Abstract
Etomidate is a potent and rapidly acting anesthetic with high therapeutic index (TI) and superior hemodynamic stability. However, side effect of suppressing adrenocortical function limits its clinical use. To overcome this side effect, we designed a novel etomidate analog, EL-0052, aiming to retain beneficial properties of etomidate and avoid its disadvantage of suppressing adrenocortical steroid synthesis. Results exhibited that EL-0052 enhanced GABAA receptors currents with a concentration for EC50 of 0.98 ± 0.02 μM, which was about three times more potent than etomidate (3.07 ± 1.67 μM). Similar to hypnotic potency of etomidate, EL-0052 exhibited loss of righting reflex with ED50s of 1.02 (0.93–1.20) mg/kg in rats and 0.5 (0.45–0.56) mg/kg in dogs. The TI of EL-0052 in rats was 28, which was higher than 22 of etomidate. There was no significant difference in hypnotic onset time, recovery time, and walking time between EL-0052 and etomidate in rats. Both of them had minor effects on mean arterial pressure in dogs. EL-0052 had no significant effect on adrenocortical function in dogs even at a high dose (4.3 × ED50), whereas etomidate significantly inhibited corticosteroid secretion. The inhibition of cortisol synthesis assay showed that EL-0052 had a weak inhibition on cortisol biosynthesis in human H259 cells with an IC50 of 1050 ± 100 nM, which was 2.09 ± 0.27 nM for etomidate. EL-0052 retains the favorable properties of etomidate, including potent hypnotic effect, rapid onset and recovery, stable hemodynamics, and high therapeutic index without suppression of adrenocortical function.
SIGNIFICANCE STATEMENT The novel etomidate analog EL-0052 retains the favorable properties of etomidate without suppressing adrenocortical function and provides a new strategy to optimize the structure of etomidate.
Introduction
As a potent and rapidly acting anesthetic, etomidate displays many favorable clinical properties, such as high therapeutic index and superior hemodynamic stability. However, the inhibitory effect of etomidate on adrenocortical increases the morbidity and mortality of patients, especially for those undergoing intensive care or receiving continuous infusion (Albert et al., 2011; Forman, 2011; Chan et al., 2012; Komatsu et al., 2013), which limits its clinical applications. Therefore, it is necessary to chemically modify etomidate to maintain its advantages and overcome its shortcomings.
Previous structure-activity relationship studies on etomidate have indicated that imidazole ring and ester moiety are the main groups that inhibit the biosynthesis of adrenocortical steroid (Ouellet et al., 2008; Atucha et al., 2009; Gay et al., 2009; Sneyd, 2012; Pejo et al., 2016). Currently, replacing the nitrogen atom in the imidazole ring with carbon atoms or modifying the ester group are the most commonly used strategies to reduce corticosteroid toxicity of etomidate. The corresponding chemical entities that have been successfully discovered include carboetomidate, methoxycarbonyl etomidate, etc. (Cotten et al., 2009, 2010; Pejo et al., 2012; Campagna et al., 2014; Wang et al., 2017). These etomidate analogs can significantly reduce the activity of adrenocortical suppression, but most of them have a lower hypnotic potency than etomidate, which may increase the safety risk and bring some difficulties in formulation (Cotten et al., 2009, 2010; Pejo et al., 2012; Sneyd, 2012; Campagna et al., 2014; Wang et al., 2017).
