Abstract
Cariprazine is an approved antipsychotic and antidepressant which is a dopamine (DA) D3-preferring D3/D2 receptor partial agonist, serotonin (5-HT) 5-HT1A receptor partial agonist, and 5-HT2B and 5-HT2A receptor antagonist, a profile unique for atypical antipsychotic drugs. The purpose of this study was to clarify the effects of cariprazine and selective D3 receptor ligands on neurotransmitter efflux in the rat nucleus accumbens (NAC) and ventral hippocampus (HIP), brain regions important for reality testing, rewarded behavior, and cognition. In vivo microdialysis was performed in awake, freely moving rats after administration of cariprazine; (+)-PD-128907 [(4aR,10bR)-3,4a,4,10b-tetrahydro-4-propyl-2H,5H-[1]benzopyrano-[4,3-b]-1,4-oxazin-9-ol hydrochloride], a D3 receptor–preferring agonist; and SB-277011A [trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinolininecarboxamide hydrochloride], a selective D3 receptor antagonist, alone or combined, and extracellular levels of multiple neurotransmitters and metabolites were measured in the NAC and HIP by ultraperformance liquid chromatography with tandem mass spectrometry. Cariprazine increased DA, norepinephrine (NE), and 5-HT efflux in both regions, whereas it increased glycine (Gly) and glutamate efflux only in the NAC and efflux of DA metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) only in the HIP. Similarly, SB-277011A increased DA, NE, DOPAC, and HVA, but not 5-HT, efflux in the NAC and HIP, and acetylcholine efflux in the HIP. Most of these effects of cariprazine and SB-277011A were fully or partially attenuated by the D3 receptor agonist (+)-PD-128907, suggesting these effects of cariprazine are related to its D3 receptor partial agonism, and that this mechanism, leading to diminished stimulation of D3 receptors, may contribute to its efficacy in both schizophrenia and bipolar disorder. The possible role of Gly in the action of cariprazine is discussed.
SIGNIFICANCE STATEMENT The novel atypical antipsychotic drug cariprazine increased nucleus accumbens and hippocampal neurotransmitter efflux, similar to the actions of the D3 receptor antagonist SB-277011A [trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinolininecarboxamide hydrochloride]. The D3 receptor–preferring agonist (+)-PD-128907 [(4aR, 10bR)-3,4a,4,10b-tetrahydro-4-propyl-2H,5H-[1]benzopyrano-[4,3-b]-1,4-oxazin-9-ol hydrochloride], diminished the effects of both compounds on neurotransmitter efflux in both regions. These results suggested D3 receptor partial agonist activity of cariprazine, producing functional antagonism, may contribute to its efficacy in schizophrenia and bipolar disorder.
Introduction
Cariprazine (United States: Vraylar, Allergan; Europe: Reagila, Gedeon Richter Plc), an orally active and potent dopamine (DA) D3-preferring D3/D2 receptor partial agonist, is a novel atypical antipsychotic drug (AAPD) approved to treat adults with schizophrenia and bipolar I disorder (De Deurwaerdère, 2016; Stahl, 2016; Garnock-Jones, 2017). The DA D3 receptor, through pre- and postsynaptic action in brain areas, has been suggested to play a key role in depression and negative symptoms (Leggio et al., 2013). Most of the approved AAPDs are more potent serotonin (5-HT) 5-HT2A receptor antagonists than DA D2 receptor antagonists at clinically effective doses, with additional direct or indirect actions on 5-HT1A, 5-HT6, 5-HT7, D1, D3, D4, adrenergic, histaminergic, glutamatergic, and muscarinic or nicotinic receptors (Meltzer and Huang, 2008). These, and other indirect effects, may result in increased 5-HT, DA, norepinephrine (NE), acetylcholine (ACh), or glutamate (Glu) efflux (Kuroki et al., 1999; Meltzer and Huang, 2008; Huang et al., 2014), as well as neurotrophin, e.g., brain-derived neurotrophic factor, efflux (Angelucci et al., 2004; Leggio et al., 2013) at multiple sites. The combined effects of these actions may be the basis for their ability to treat psychosis, negative symptoms, cognitive dysfunction, and mood disturbances (Meltzer and Huang, 2008; Meltzer, 2015; Nucifora et al., 2017). The main actions of cariprazine are DA D3 and D2 receptor partial agonism, with approximately 10-fold greater binding affinity for the D3 receptor (pKi 10.07 and 9.16–9.31 for human D3 and D2L-S receptors, respectively) (Kiss et al., 2010).
