The March issue of the Journal of Pharmacology and Experimental Therapeutics offers an interesting read on palonosetron for managing nausea and vomiting in beagle dogs treated with the anticancer agent cisplatin (Levijoki et al., 2023). The subject matter is anything but novel. Chemotherapy-induced nausea and vomiting (CINV) is sadly known to patients with cancer (Lorusso et al., 2017), beagles and cisplatin have already been used to decipher the mechanisms of CINV (Kenward et al., 2014), and injectable and oral formulations of palonosetron rank very high in the pharmacological armamentarium against CINV (Smith et al., 2020). So, what is new in this report? Novelty rests with the formulation of palonosetron, which was used as eye drops and worked well from both pharmacokinetic and efficacy viewpoints. A critical look at clinical facts and preclinical findings is nonetheless much needed before this report can be considered as breakthrough or preliminary finding.
CINV is a big problem in clinical oncology. Although the last decades have witnessed remarkable improvements in antiemetic medication, too many patients with cancer still develop some degree of nausea and vomiting (Piechotta et al., 2021). CINV causes physical discomfort to the patient with cancer, and psychologic conditioned responses may be strong enough to make an “anticipatory” CINV occur before chemotherapy is administered (Aapro, 2018). Not all cancer drugs stand shoulder to shoulder with respect to CINV. Some drugs, whether taken in isolation or incorporated in multiagent regimens, are considered as highly emetogenic, whereas others are considered as moderately or minimally emetogenic. Statements and consensus documents from the Multinational Association of Supportive Care in Cancer, the European Society of Medical Oncology, and the American Society of Clinical Oncology constantly update the emetogenicity of cancer drugs as comprehensive reviews and metanalyses are published by research groups (Razvi et al., 2019; Piechotta et al., 2021). Cisplatin, which was used in the paper under scrutiny, is unanimously classified as highly emetogenic.
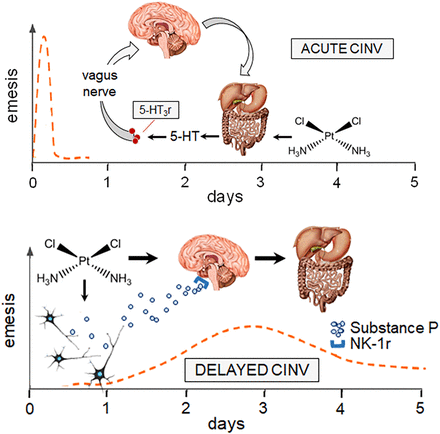
Highly emetogenic drugs can induce a biphasic pattern of CINV, characterized by acute and delayed phases that develop, respectively, within 24 hours or 5 days from chemotherapy administration (Aapro, 2018). Delayed CINV is particularly insidious and distressful to cope with. When patients are treated with cisplatin, there may be no early CINV in as many as 62% of them, but the cumulative incidence of CINV can be as high as 93%, with the highest incidence of delayed CINV occurring 48 to 72 hours after cisplatin infusion (Kris et al., 1985). Acute and delayed CINV reflect peripheral or central emetogenic pathways mediated by distinct neurotransmitters. Acute CINV occurs when cancer drugs cause enterochromaffin cells in the gastrointestinal (GI) tract to release serotonin (5-HT); activation of 5-HT3 receptors in vagal afferents fibers then triggers ascending inputs toward the vomiting center of the brain, from which descending inputs originate and cause contractions of abdominal muscles, stomach, and diaphragm (Chen et al., 2022). The circuit between GI tract, brain, and the effectors of CINV is consolidated by signals that project from chemoreceptors of the trigger zone of area postrema toward the vomiting center. The trigger zone is not protected by the blood-brain barrier, which makes it liable to direct activation by cancer drugs. Delayed CINV builds on substance P, a neuropeptide that is largely expressed in both peripheral and central nervous systems. Cancer drugs mobilize substance P, which then activates neurokinin-1 (NK-1) receptors in the central nervous system (Aapro, 2018). Kinetics and mechanisms of acute and delayed CINV are illustrated in Fig. 1, with cisplatin serving as prototypic trigger of both types of CINV.
Conceptual representation of acute and delayed CINV. The upper panel shows that acute CINV (dashed line) occurs within 1 hour from chemotherapy administration. The same panel incorporates a representation of the GI tract-brain circuit that mediates acute CINV. The chemical structure of cisplatin as a trigger of 5-HT release in the GI tract is reported. The lower panel shows cisplatin-induced substance P mobilization from neurons and central induction of delayed CINV, which occurs within 5 days (dashed line). 5-HT3r, serotonin type 3 receptor; NK-1r, neurokinin type 1 receptor.
Combining 5-HT3 and NK-1 receptor antagonists is an intuitive strategy to manage both acute and delayed CINV, especially if one appreciates that NK-1 receptors may at least in part also contribute to acute CINV, and crosstalks between 5-HT and substance P may well occur (Aapro, 2018). Keeping these pharmacological premises in mind, it is no surprise that Levijoki and colleagues (2023) selected palonosetron as the active principle of antiemetic eye drops. Palonosetron is in fact a versatile drug that is best known as a second-generation allosteric 5-HT3 receptor antagonist but that also mitigates serotonin potentiation of substance P responses, something that is not observed with first-generation 5-HT3 receptor antagonists such as granisetron or ondansetron (Rojas et al., 2010a). Palonosetron would therefore be perfect to blunt acute CINV and mitigate delayed CINV induced by cisplatin in the beagle model.