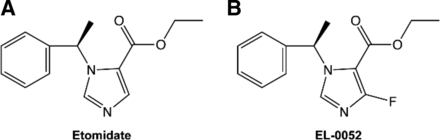
It has been reported that many imidazole-containing medications (e.g., cimetidine, ketoconazole) inhibit the specific isozymes of cytochrome P450 by binding to the heme iron atom and blocking oxygen binding (Seward et al., 2006; Ouellet et al., 2008). 11-β hydroxylase (CYP11B1), a member of the cytochrome P450 superfamily, is a key enzyme for the synthesis of corticosteroids. Studies have shown that the basic nitrogen on the imidazole ring of etomidate can form a coordination bond with heme iron in the active center of 11-β hydroxylase (Roumen et al., 2007), thus inhibiting the bioactivity of 11-β hydroxylase and ultimately leading to a decrease in corticosteroid synthesis (Pejo et al., 2016). It has been shown that reducing the coordination of nitrogen with heme iron can reduce etomidate’s inhibitory effect on adrenocortical function (Cotten et al., 2010). Based on these findings, we hypothesized that replacing the hydrogen atom beside the imidazole nitrogen with a highly electronegative group could reduce the electron cloud density of nitrogen in the imidazole ring, thereby weakening the coordination of nitrogen with heme iron and ultimately reducing etomidate’s inhibitory effect on 11-β hydroxylase. In addition, such modification might retain hypnotic effects due to the preservation of imidazole nitrogen and ester moiety. To test this hypothesis, we synthesized a series of compounds and screened out a lead compound ethyl R-(+)-4-fluoro-1-(1-phenylethyl)-1H- imidazole-5-carboxylate (EL-0052) (Fig. 1B). Furthermore, we examined the hypnotic effect, hemodynamic stability, and adrenocortical toxicity of EL-0052 in comparison with etomidate.
Chemical structure of etomidate (A) and EL-0052 (B).
Materials and Methods
Materials
Etomidate and propofol were provided by Jiangsu Nhwa Pharmaceutical Corporation Ltd. (Xuzhou, China). EL-0052 was provided by Jiangsu Nhwa-Luokang Pharma R&D Ltd. (Chongqing, China). The information about synthesis and purity methods of EL-0052 was described in the patent US10392352, and the data can be freely accessed. Hypnotics were dispensed in 20% medium and long-chain fat-emulsion injection (80KG045, Huarui Pharmaceutical Company Ltd.) for in vivo tests and dissolved in 0.5% DMSO for in vitro tests. The chemical structures of etomidate and EL-0052 are shown in Fig. 1.
Animals
Wistar rats (280–330 g) were purchased from Shanghai SIPPR-BK Laboratory Animal Co., Ltd. Beagle dogs were purchased from Department of Laboratory Animal Science, Shanghai Jiao Tong School of Medicine. All animal experiments were conducted in accordance with Guide for Care and Use of Laboratory Animals (8th edition) and approved by Institutional Animal Care and Use Committee of Jiangsu Nhwa Pharmaceutical Corporation Ltd. (Xuzhou, China).
GABAA Receptor Electrophysiology
HEK293 cells stably expressing human GABAA receptors (α1β2γ2) were provided by ICE Bioscience Co. Ltd (Beijing, China). A total of 5 × 103 HEK293 cells were planted on a piece of cover glass and incubated in buffer solution (140 mM NaCl, 5 mM CsCl, 2 mM CaCl2,1 mM MgCl2, 5 mM HEPES, 10 mM glucose, pH = 7.4) for 18 hours before electrophysiologic experiments. Whole-cell patch-clamp technique was used to record the changes in average whole-cell currents. Etomidate and EL-0052 were dissolved in DMSO as stock solutions. The final working solutions contained 2 μM GABA and etomidate or EL-0052 of 0.01, 0.1, 1, 10, and 100 μM. GABA current activated by 2 μM GABA was stably recorded for 30 seconds before a cell was continuously perfused with the working solutions containing 2 μM GABA and tested drugs in increasing concentrations. The washout interval was 2 minutes. Then 2 μM GABA was given again to test the reversibility of GABAA receptors after the perfusion of 100 μM drug solution. For each concentration of tested drugs, at least three independent assays were performed. The peak current amplitudes were normalized to control currents elicited by 2 μM GABA. EC50s of etomidate and EL-0052 were calculated using GraphPad Prime 5.0.
Determination of ED50 for Loss of Righting Reflex and Median Lethal Dose
The Dixon’s up-and-down sequential allocation method was used to determine the ED50 for loss of righting reflex (LORR) and median lethal dose (LD50) of EL-0052, etomidate, or propofol (Dixon, 1991).