Cariprazine is also a potent partial agonist at the 5-HT1A receptor (pKi 8.59 and 8.34 for human and rat 5-HT1A receptors, respectively) and an antagonist at the 5-HT2B receptor (pKi 9.24 for human 5-HT2B receptor) and 5-HT2A receptor with a moderate binding affinity (pKi 7.73 for human 5-HT2A receptor) (Kiss et al., 2010). 5-HT2A receptor antagonism may contribute to its low burden of extrapyramidal side effects and ability to improve cognition (Meltzer, 2015).
The pharmacologic action of cariprazine receiving the greatest attention is its potent partial agonism activity at the D3 receptor (Kiss et al., 2010; Tadori et al., 2011). D3 receptors are predominantly located in the nucleus accumbens (NAC), with lower expression in the thalamus, hippocampus (HIP), and cortex, areas important to the development of psychotic and negative symptoms and the cognitive impairment associated with schizophrenia (CIAS). They are both pre- and postsynaptical (Centonze et al., 2003; Sokoloff et al., 2013; Maramai et al., 2016). In preclinical studies, DA D3 receptor blockade enhanced while D3 receptor agonism impaired learning, memory, attention, speed of processing, social recognition, and executive function (Leggio et al., 2013, 2016; Zimnisky et al., 2013; Pich and Collo, 2015; Magnard et al., 2016; Maramai et al., 2016).
Indicative of its antipsychotic-like activity, cariprazine inhibited the locomotor-stimulating effects of the noncompetitive N-methyl-d-aspartate receptor (NMDAR) antagonists, including dizocilpine (MK-801, (5S,10R)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine) and phencyclidine (PCP) (Gyertyán et al., 2011). Cariprazine also ameliorated the deficits in animal models of CIAS (Rajagopal et al., 2014; Neill et al., 2016; Watson et al., 2016; Barnes et al., 2018). The ability of cariprazine to attenuate PCP-induced disruption in memory was significantly diminished in DA D3 receptor knockout mice (Zimnisky et al., 2013).
In microdialysis studies, AAPDs that are more potent 5-HT2A than DA D2 receptor antagonists, e.g., clozapine, olanzapine, risperidone, and lurasidone, increase prefrontal cortical (PFC) and HIP ACh and DA efflux and striatal (STR) DA efflux and have variable effects on Glu efflux (López-Gil et al., 2010; Huang et al., 2014). These AAPDs suppress MK-801- or PCP-induced Glu or 5-HT efflux (Gobert et al., 1996; López-Gil et al., 2007, 2010; Huang et al., 2014, 2015). DA D3 receptor full antagonists or partial agonists with low intrinsic activity would be expected to function as antagonists when synaptic DA concentrations are elevated. This effect has been suggested to enhance cognition via promoting PFC ACh release, enhancing the release of DA in the STR and PFC, or activating cAMP-response-element binding protein signaling in the HIP, whereas D3 receptor agonists would have the opposite effect (Nakajima et al., 2013; Huang et al., 2015). In support of this, we reported that the selective D3 receptor antagonist NGB-2904 (N-[4-[4-(2,3-Dichlorophenyl)-1-piperazinyl]butyl]-9H-fluorene-2-carboxamide) increased PFC DA and ACh and STR DA efflux (Huang et al., 2015). This study aimed to compare the effects of cariprazine and the selective DA D3 receptor antagonist SB-277011A [trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinolininecarboxamide hydrochloride] in the absence and presence of the DA D3 receptor–preferring agonist (+)-PD-128907 [(4aR, 10bR)-3,4a,4,10b-tetrahydro-4-propyl-2H,5H-[1]benzopyrano-[4,3-b]-1,4-oxazin-9-ol hydrochloride] on neurotransmitter efflux in the ventral HIP and NAC.
