE). In earlier studies, the team demonstrated that PAR2-null mice show reductions in myelin oligodendrocyte glycoprotein (MOG35-55)–driven EAE (Noorbakhsh et al., 2006) and in HIV-encephalitis (Noorbakhsh et al., 2005). Taking an important step forward, here a pharmacological approach using P2pal-18S was deployed to interfere with PAR2 signaling in adult mice during EAE development. Convincingly, the team demonstrates that including P2pal-18S along with the disease initiating antigen (MOG35-55) in complete adjuvant at the time of priming, followed by an additional subcutaneous dose in olive oil 10 days later, reduces disease severity in a mild EAE model. Improvements in functional outcomes were reflected in reductions in the accumulation of CD4+ T cells and CD11b/CD45high bone marrow–derived macrophages (BMDMs) as well as preserved spinal cord myelin basic protein. These results were complemented by evidence of reduced granulocyte-macrophage colony-stimulating factor (GM-CSF), a cytokine that activates macrophages and dendritic cells, and eotaxin, a chemokine that recruits eosinophils, in the serum of treated mice. Together these findings convincingly highlight the ability of P2pal-18S to reduce the development of MOG35-55–driven neuroinflammation when included in the adjuvant at the time of priming.
Building on the ability of P2pal-18S to attenuate the manifestation of MOG35-55 EAE, the physiologic roles of PAR2 in T cell and monocyte responses were investigated in vitro. First, the functional activation of PAR2 in splenic CD4 T cells was clearly established using sensitive measurements of increases in intracellular Ca2+ upon PAR2 agonist application. P2pal-18S reduced cytokine secretion by mixed splenocyte cultures and limited CD4 T cell proliferation after anti-CD3/CD28 activation. P2pal-18S mediated reductions in cytokines, including proinflammatory cytokines [interleukin (IL)-1β, IL-6, tumor necrosis factor alpha (TNFα), GM-CSF, and IL-17A] in addition to anti-inflammatory Th2 cytokines (IL-4, IL-5, and IL-13). Since anti-CD3/CD28 broadly activates T cells, it will be of interest to determine if in vivo delivery of P2pal-18S reduced lymphocyte recall responses to MOG35-55. In this case, determining any differential impact on proinflammatory versus anti-inflammatory Th2 responses would be important since Th2 responses promote EAE remission (Cua et al., 1995; Kokubo et al., 2022).
Given the impact of P2pal-18S on monocytes, the Eftekhari team performed in vitro studies to understand potential effects on the polarization of BMDM to M1 (proinflammatory) or M2 (anti-inflammatory/tissue reparative) phenotypes. Macrophage polarization occurs in response to microenvironmental signals, permitting response to injury while maintaining tissue homeostasis. Interestingly, treatment of macrophages with P2pal-18S resulted in reduced expression of both M1 (iNOS) and M2 (arginase) RNA with results mirrored in BMDM from PAR2-null mice. P2pal-18S also reduced the secretion of IL-4 from M2 polarized BMDM. Overall, these results, taken with the wide impact of blocking PAR2 on anti-CD3/CD28 activated T cells, suggest that P2pal-18S imparts broadly immunosuppressive effects in the in vitro assays examined.
By binding to the intracellular domain of PAR2, P2pal-18S is positioned to interfere with coupling to intracellular G proteins and subsequent signaling (Shpakov, 2023). For example, P2pal-18S reduces the stability of the Bcl-2 homolog Bcl-xL in colorectal carcinoma cells (Li et al., 2021), ERK and STAT3 signaling in cholangiocarcinoma cells (Kaufmann et al., 2012), and Bim expression as well as ERK signaling in murine neuronal cultures (Yoon et al., 2013). Which signaling cascades are modified by P2pal-18S that are responsible for the immunosuppressive effects observed in the current study remains to be clarified.
This identification of the efficacy of PAR2 cell-penetrating lipopeptides in the MOG35-55 EAE model (Eftekhari et al., 2023) further highlights the linkage of PAR2 with inflammatory events across organ systems. This includes roles for PAR2 activation in promoting inflammation in models of arthritis, asthma, colitis, glomerulonephritis, dermatitis, spinal cord injury, and inflammatory pain [see (Peach et al., 2023) for review]. Previously, P2pal-18S pepducins were delivered to successfully reduce carrageenan-induced edema (Sevigny et al., 2011), caerulein-driven acute biliary pancreatitis (pancreatic dysfunction) (Michael et al., 2013), and bleomycin-induced pulmonary fibrosis (Lin et al., 2015) as well as inflammation and itch in atopic dermatitis models (Barr et al., 2019). Collectively, these studies point to the need for additional efforts to test the ability of P2pal-18S to reduce inflammation in additional experimental systems, including models of progressive MS.