The ED50 test for LORR was performed in male rats and male dogs. We first conducted a preliminary test to find out the approximate dose range of ED50 and LD50 and then set a series of doses with an interval of 1.25 times between each dose group for the formal test. In the formal test, the desired doses of EL-0052 (0.82, 1.02, and 1.28 mg/kg for rats and 0.45, 0.56, and 0.7 mg/kg for dogs), etomidate (0.66, 0.82, and 1.02 mg/kg for rats and 0.30, 0.38, 0.47, and 0.59 mg/kg for dogs), and propofol (4, 5, and 6.25 mg/kg for rats and 1.94, 2.43, and 3.04 mg/kg for dogs) were injected intravenously within 10 seconds (rats) or 30 seconds (dogs) and followed by a normal saline flush. The volume of saline flush was 1 ml, and it was injected within 10 seconds. After injection, animals were placed with their back on a table (for rats) or laid on their sides (for dogs). An animal was judged to have LORR if it lost its ability to right itself (onto all four paws) immediately.
In the LD50 test, male rats were injected intravenously with a series of doses of EL-0052 (25.6, 32, and 40 mg/kg) or etomidate (16.4 and 20.5 mg/kg) through tail vein within 10 seconds. The death rate of rats was observed in 30 minutes after injection.
Duration of LORR in Rats
Male Wistar rats weighing 280–330 g were first placed in plastic restrainers before intravenously injected with 20% medium and long-chain fat-emulsion injection (as a negative control), EL-0052 (2.04 mg/kg), or etomidate (1.64 mg/kg) at 2 × ED50 doses, and the duration of injection was 10 seconds. Animals were removed from restraint devices immediately after injection and turned gently on their backs to assess the duration of LORR. A stopwatch was used to record the time from the injection to recovery. The onset time was defined as the time from injection to LORR. The recovery time was defined as the time when animals regained the ability to right themselves after LORR. The walking time was defined as the time when any hind limb of animals took the first step after the recovery of righting reflex.
To avoid subjective differences, all behavioral experiments (ED50, LD50, and LORR tests) were conducted blindly. Behavioral observations and recording were performed by a specially trained individual who was blinded to different treatments.
Mean Arterial Pressure Measurement in Beagle Dogs
Twenty-four beagle dogs with stable blood pressure were screened out by noninvasive blood pressure monitor (BP-2010E, Softron, Japan), and randomly divided into six groups: vehicle, propofol (6 mg/kg, 2.5×ED50), etomidate (1.15 mg/kg, 2.5 × ED50), etomidate (2 mg/kg, 4.3 × ED50), EL-0052 (1.26 mg/kg, 2.5 × ED50), and EL-0052 (2.17 mg/kg, 4.3 × ED50) groups, with two male dogs and two female dogs in each group. The doses of propofol (6 mg/kg) and etomidate (2 mg/kg) were set according to that of Campagna (Campagna et al., 2014). Mean arterial pressure (MAP) was measured by a tail-cuff method using noninvasive blood pressure monitor in conscious dogs. Each dog with a tail cuff was placed in casting harness and allowed to acclimate for at least 10 minutes. The desired doses of anesthetic agents or vehicle (20% medium and long-chain fat-emulsion injection, as a negative control) were injected via the forelimb cephalic vein in 30 seconds. The blood pressure was recorded every 1 minute for 5 minutes prior to drug administration and every 1 minute for 20 minutes thereafter. The MAP at each time point after drug administration was compared with the baseline (mean of MAP recorded 5 minutes before drug administration).