Materials and Methods
Animals and Drugs.
Male Sprague-Dawley rats (250–300 g; Harlan Laboratories, Indianapolis, IN) were used throughout the study. They were housed three to four per cage in a controlled 14:10-hour light:dark cycle with free access to food and water. Cariprazine (provided by Allergan, Madison, NJ) was dissolved in 0.5% methylcellulose and 0.2% Tween 80 solution and was administered by oral administration (PO), whereas (+)-PD-128907 and SB-277011A (Tocris, Ellisville, MO) were prepared in aqueous solution and administered intraperitoneally (IP). The vehicle (data for vehicle in Fig. 1 and vehicle+vehicle in Figs. 2 and 3 are from the same group of animals: vehicle for (+)-PD-128907 i.p. + vehicle for cariprazine PO) or drugs were administered in a volume of 1.0 ml/kg to randomly assigned rats (N = 8 per group).
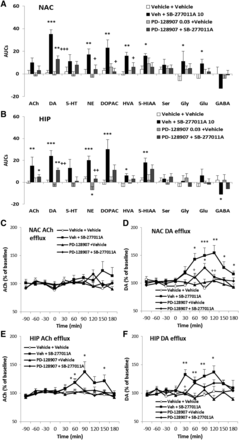
Effects of cariprazine on the extracellular levels of neurotransmitters and metabolites in the rat NAC and HIP. (A and B) Area under the curve values. N = 8 per group. Cariprazine was administered at the 0-minute time point. *P < 0.05; **P < 0.01; ***P < 0.001 vs. vehicle; one-way ANOVA least significant difference. Cariprazine 0.1 mg/kg, but not 0.03 mg/kg (PO), significantly increased DA [P = 0.001 (C)], NE (P = 0.040), 5-HT [P = 0.035 (D)], and Gly (P = 0.021) efflux in the NAC; cariprazine also increased DA [P = 0.001 (E)], 5-HT [P = 0.006 (F)], DOPAC (P = 0.027), and HVA (P = 0.025) efflux in the HIP. Cariprazine 0.3 mg/kg increased DA [P < 0.001 (C)], NE (P = 0.003), Gly (P = 0.026), and Glu (P = 0.021) efflux in the NAC. It also increased HIP DA [P = 0.004 (E)] and NE (P = 0.025) as well as DOPAC (P = 0.002) efflux. Cariprazine had no effect on ACh, Ser, or GABA efflux in either region. AUC, area under the curve.
Effect of SB-277011A and (+)-PD-128907 on neurotransmitter levels in the rat NAC and HIP. (A and B) Area under the curve values. N = 8 per group. SB-277011A was administered at the 0-minute time point, and (+)-PD-128907 was administered at the −30-minute time point. *P < 0.05; **P < 0.01; ***P < 0.001 vs. vehicle; +P < 0.05; ++P < 0.01; +++P < 0.001 vs. SB-277011A in one-way ANOVA least significant difference. The DA D3 receptor antagonist SB-277011A (10 mg/kg, i.p.) significantly increased DA [P < 0.001 (D)], NE (P = 0.003), DOPAC (P = 0.003), HVA (P = 0.001), 5-HIAA (P = 0.018), Gly (P = 0.039), and Glu (P = 0.016), but not ACh (C) efflux in the NAC (A), as well as ACh [P = 0.007 (E)], DA [P < 0.001 (F)], NE (P < 0.001), DOPAC (P < 0.001), HVA (P = 0.026), and 5-HIAA (P = 0.005) efflux in the HIP (B). The DA D3 receptor agonist (+)-PD-128907 (0.03 mg/kg, i.p.) slightly decreased HIP NE (P = 0.022) efflux, with no effect on other neurotransmitters. (+)-PD-128907 partially, but significantly, blocked the SB-277011A–induced increase on DA efflux in the NAC [P < 0.001 (A)] and HIP [P = 0.004 (B)] and NE efflux in the NAC [P = 0.022 (A)] and HIP [P = 0.001 (B)]. AUC, area under the curve.
