Visual Overview
Abstract
The upregulation of kidney injury molecule-1 (KIM-1) has been extensively studied in various renal diseases and following acute injury; however, the initial mechanisms controlling KIM-1 expression remain limited. In this study, KIM-1 expression was examined in mouse renal cell cultures and in two different models of acute kidney injury (AKI), ischemia reperfusion (IR)–induced and lipopolysaccharide (LPS)-induced sepsis. KIM-1 mRNA increased in both AKI models, and pharmacological inhibition of extracellular signal–regulated kinase 1/2 (ERK1/2) signaling attenuated injury-induced KIM-1 expression in the renal cortex. Toll-like receptor 4 knockout (TLR4KO) mice exhibited reduced ERK1/2 phosphorylation and attenuated KIM-1 mRNA after LPS exposure. TLR4KO mice were not protected from IR-induced ERK1/2 phosphorylation and upregulation of KIM-1 mRNA. Following renal IR injury, phosphorylation of signal transducer and activator of transcription 3 (STAT3) at serine 727 and tyrosine 705 increased downstream from ERK1/2 activation. Because phosphorylated STAT3 is a transcriptional upregulator of KIM-1 and inhibition of ERK1/2 attenuated increases in STAT3 phosphorylation, we suggest an ERK1/2-STAT3-KIM-1 pathway following renal injury. Finally, ERK1/2 inhibition in naive mice decreased KIM-1 mRNA and nuclear STAT3 phosphorylation in the cortex, indicating homeostatic regulation of KIM-1. These findings reveal renal ERK1/2 as an important initial regulator of KIM-1 expression in IR and septic AKI and at a physiologic level.
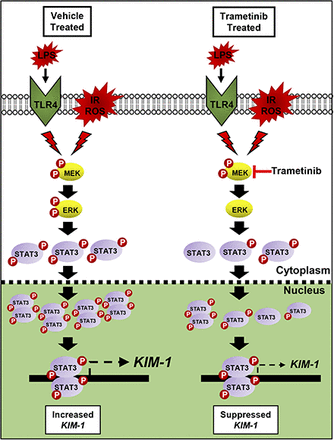
Visual Abstract.Proposed mechanism of IR, LPS, and ROS-induced renal damage that initiates ERK1/2 and STAT3 phosphorylation. STAT3 then binds to the KIM-1 promoter and increases KIM-1 mRNA. By preventing ERK1/2 phosphorylation following renal injury, STAT3 phosphorylation is decreased, leading to less phosphorylated STAT3 within the nucleus, and subsequently less KIM-1 mRNA increases post injury.
Footnotes
- Received July 21, 2017.
- Accepted September 27, 2017.
↵1 Current affiliation: University of Arizona, Tucson, Arizona.
This study was funded by National Institutes of Health National Institute of General Medical Sciences [Grant R01GM084147] award to R.G.S.; the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs [Grant BX000851] awarded to R.G.S.; and the Ruth L. Kirschstein National Research Service Award Individual Predoctoral Fellowship through the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant F31DK105782] awarded to J.B.C.
- U.S. Government work not protected by U.S. copyright
JPET articles become freely available 12 months after publication, and remain freely available for 5 years.Non-open access articles that fall outside this five year window are available only to institutional subscribers and current ASPET members, or through the article purchase feature at the bottom of the page.
|