Suppression of Cortisol Synthesis in H259R Cells
The in vitro effect of EL-0052 on the cortisol synthesis was investigated using human adrenocortical cell line H259R (NCI-H295R, Cell Culture Center, Chinese Academy of Medical Science). H259R cells were suspended in growth medium (Dulbecco’s modified Eagle’s medium/F12 supplement with 1% insulin-transferrin-selenium, 1.25 mg/ml bovine serum albumin, 2.5% FBS, 15 mM HEPES, and 0.0053 mg/ml linoleic acid), and the cell density was adjusted to 105 cells/ml. The cell suspension was seeded in a 12-well plate with 2 ml in each well and cultured at 37°C under 5% CO2. The growth medium was replaced with maintenance medium (Dulbecco’s modified Eagle’s medium/F12 supplement with 1% insulin-transferrin-selenium and 20 µM Forskolin) when cell density reached about 80%. Each well was added with 1.98 ml of maintenance medium and 0.02 ml of etomidate (final concentrations: 0.032, 0.16, 0.8, 4, 20, and 100 nM) or EL-0052 (final concentrations: 3.2, 16, 80, 400, 2000, and 10,000 nM) with triple duplication for each concentration. After incubation at 37°C under 5% CO2 for 48 hours, 1.2 ml maintenance medium was collected and centrifuged at 1000 rpm for 5 minutes. The concentrations of cortisol in the supernatant were determined by ELISA kits (R&D SYSTEMS, USA). IC50 was calculated using GraphPad Prime 5.
Adrenocortical Suppression Test in Dogs
Suppression of adrenocortical synthesis in dogs was performed as previously reported (Cotten et al., 2010). Briefly, 18 beagle dogs were randomly divided into vehicle, etomidate (2 mg/kg, 4.3 × ED50), and EL-0052 (2.17 mg/kg, 4.3 × ED50) groups with three male and three female dogs in each group. Dexamethasone (0.1 mg/kg) was intravenously administered to each dog to suppress corticosterone production. Then 4 ml blood was sampled 2 hours later. Then vehicle (20% medium and long-chain fat-emulsion injection, as a negative control) or tested drug along with adrenocorticotropic hormone (ACTH1-24) (20 µg/kg, Sigma) was injected successively to stimulate corticosterone production. Another 4 ml blood was collected 15 minutes after ACTH injection. The concentrations of cortisol and corticosterone were quantified by ELISA kits (R&D SYSTEMS, USA).
Statistical Analysis
All data were expressed as the mean ± S.E.M. unless otherwise noted. The EC50s for GABAA receptor function assay and the IC50s for the H259R cell cortisol suppression assay were calculated using nonlinear regression analysis in GraphPad Prism 5 according to the following equation: Y = Bottom + (Top − Bottom)/(1 + 10^((LogEC50 − X) *Hillslope)). Results were reported as EC50s or IC50s and S.E.M. The ED50s for LORR test and LD50s for acute toxicity test were determined using the up-and-down procedure according to the OECD (Organization for Economic Cooperation and Development) test guidelines on acute oral toxicity under a computer-guided statistical program, AOT425statPgm, version 1.0. Results were expressed as ED50s or LD50s with 95% confidence intervals (CIs).
Data for behavioral and physiologic were compared using one-way or two-way ANOVA followed by a Tukey multiple comparison test. Data for serum corticosterone concentrations or MAP values were analyzed using two-way repeated measures ANOVA followed by a Bonferroni post-test. The statistical analysis was performed with SPSS 19.0. The value of P < 0.05 was considered to be statistically significant.
Results
Activation of GABAA Receptor Currents by EL-0052
It has been reported that etomidate exerts hypnotic effects by modulating GABAA receptors (Belelli et al., 2003). To test whether EL-0052 modulates GABAA receptors, the effects of EL-0052 on human α1β2γ2 GABAA receptors were evaluated. Both EL-0052 and etomidate enhanced the currents of GABAA receptors activated by 2 μM GABA in a concentration-dependent manner, and their EC50s were 0.98 ± 0.02 μM and 3.07 ± 1.67 μM, respectively (Fig. 2, A–C). The results indicated that EL-0052 was three times more potent than etomidate. However, the maximum effect of EL-0052 on enhancing the currents elicited by 2 μM GABA was about half that of etomidate.