Effect of cariprazine and (+)-PD-128907 on neurotransmitter levels in the rat NAC and HIP. (A and B) Area under the curve values. N = 8 per group, except N = 7 for (+)-PD-128907+cariprazine group. Cariprazine was administered at the 0-minute time point, and (+)-PD-128907 was administered at the −30-minute time point. *P < 0.05; **P < 0.01 vs. vehicle; +P < 0.05; ++P < 0.01 vs. cariprazine in one-way ANOVA least significant difference. (+)-PD-128907 partially but significantly blocked the effects of cariprazine (0.1 mg/kg) on DA efflux (P = 0.033) in the NAC (A) and NE efflux (P = 0.009) in the HIP (B). AUC, area under the curve. Time response curves for DA and 5-HT in NAC and HIP are given in C, D, and E, F, respectively.
Surgery and Microdialysis.
The rats were anesthetized with 2% isoflurane (Isothesia; Butler Schein, Dublin, OH). A stainless-steel guide cannula (21 G) with dummy probes was placed and fixed by cranioplastic cement to the NAC and HIP. Relative to the bregma, the stereotaxic coordinate of the implanted probe was A +2.0, L −1.4, V –8.0 mm for the NAC, and A −5.6, L + 5.0, V –7.5 mm for the HIP with an incision bar level of –3.0 mm, according to the atlas (Paxinos and Watson, 1998).
Approximately 2 to 3 days after cannulation, anesthetized animals were implanted with concentric-shaped dual dialysis probes having 2.0 mm of membrane surface (Synaptech Co., Marquette, MI). Rats were then housed individually in dialysis cages with overnight perfusion (0.2 μl/min) of the probes with Dulbecco’s phosphate-buffered saline (Sigma-Aldrich, St. Louis, MO), including Ca2+ (138 mM NaCl, 8.1 mM Na2HPO4, 2.7 mM KCl, 1.5 mM KH2PO4, 0.5 mM MgCl2, 1.2 mM CaCl2, pH 7.4). In the morning on the day of microdialysis, the perfusion rate was increased to 1.2 μl/min, and samples were collected every 30 minutes after a 1-hour stabilization period to achieve stable baseline values. The rats were then administered vehicle, cariprazine (0.03, 0.1, 0.3 mg/kg, PO), or SB-277011A (10 mg/kg, IP.), and/or (+)-PD-128907 (0.03 mg/kg, IP). The doses of SB-277011A and (+)-PD-128907 were selected based on previous reports (Zapata et al., 2001; Lacroix et al., 2006; Huang et al., 2015). A 30-minute interval was maintained for the coadministration. The effects of the drug(s) on extracellular neurotransmitter and metabolite levels were monitored for an additional 180 minutes after the second injection. Extracellular neurotransmitter levels of ACh, DA, 5-HT, NE, metabolites of DA [namely, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA)], metabolite of 5-HT [namely, 5-hydroxyindole acetic acid (5-HIAA)], glycine (Gly), serine (Ser), Glu, and γ-aminobutyric acid (GABA) were measured by ultraperformance liquid chromatography with tandem mass spectrometry (Huang et al., 2014). The procedures applied in this experiment were approved by the Institutional Animal Care and Use Committee of Northwestern University (Chicago, IL).
Data Analysis.
Only results derived from healthy rats with correctly positioned dialysis probes were used in the data analysis. One rat in the (+)-PD-128907+cariprazine group was eliminated. Baseline levels, measured for 90 minutes prior to any drug administration, were averaged and normalized to 100%. Effects of the drugs on extracellular neurotransmitter and metabolite levels are expressed relative to baseline in time-response curves. Area under the curve from 0 to 180 minutes was calculated and analyzed by ANOVA followed by Fisher’s least significant difference post hoc tests. Two-way ANOVA was used for the pretreatment and treatment interaction on neurotransmitter efflux. Repeated-measure ANOVA was used for the time-point difference in the time-response curve. Results are presented as the mean ± S.E., and P < 0.05 was considered significant.