The results reported by Levijoki and colleagues (2023) are promising in many respects. At the dose of 30 μg/kg, palonosetron eye drops reduced nausea and discomfort-related behavior by 98% and 95%, respectively. At the dose of 120 μg/kg, vomiting was completely suppressed. If we now look at our watch, we can say that the effects of palonosetron were surprisingly long lasting. Palonosetron was rapidly absorbed into systemic circulation, with a time to plasma Cmax of approximately 5 minutes when polyvinylpyrrolidone (PVP) was used as the vehicle. Plasma levels then decreased by one log in just 4 hours, yet dogs treated with 120 μg of palonosetron per kg had essentially no vomiting for ∼6 hours. Inasmuch as dogs treated with vehicle drops started to vomit ∼2.5 hours after cisplatin infusion and showed a vomiting peak at ∼3.5 hours, long lasting antiemetic effects of palonosetron can only be reconciled with its unique effects on 5-HT3 receptors (high affinity, potent inhibition, induction of receptor internalization) (Rojas et al., 2010a,b). The work by Levijoki and colleagues (2023) shows further strengths that merit consideration. Systemic pharmacokinetics of the ocular palonosetron formulation were much similar to those of an intravenous formulation, particularly when PVP was used as a vehicle. Replacing PVP with hydroxypropyl-β-cyclodextrin (HP-β-CD) resulted in somewhat longer time to Cmax (∼20 minutes), but the subsequent decay of palonosetron levels was significantly delayed over time, such that one log decrease from Cmax occurred in >6 hours. These facts anticipate that HP-β-CD might be exploited to obtain palonosetron ocular formulations with furtherly extended antiemetic effects. Replacing PVP with HP-β-CD also improved local tolerability of the eye drops, although both PVP and HP-β-CD formulations were generally well tolerated.
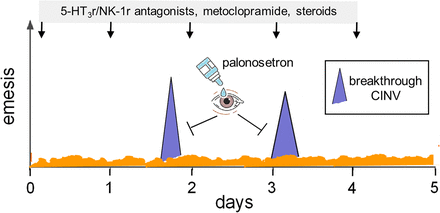
But not all that glitters is gold, and some study limitations must be brought into focus. The authors acknowledge weaknesses such as small sample size, lack of comparisons between beagles and other animal species, and lack of shoulder-to-shoulder comparisons between palonosetron eye drops and similar formulations of other 5-HT3 receptor antagonists. All such concerns are reasonable, but I would not make an insurmountable issue out of them. My major concern pertains to a seemingly naive question: why was this study conducted and why were eye drops thought to be so important for improving clinical management of CINV? The authors did explain the rationale for their work and focused very much on “breakthrough” CINV [i.e., nausea and vomiting that occur within 5 days after chemotherapy in a patient receiving optimal prophylaxis with 5-HT3 and/or NK-1 receptor antagonists, steroids, metoclopramide (dopamine receptor antagonist) and other ancillary drugs (Gupta et al., 2021)]. They correctly state that oral palonosetron and other antiemetics would be ineffective due to vomiting at that time. Rectal, subcutaneous, or intravenous administration of antiemetics would instead be needed, but this would also require patient assistance by a caregiver. This is where rapidly absorbed palonosetron eye drops might change the game and help the patient to self-manage breakthrough CINV. Although absolutely correct, this rationale may only partially be translated into experimental facts.
In earlier studies of CINV in beagles, other investigators found that a “clinical” dose of cisplatin (70 mg/kg) caused significantly earlier CINV compared with the “subclinical” dose adopted in the present study (15 mg/kg), with absolute vomit counts also being significantly higher after 70 mg/kg than 15 mg/kg (Kenward et al., 2014). On these grounds, the reader would ask if low-dose cisplatin actually induced a kind of moderate delayed CINV rather than authentic breakthrough CINV. To answer this question, the experimental model should be reshaped to accommodate both acute and delayed and breakthrough CINV, and the efficacy of ocular palonosetron against breakthrough CINV should be documented in animals that received optimal prophylaxis of acute and delayed CINV. This is probably easier said than done, but an experimental effort in that direction would be required for clinical development of ocular palonosetron. Currently, breakthrough CINV is treated with drugs that were not administered as prophylactic antiemetics (atypical antipsychotics, benzodiazepines, cannabinoids) (Gupta et al., 2021). Would ocular palonosetron relieve breakthrough CINV in animals that already received prophylactic doses of its intravenous formulation? This is an important scenario to explore. Should ocular palonosetron work well in this setting, its clinical development as a user-friendly rescue agent would be warranted and psychologic discomfort from using too many classes of drugs would be minimized. The prospective place in therapy for ocular palonosetron is shown in Fig. 2.
Palonosetron eye drops to treat breakthrough CINV. 5-HT3r, serotonin type 3 receptor; NK-1r, neurokinin type 1 receptor.
In sum, the work by Levijoki and colleagues (2023) on palonosetron eye drops against CINV must be considered in perspective, which is more than obvious when one speaks of eyes. In the words of the French novelist Marcel Proust, “the real voyage of discovery consists not in seeking new landscapes, but in having new eyes.”
Authorship Contributions
Performed data analysis: Minotti.
Wrote or contributed to the writing of the manuscript: Minotti.
Footnotes
- Received February 24, 2023.
- Accepted April 3, 2023.
This work received no outside funding.
The author has no actual or perceived conflict of interest with the contents of this article.
ABBREVIATION
- CINV
- chemotherapy-induced nausea and vomiting
- GI
- gastrointestinal
- HP-β-CD
- hydroxypropyl-β-cyclodextrin
- 5-HT
- serotonin
- 5-HT3
- serotonin type 3
- NK-1
- neurokinin-1
- PVP
- polyvinylpyrrolidone
- Copyright © 2023 by The American Society for Pharmacology and Experimental Therapeutics