Enhancement effects of EL-0052 and etomidate on human GABAA receptor current. (A) Representative traces showing enhancement of currents activated by 2 μM GABA by different concentrations of etomidate and EL-0052. Direct activation of GABAA receptors by etomidate (B) and EL-0052 (C). Current amplitudes were normalized to that activated by 2 μM GABA. The data of curves were fitted to Hill equation yielding an EC50s of 3.07 ± 1.67 μM for etomidate and 0.98 ± 0.02 μM for EL-0052, respectively. Each data point represents the mean ± S.E.M. from three independent experiments.
Hypnotic Properties of EL-0052 in Rats and Dogs
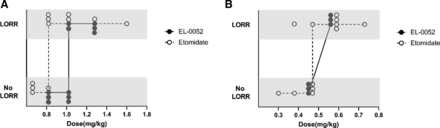
To reduce etomidate-induced corticosteroid suppression, a series of etomidate analogs were developed, but most of them reported compromised hypnotic properties (Cotten et al., 2009, 2010; Ge et al., 2012, 2014; Pejo et al., 2012; Sneyd, 2012; Wang et al., 2017). Therefore, tests were conducted to clarify whether EL-0052 could retain the hypnotic properties of etomidate in rats and dogs. The ED50s of EL-0052, etomidate, and propofol in rats were 1.02 mg/kg (95% CI: 0.93–1.20 mg/kg), 0.82 mg/kg (95% CI: 0.68–0.88 mg/kg), and 5.12 mg/kg (95% CI: 4.40–5.71 mg/kg), respectively (Fig. 3, A and B; Table 1). The ED50s of EL-0052, etomidate, and propofol in dogs were 0.50 mg/kg (95% CI: 0.45–0.56 mg/kg), 0.46 mg/kg (95% CI: 0.27–0.58 mg/kg), and 2.43 mg/kg (95% CI: 1.94–3.1 mg/kg), respectively (Fig. 3, A and B; Table 1). The hypnotic potency of EL-0052 was similar to that of etomidate in rats and dogs. The dose of 2 × ED50 was sufficient to produce LORR in rats, and there was no significant difference between EL-0052 and etomidate in hypnotic onset time (P = 0.558), recovery time (P = 0.082), and walking time (P = 0.801) (Fig. 4, A and B). These results demonstrated that EL-0052 retained etomidate’s favorable hypnotic properties of rapid onset and fast recovery after a single-dose administration.
EL-0052 and etomidate dose-response curves for LORR in rats (A) and dogs (B). Each data point represents the results from a single animal. The ED50s were determined using the up-and-down procedure according to OECD test guidelines on acute oral toxicity under a computer-guided statistical program, AOT425statPgm, version 1.0. The ED50s of EL-0052 and etomidate in rats were 1.02 mg/kg (95% CI: 0.93–1.20 mg/kg) and 0.82 mg/kg (95% CI: 0.68–0.88 mg/kg), respectively. The ED50s of EL-0052 and etomidate in dogs were 0.50 mg/kg (95% CI: 0.45–0.56 mg/kg) and 0.46 mg/kg (95% CI: 0.27–0.58 mg/kg), respectively.
The onset time (A), recovery time, and walking time (B) observed after intravenous administration of 2 × ED50 EL-0052 or etomidate in rats (n = 10). The doses of EL-0052 and etomidate were 2.04 mg/kg and 1.64 mg/kg, respectively. Under this equivalent dose, there was no significant difference in hypnotic efficacy between the two groups.
The ED50 for LORR, LD50, and therapeutic index of EL-0052 and etomidate in rats and dogs
To calculate the TI, we further performed the acute toxicity test to obtain the LD50 value of EL-0052 and etomidate in rats. The LD50s of EL-0052 and etomidate in rats were 29 mg/kg (95% CI: 26–32 mg/kg) and 18 mg/kg (95% CI: 16–21 mg/kg), respectively. Accordingly, the TI of EL-0052 was 28, which was higher than 22 of etomidate (Table 1).