Results
Table 1 summarizes the mean basal neurotransmitter levels in study rats (N = 63). There were no significant differences among the treatment groups. N = 8 per group, with the exception of N = 7 for the (+)-PD-128907+cariprazine group.
Basal levels of neurotransmitters in dialysates (mean ± S.E.; N = 63)
Cariprazine enhanced DA, 5-HT, and NE efflux in both the NAC (Fig. 1A) and HIP (Fig. 1B). Cariprazine 0.1 mg/kg, but not 0.03 mg/kg (PO), significantly increased DA (P = 0.001; Fig. 1C), NE (P = 0.040), 5-HT (P = 0.035; Fig. 1D), and Gly (P = 0.021) efflux in the NAC. Cariprazine also increased DA (P = 0.001; Fig. 1E), 5-HT (P = 0.006; Fig. 1F), DOPAC (P = 0.027), and HVA (P = 0.025) efflux in the HIP. Cariprazine 0.3 mg/kg increased DA (P < 0.001; Fig. 1C), NE (P = 0.003), Gly (P = 0.026), and Glu (P = 0.021) efflux in the NAC, and HIP DA (P = 0.004; Fig. 1E) and NE (P = 0.025), as well as DOPAC (P = 0.002) efflux. Cariprazine had no effect on ACh, Ser, or GABA efflux in either region.
The DA D3 receptor antagonist SB-277011A (10 mg/kg, i.p.) significantly increased DA (P < 0.001; Fig. 2D), NE (P = 0.003), DOPAC (P = 0.003), HVA (P = 0.001), 5-HIAA (P = 0.018), Gly (P = 0.039), and Glu (P = 0.016) efflux in the NAC (Fig. 2A), as well as ACh (P = 0.007; Fig. 2E), DA (P < 0.001; Fig. 2F), NE (P < 0.001), DOPAC (P < 0.001), HVA (P = 0.026), and 5-HIAA (P = 0.005) efflux in the HIP (Fig. 2B).
The DA D3 receptor–preferring agonist (+)-PD-128907 (0.03 mg/kg, i.p.) slightly decreased HIP NE (P = 0.022) efflux, with no effect on other neurotransmitters. (+)-PD-128907 partially, but significantly, attenuated the SB-277011A–induced increase in DA efflux in the NAC (P < 0.001; Fig. 2A) and HIP (P = 0.004; Fig. 2B) and NE efflux in the NAC (P = 0.022; Fig. 2A) and HIP (P = 0.001; Fig. 2B). Two-way ANOVA demonstrated significant interactions between (+)-PD-128907 and SB-277011A in NAC DA (P = 0.003), DOPAC (P = 0.011), and 5-HIAA (P = 0.037) efflux and HIP DOPAC efflux (P = 0.050). Based on these studies with selective ligands, it was observed that DA D3 receptor antagonism stimulated both NAC and HIP DA and NE, but not 5-HT, efflux. (+)-PD-128907 partially but significantly attenuated the effects of cariprazine (0.1 mg/kg) on DA efflux (P = 0.033) in the NAC (Fig. 3A) and NE efflux (P = 0.009) in the HIP (Fig. 3B), consistent with the conclusion that the actions of cariprazine on DA and NE efflux were due, at least in part, to its D3 receptor partial agonist activity. Table 2 summarizes the results for the neurotransmitter efflux in the NAC and HIP.
Summary of the effects of the tested compounds on neurotransmitter efflux
Discussion
The major finding of this study is that cariprazine increased DA, NE, 5-HT, Glu, and Gly efflux in the rat NAC or HIP, or both, as did the DA D3 receptor antagonist SB-277011A. These increases were attenuated by the D3 receptor–preferring agonist (+)-PD-128907, providing further support that the antipsychotic and antidepressant effects of cariprazine, which may be based on its action to modulate dopaminergic, noradrenergic, serotonergic, and glutamatergic activity, are significantly dependent on DA D3 receptor blockade. No effects on ACh, Ser, or GABA efflux were detected after cariprazine, SB-277011A, or (+)-PD-128907.