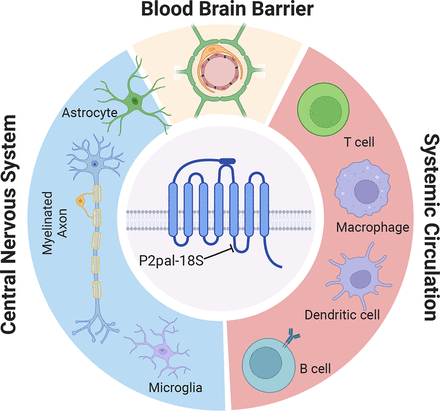
Since PAR2 is widely expressed under homeostatic conditions and frequently noted as upregulated with pathology (Radulovic et al., 2015; Kalogera et al., 2021), including in MS lesions (Noorbakhsh et al., 2006), clarifying the range of cellular interactions of P2pal-18S in EAE will be important. This understanding may enable the pursuit of strategies that confer neuroprotective and proregenerative outcomes in addition to quelling the inflammatory aspects of MS. In this regard, it is noteworthy that in addition to immune cells, PAR2 is expressed by other cell types relevant to MS, including endothelia (Klarenbach et al., 2003; Göbel et al., 2019), neurons (Yoon et al., 2013; Liu et al., 2014), microglia (Sun et al., 2014), astrocytes (Vandell et al., 2008; Yoon et al., 2018), myelinating oligodendrocytes, and their progenitors (Yoon et al., 2017) (Fig. 1). Of interest, blocking PAR2 function attenuates astroglial reactivity (Radulovic et al., 2015). Also, in combination with P1pal-7, a lipopeptide inhibitor of PAR1, P2pal-18S blocks glutamate-mediated excitotoxicity in primary neuron cultures (Yoon et al., 2013). Finally, PAR2-null mice show accelerated CNS myelination, increased myelin resiliency after spinal cord injury, and improved myelin regeneration in an acute model of demyelinating disease (Yoon et al., 2017). A small molecule inhibitor of PAR2 (GB88) also increases expression of the major myelin proteins, proteolipid protein and myelin basic protein (MBP), by oligodendrocytes (Yoon et al., 2017). These findings, taken with the results of Eftekhari et al. (2023), further highlight PAR2 as a single target capable of impacting multiple aspects of MS pathology.
Schematic depicts protease activated receptor 2 (PAR2), a seven-transmembrane G protein–coupled receptor that is expressed across immune cells, CNS neurons, glia, and elements of the blood-brain barrier relevant to neuroinflammation. PAR2 is activated by trypsin-like serine proteases that cleave near its N terminus to reveal a tethered ligand that binds to the second extracellular loop of the receptor. P2pal-18S is a lipopeptide allosteric inhibitor of PAR2 that binds to intracellular loop 3 to impede coupling to G proteins and thereby interferes with downstream signaling. PAR2 is expressed by many of the cellular elements relevant to MS pathogenesis, including immune cells [T cell subsets (Th1, Th2, Th17, macrophages, dendritic cells, and B cells)] in the systemic circulation; cells that comprise the neurovascular unit forming the blood-brain barrier (endothelial cells, pericytes, and astrocytes); and CNS neurons and glia (myelinating oligodendrocytes, astrocytes, and microglia). Effective therapies for MS will require not only restoring inflammatory homeostasis but also protecting neurons and promoting regeneration of the myelin sheaths that wrap and insulate axons to facilitate the conduction of electrical impulses (created in BioRender.com).
The findings presented by Eftekhari et al. (2023) are exciting since they demonstrate for the first time that administration of P2pal-18S in adjuvant at the time of EAE induction largely prevents the development of functional deficits. There remain questions and some potential limitations. Importantly, as the authors point out, future studies to determine if PAR2 can be targeted to reduce established neuroinflammation and disease progression in chronic neuroinflammatory models will be a significant line of future investigation. P2pal-18S was delivered subcutaneously in complete Freund's at the time of MOG immunization and 10 days later emulsified in olive oil leaving open questions regarding the site and mechanism of action. Among the possibilities, the peptide could be slowly released into the systemic circulation. Alternatively, the presence of P2pal-18S in the immunizing emulsion may impact monocytes as they uptake the MOG35-55 peptide, subsequent antigen presentation, and T cell priming, resulting in reduced CD4 T cell reactivity to MOG35-55 and diminished T cell and monocyte CNS infiltration. The potential impact on T cell priming could be addressed by quantifying MOG35-55 recall responses by lymph node/splenocyte cultures prepared from treated and control mice at early stages of disease. In this regard, it will be of interest to determine if the T cells were permanently altered by P2pal-18S treatment, potentially rendering them unable to initiate disease in an adoptive transfer model.
In summary, the findings of Eftekhari et al. (2023) shed new light on PAR2 as an important mediator of inflammatory events in the development of MOG35-55 EAE and show that a lipopeptide inhibitor mitigates disease. Continued study of lipopeptides targeting PAR2 or other PARs (PAR1 and PAR4) known to drive neuroinflammatory cascades (O’Callaghan et al., 2012) is an exciting avenue for additional translational studies. Future efforts investigating PAR-directed lipopeptides alone or as an adjunctive therapy in a model of progressive MS, for which there are few therapeutic options, will be critically important. As the field moves forward, continued efforts to identify new targets to selectively modulate key aspects of the neuroinflammatory cascade, to prevent neurodegeneration, and to promote regeneration will all be essential to realize the promise of precision medicine for the effective treatment of MS.
Footnotes
- Received August 22, 2023.
- Accepted September 26, 2023.
This work received no external funding.
No author has an actual or perceived conflict of interest with the contents of this article.
Abbreviations
- BMDM
- bone marrow–derived macrophage
- CNS
- central nervous system
- EAE
- experimental autoimmune encephalomyelitis
- MOG
- myelin oligodendrocyte glycoprotein
- MS
- multiple sclerosis
- PAR
- protease-activated receptor
- TNFα
- tumor necrosis factor
- Copyright © 2023 by The American Society for Pharmacology and Experimental Therapeutics