Hemodynamic Actions of EL-0052 in Beagle Dogs
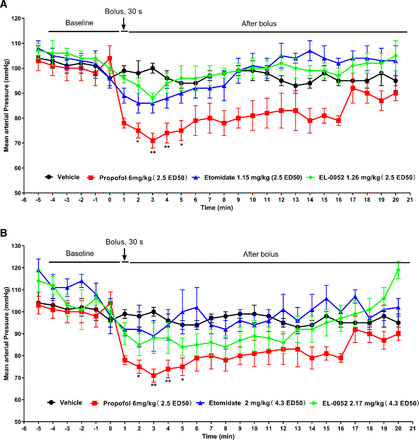
In clinical applications, one of the superior properties of etomidate over propofol is hemodynamic stability. To determine whether EL-0052 maintained the excellent properties of etomidate, the effects of EL-0052, etomidate, and propofol on hemodynamics were compared in beagle dogs. Propofol significantly reduced MAP in dogs. Etomidate and EL-0052 also produced a brief reduction in MAP, but there was no significant difference compared with the baseline (Fig. 5, A and B). At the dose of 2.5 × ED50, the maximum inhibition rates of propofol, etomidate, and EL-0052 on MAP were 30%, 17%, and 16%, respectively, indicating that the inhibitory effects of etomidate and EL-0052 on MAP were weaker than that of propofol at equivalent doses. At the high dose of 4.3 × ED50, the maximum inhibition rates of etomidate and EL-0052 on MAP were 21% and 22%, respectively, which were similar to that of propofol at the dose of 2.5 × ED50, but the duration of blood pressure inhibition of these two drugs was shorter than propofol.
Effects of etomidate, propofol and EL-0052 on MAP in beagle dogs. (A) Effects of a bolus injection of 2.5 × ED50 etomidate, propofol, and EL-0052 on MAP in dogs (n = 4). (B) Effects of a bolus injection of 4.3 × ED50 etomidate and EL-0052 on MAP in dogs (n = 4). The doses of propofol (6 mg/kg, 2.5 × ED50) and etomidate (2 mg/kg, 4.3 × ED50) were determined according to that of Campagna et al. (2014) Each data point represents the mean ± S.E.M. of MAP recorded every 1 minute using noninvasive blood pressure monitor. * p < 0.05, ** p < 0.01 vs. the baseline (mean of MAP recorded 5 minutes before drug administration).
Comparison of Adrenocortical Suppression between EL-0052 and Etomidate Both in Cells and Dogs
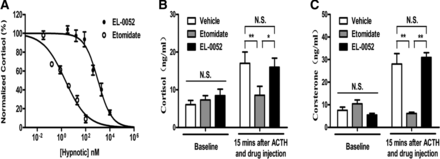
EL-0052 was designed to be of no inhibition of corticosteroids. To verify whether EL-0052 had any effects on corticosteroid suppression, the inhibitory effects of EL-0052 and etomidate on cortisol biosynthesis in human H259R cells were examined. The IC50 of EL-0052 inhibiting cortisol was 1050 ± 100 nM, which was about 500-fold less potent than etomidate (2.09 ± 0.27 nM) (Fig. 6A). In dog adrenocortical suppression test, the baseline levels of serum cortisol and corticosterone concentrations were similar among the groups of vehicle (6.06 ± 1.13 ng/ml and 7.56 ± 1.40 ng/ml), etomidate (7.30 ± 1.16 ng/ml and 10.45 ± 1.69 ng/ml), and EL-0052 (8.46 ± 1.71 ng/ml and 5.51 ± 0.72 ng/ml) under dexamethasone inhibition (Fig. 6, B and C). Fifteen minutes after ACTH1-24 injection, the serum cortisol and corticosterone levels were 17 ± 3.04 ng/ml and 28.04 ± 4.59 ng/ml in the vehicle group, 8.54 ± 2.38 ng/ml and 6.13 ± 0.56 ng/ml in the etomidate group, and 15.99 ± 2.40 ng/ml and 30.95 ± 2.12 ng/ml in the EL-0052 group (Fig. 6, B and C). Compared with the vehicle group, etomidate significantly inhibited ACTH-induced increase of corticosteroids (P = 0.021 for cortisol and P = 0.001 for corticosterone). In contrast, EL-0052 had no significant effect on ACTH1-24–induced corticosteroid elevation (P = 0.817 for cortisol and P = 0.611 for corticosterone) (Fig. 6, B and C), indicating that EL-0052 had no effect on adrenocortical suppression in dogs after a single-dose administration.
The effects of etomidate and EL-0052 on adrenocortical function in vitro and in vivo. (A) The effects of etomidate and EL-0052 on biosynthesis of cortisol in human adrenocortical tumor cell line H259R. The data of curves are fitted to Hill equation. The IC50 was 1050 ± 100 nM and 2.09 ± 0.27 nM for EL-0052 and etomidate, respectively. Each data point represents the mean ± S.E.M. from three independent experiments. The results from dog adrenocortical suppression tests showed that 4.3 × ED50 of EL-0052 (at 2.17 mg/kg) did not decrease the concentrations of cortisol (B) and corticosterone (C) in dog plasma 15 minutes after adrenocorticotropic hormone stimulation. Six dogs were studied in each group. *, P < 0.05; **, P < 0.01. N.S., no significant difference.
Discussion
In this study, we describe the pharmacological properties of EL-0052, an etomidate analog, in which a hydrogen atom in the fourth position of imidazole ring of etomidate is replaced with a fluorine atom. We find that EL-0052 potently enhances GABAA receptor’s function; displays excellent hypnotic properties with high hypnotic potency, rapid onset, and fast recovery; and maintains hemodynamics stability. Most importantly, EL-0052 has no significant effect on corticosteroid secretion in dogs even at very high doses.
The adrenal function suppression is a major side effect of etomidate, which limits its clinical applications (Annane, 2005). It has been identified that the inhibition of 11-β hydroxylase is the main cause of etomidate-induced adrenocortical suppression. The basic imidazole nitrogen is also considered to be responsible for the binding of etomidate and 11-β hydroxylase (Roumen et al., 2007). To reduce the adrenocortical toxicity of etomidate, our strategy is to replace the hydrogen atom with a highly electronegative fluorine atom on fourth position of imidazole ring of etomidate. Selective replacement of the hydrogen atom with a fluorine atom is a strategy widely used in bio-organic and medicinal chemistry (Richardson, 2016). The high electronegativity of the fluorine can have significant electronic effect on the properties of organic compounds (Yerien et al., 2016). Theoretically, after introducing a fluorine atom into the imidazole ring of etomidate, the electron cloud density of the nitrogen atom on the imidazole ring of EL-0052 will be greatly reduced, and the coordination effect with heme iron will be correspondingly reduced, thus reducing the suppression of adrenocortical function. Our study on human H259R cells demonstrates that the inhibitory potency of EL-0052 on corticosteroid synthesis is only 1/500 compared with etomidate. The assays in dogs further confirm that the adrenocortical suppression of EL-0052 is remarkably lower than that of etomidate, as EL-0052 has no significant effect on adrenocortical function (P = 0.817 for cortisol and P = 0.611 for corticosterone), whereas etomidate significantly inhibits corticosteroids secretion (P = 0.021 for cortisol and P = 0.001 for corticosterone). These results indicate that EL-0052 avoids the corticosteroid inhibitory effects that are common for etomidate. Therefore, EL-0052 has potential for clinical use as a maintenance anesthetic.
Maintaining stable cardiovascular function is a key factor for guaranteeing success of surgery. Our study finds that EL-0052 and etomidate exhibit no significant impact on MAP in dogs even at a very high dose (4.3 × ED50). In contrast, propofol markedly lowered blood pressure at the hypnotic dose (2.5 × ED50). This finding suggests that EL-0052 may have a significant advantage over propofol in maintaining cardiovascular stability in clinical applications.