The present data showed that cariprazine, similar to other AAPDs, increased NAC and HIP DA efflux (Meltzer and Huang, 2008; Huang et al., 2014). Cariprazine enhanced the turnover of DA and slightly reduced 5-HT turnover in the mouse STR and frontal cortex after oral administration (Kiss et al., 2010). Acute oral (0.05, 0.2, or 0.8 mg/kg) treatment with cariprazine dose-dependently attenuated the PCP-induced Glu, DA, NE, and 5-HT efflux in the medial PFC (mPFC), whereas cariprazine by itself (only 0.2 mg/kg tested) had no effect on efflux of any of these neurotransmitters (Kehr et al., 2018). Other AAPDs normalize the activity of principal cortical neurons following their activation by the NMDAR antagonists PCP and MK-801, which enhance the activity of parvalbumin-positive GABAergic interneurons in the mPFC. The AAPDs prevent excessive activation of these neurons (Amargós-Bosch et al., 2006; López-Gil et al., 2007, 2010; Carli et al., 2011).
Aripiprazole, a widely used AAPD, similar to cariprazine, is also a DA D2/D3 receptor partial agonist, which increases DA efflux in the HIP but not the NAC (Li et al., 2004). However, cariprazine has much greater affinity and selectivity for D3 versus D2 receptors compared with other AAPDs, including aripiprazole (3- to 10-fold greater D3 vs. D2 selectivity) (Kiss et al., 2010). Further, at antipsychotic-like effective doses (around 0.1–1 mg/kg, orally in rats), cariprazine occupies both DA D3 and D2 receptors, whereas aripiprazole occupies D2 but not D3 receptors to a significant level (Gyertyán et al., 2011). The DA D3 receptor–preferring agonist (+)-PD-128907 attenuated the effect of the D3 receptor antagonist SB-277011A on rat HIP ACh efflux. We previously reported that the D3 receptor antagonist NGB-2904 increased DA and ACh efflux in mouse mPFC and dorsal STR (Huang et al., 2015). SB-277011A and U99194 (2,3-Dihydro-5,6-dimethoxy-N, N-dipropyl-1H-inden-2-amine), both D3 receptor antagonists, increase rat cortical ACh efflux (Lacroix et al., 2006; Barth et al., 2013). On the other hand, (+)-PD-128907 has been reported to decrease DA efflux in the NAC and PFC (Pugsley et al., 1995; Gobert et al., 1996; Zapata and Shippenberg, 2005). Typical antipsychotic drugs, including S-(−)-sulpiride and haloperidol, which are D2 receptor antagonists, increase DA efflux in the NAC (Kuroki et al., 1999; Huang et al., 2014). Selective DA D3 receptor antagonists, such as SB-277011A studied here, also increased DA efflux in the NAC as well as the HIP. Therefore, the D2 as well as the D3 receptor actions of cariprazine may contribute to increased DA efflux in the regions studied (Kuroki et al., 1999; Huang et al., 2014). However, at the dose (0.03 mg/kg, i.p.) in the present study, (+)-PD-128907 is more likely to be selective for D3 compared with D2 receptors. This dose of (+)-PD-128907 was reported to slightly decrease (about 20%) NAC DA release in wild-type but not D3 receptor knockout mice (Pugsley et al., 1995; Zapata et al., 2001). Moreover, the effect in rats of (+)-PD-128907, at a dose of 0.2 mg/kg (s.c.), on prepulse inhibition was blocked by the selective D3 receptor antagonists SB-277011 and A-691990 (2-tert-butyl-4-{4-[3-(4,5-dimethyl-4H-[1,2,4]triazol-3-ylsulfanyl)-propyl]-piperazin-1-yl}-6-trifluoromethyl-pyrimidine), but not by the D2 receptor antagonist haloperidol (Zhang et al., 2007). AAPDs, including olanzapine and lurasidone, which are more potent 5-HT2A receptor antagonists than D2 receptor antagonists, also increased cortical ACh efflux in mice (Huang et al., 2014). Blonanserin is another potent D2 and D3 receptor antagonist AAPD (Baba et al., 2015). Neither blonanserin in mice nor cariprazine in rats enhanced HIP ACh efflux (Huang et al., 2015; and present data), which may result from their limited efficacy as direct or indirect 5-HT1A receptor partial agonists (Ichikawa et al., 2002; Huang et al., 2014). However, inability to enhance ACh efflux in the HIP did not preclude the efficacy of cariprazine to restore novel object recognition (NOR) and other cognitive deficits in rodents treated with subchronic PCP or MK-801 (Neill et al., 2016; Watson et al., 2016).
The effects of 0.1 and 0.3 mg/kg cariprazine on neurotransmitter efflux in the HIP did not significantly differ from each other, although the higher dose would be expected to occupy a significantly higher proportion of HIP DA D2 receptors. Bilateral microinjection of the D3 receptor antagonist S33084 into the rat PFC caused a dose-related improvement in NOR; on the other hand, bilateral microinjection of the D2 receptor antagonist L741626 caused a dose-related impairment of NOR, suggesting that blockade of D3 receptors enhances NOR, whereas antagonism of the D2 receptor impairs cognition (Watson et al., 2012).
Extensive evidence supports the role of glutamatergic and GABAergic neurotransmission in the development of CIAS. Cariprazine, 0.3 but not 0.1 mg/kg, slightly but significantly increased Glu efflux in the NAC. Because we only showed the effect of (+)-PD-128907 pretreatment with 0.1 mg/kg cariprazine, we were unable to determine if the Glu efflux could be attenuated by (+)-PD-128907. However, the D3 receptor antagonist SB-277011A significantly increased Glu efflux in the NAC, and this effect was significantly attenuated by (+)-PD-128907. It seems likely that the increase in Glu in the NAC induced by cariprazine and SB-277011A resulted from functional antagonist activity at the DA D3 receptor. However, S33138, a preferential D3 versus D2 receptor antagonist, failed to affect cortical Glu, Gly, or GABA efflux (Millan et al., 2008). The small effects of cariprazine and SB-277011A on NAC Glu efflux contrast with those of lurasidone, which produces a greater increase in the NAC and mPFC (Huang et al., 2014). Other AAPDs have shown variable effects on Glu efflux (López-Gil et al., 2007, 2010; Carli et al., 2011; Huang et al., 2014). Moreover, selective D3 receptor partial agonists and antagonists have been reported to prevent the locomotor-stimulating effects of MK-801, suggesting that the antipsychotic-like effect of DA D3 receptor blockers might result from normalizing Glu function by modulating the release of Glu (Leriche et al., 2003; Sokoloff et al., 2013). The D3/D2 receptor agonist quinelorane was reported to selectively decrease dialysate GABA levels in NAC core (Hemmati et al., 2001). Activation of DA D3 receptors can modulate GABAA receptor endocytosis and suppress synaptic GABAergic transmission in the HIP and NAC (Chen et al., 2006; Swant et al., 2008; Kohnomi and Konishi, 2015). We noted no effect of (+)-PD-128907 on GABA efflux in either the NAC or HIP. Further study is indicated to clarify the effect of cariprazine on synaptic GABAergic transmission, as there is evidence that decreased GABAergic activity may contribute to the development of CIAS (Tse et al., 2015).