Although currently reported etomidate analogs can reduce the inhibitory effect of corticosteroids and maintaining cardiovascular stability, such as carboetomidate, MOC-etomidate, and MOC-carboetomidate, their hypnotic potency is greatly reduced. The ED50s of carboetomidate, MOC-etomidate, MOC-carboetomidate, and ET26 in rats are 7.2 mg/kg, 5.2 mg/kg, 13.5 mg/kg, and 2.35 mg/kg, respectively, which are much lower than that of etomidate (1 mg/kg) (Cotten et al., 2009, 2010; Pejo et al., 2012; Sneyd, 2012; Wang et al., 2017). In contrast, our results show that EL-0052 not only maintains cardiovascular stability and eliminates adrenocortical inhibition but also retains the potent hypnotic efficacy and excellent hypnotic properties of etomidate, indicating that the imidazole nitrogen and the ester moiety are vital for inhibiting the synthesis of corticosteroids and for producing the anesthesia effect. The ED50s of EL-0052 in rats and dogs are 1.02 mg/kg (95% CI: 0.93–1.20 mg/kg) and 0.50 mg/kg (95% CI: 0.45–0.56 mg/kg), respectively, which are similar to those of etomidate with ED50s of 0.82 mg/kg (95% CI: 0.68–0.88 mg/kg) and 0.46 mg/kg (95% CI: 0.27–0.58 mg/kg). At the hypnotic doses, there are no significant differences in hypnotic onset time (P = 0.558), recovery time (P = 0.082), and walking time (P = 0.801) between EL-0052 and etomidate in rat LORR tests, indicating that EL-0052 retains the favorable hypnotic properties of etomidate with rapid onset and fast recovery after a single-dose administration.
In addition to the aforementioned properties, we also find that EL-0052 has a TI of 28, which is higher than etomidate (22) and propofol (3.4) (Forman, 2011), suggesting that EL-0052 may be safer than etomidate and propofol in clinical applications.
There are some limitations in our study. We only evaluated the hypnotic effect of EL-0052 in a single administration but did not conduct the hypnotic tests under continuous infusion. In addition, the pharmacokinetic properties of EL-0052 were not explored. We will carry out these experiments to evaluate more properties of EL-0052 in further research.
In summary, EL-0052 not only retains the favorable properties of etomidate, including potent hypnotic effect, rapid onset and recovery, stable hemodynamics and high therapeutic index, but also avoids adrenocortical function suppression. Our findings demonstrate a feasibility to modify etomidate by replacing hydrogen atoms beside the imidazole nitrogen with other substitution groups for reduction of adrenocortical toxicity while maintaining the hypnotic effect.
Authorship Contributions
Participated in research design: Xu, Jiang, Li.
Conducted experiments: Xu, Dong, Qiu, Mei, K. Wang, Xiu, T. Wang, Zeng, Dong, Shen.
Performed data analysis: Xu, Li.
Wrote or contributed to the writing of the manuscript: Xu, Wei, Jiang, Li.
Footnotes
- Received April 24, 2021.
- Accepted August 4, 2021.
The work was supported by the National Natural Science Foundation of China [Grant 31600827].
Jiangsu Nhwa-Luokang Pharma R&D Ltd. has submitted patent applications for EL-0052 and related analogs. This study was supported by Jiangsu Nhwa-Luokang Pharma R&D Ltd. and Jiangsu Nhwa Pharmaceutical Co., Ltd., which played a role in study design, data collection and analysis, decision to publish, and preparation of the manuscript. K.W., J.X., T.W., and Q.L. are research staff of Jiangsu Nhwa-Luokang Pharma R&D Ltd. X.X., Y.D., Y.Q., and Z.M. are research staff of Jiangsu Nhwa Pharmaceutical Co., Ltd. This does not alter the authors’ adherence to all the policies of The Journal of Pharmacology and Experimental Therapeutics on sharing data and materials. Y.W. and W.J. have no conflicts of interest to declare.
Abbreviations
- ACTH
- adrenocorticotropic hormone
- CI
- confidence interval
- LD50
- median lethal dose
- LORR
- loss of righting reflex
- MAP
- mean arterial pressure
- TI
- therapeutic index
- Copyright © 2021 by The American Society for Pharmacology and Experimental Therapeutics