An interesting finding of the present study was augmented Gly efflux in the NAC in response to cariprazine 0.1 and 0.3 mg/kg and SB-277011A 10 mg/kg. (+)-PD-128907 alone was without effect on Gly. The increases in Gly efflux following cariprazine and SB-277011A, in the NAC, were reversed by (+)-PD-128907, indicating the elevation resulted from D3 receptor inhibition. Gly or D-serine are necessary coagonists with Glu to activate the NMDAR (Benveniste and Mayer, 1991). Activation of NMDAR in the NAC contributes to the induction of long-term potentiation (Schotanus and Chergui, 2008), which may be the basis for some of the beneficial effects of cariprazine in both psychosis and mood disorders. There is evidence that additional Gly in the NAC may be needed for optimization of rewarded behaviors (Saul’skaya and Solov’eva 2005). Administration of oral Gly as an adjunct to antipsychotic drug treatment may be effective to improve negative symptoms (Coyle and Tsai, 2004; Heresco-Levy et al., 2004). Further study of the importance of Gly release to the action of cariprazine is indicated.
In summary, the effects of the DA D3 receptor–preferring partial agonist cariprazine parallel those produced by the selective D3 receptor antagonist SB-277011A with regard to DA and NE efflux in the NAC and HIP. Further, significant smaller increases by both cariprazine and SB-277011A in Gly and Glu efflux were found in the NAC. These effects of cariprazine and SB-277011A were partially or fully reversed by the D3 receptor–preferring agonist (+)-PD-128907, tested at a dose that predominantly impacts D3 receptors. These data indicate that the D3 receptor mechanism contributes to the action of cariprazine in modulating the release of key neurotransmitters in the NAC and HIP, which are implicated in cognitive and reward functions. The increased DA, NE, Gly, and Glu efflux may be mediated by disinhibition of GABA interneurons produced by DA D3 receptor blockade (Diaz et al., 2011). These findings support the evidence that modulatory actions of cariprazine on monoaminergic transmission mediated by D3 receptor antagonism may contribute to its procognitive, antipsychotic, and antidepressive effects.
Acknowledgments
We thank Jiarui Zhu (college student, Department of Environmental Science, Policy, and Management, University of California, Berkeley, intern at Northwestern University during the summer of 2017) for help on animal and drug preparation and sample collection.
Authorship Contributions
Participated in research design: Meltzer, Huang, Adham, Kiss, Farkas.
Conducted experiments: Huang, He.
Performed data analysis: Huang, He.
Wrote or contributed to the writing of the manuscript: Huang, He, Kiss, Farkas, Adham, Meltzer.
Footnotes
- Received May 14, 2019.
- Accepted August 5, 2019.
This study was supported by grants from Allergan, Plc. and Gedeon Richter, Plc.
H.Y.M. has been consulting for and receives research funding from Allergan. B.K. and B.F. are employees of Gedeon Richter Plc. N.A. is an employee of Allergan. M.H. and W.H. have no conflicts of interest to declare.
This work was previously presented as follows: Meltzer HY, Huang M, He W, Kiss B, Farkas B, Adham N. Cariprazine enhances monoaminergic activity in the hippocampus and ventral striatum of rats: a possible basis for its antipsychotic effect. Society of Biological Psychiatry 73rd Annual Meeting; May 10–12, 2018; Hilton Midtown, New York, NY.
Abbreviations
- AAPD
- atypical antipsychotic drug
- ACh
- acetylcholine
- CIAS
- cognitive impairment associated with schizophrenia
- DA
- dopamine
- DOPAC
- 3,4-dihydroxyphenylacetic acid
- GABA
- γ-aminobutyric acid
- Glu
- glutamate
- Gly
- glycine
- 5-HIAA
- 5-hydroxyindole acetic acid
- HIP
- hippocampus
- 5-HT
- serotonin
- HVA
- homovanillic acid
- MK-801
- dizocilpine
- mPFC
- medial prefrontal cortex
- NAC
- nucleus accumbens
- NE
- norepinephrine
- NMDAR
- N-methyl-d-aspartate receptor
- NOR
- novel object recognition
- PCP
- phencyclidine; (+)-PD-128907, (4aR, 10bR)-3,4a,4,10b-tetrahydro-4-propyl-2H,5H-[1]benzopyrano-[4,3-b]-1,4-oxazin-9-ol hydrochloride
- PFC
- prefrontal cortex
- PO
- oral administration
- SB-277011A
- trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinolininecarboxamide hydrochloride
- Ser
- serine
- STR
- striatum
- Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics