Abstract
The binding of [3H]methoxymethyl-3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (methoxymethyl-MTEP), a potent and selective antagonist for metabotropic glutamate (mGlu)5 receptors, was characterized in rat brain both in vitro and in vivo. Nonspecific binding, as defined with 10 μM 2-methyl-6-(phenylethynyl)-pyridine (MPEP), was less than 10% of total binding in rat brain membranes. The binding of [3H]methoxymethyl-MTEP was of high affinity (K d = 20 ± 2.7 nM), saturable (B max = 487 ± 48 fmol/mg protein), and to a single site. The mGlu5 antagonists methoxymethyl-MTEP and MPEP displaced [3H]methoxymethyl-MTEP binding with IC50values of 30 and 15 nM, respectively. In vivo administration of [3H]methoxymethyl-MTEP (50 μCi/kg i.v.) revealed 12-fold higher binding in hippocampus (an area enriched in mGlu5 receptors) relative to cerebellum (an area with few mGlu5 receptors) in rats. Similarly, administration of [3H]methoxymethyl-MTEP to mGlu5-deficient mice demonstrated binding at background levels in forebrain, whereas wild-type littermates exhibited 17-fold higher binding in forebrain relative to cerebellum. Systemic administration of unlabeled mGlu5 antagonists methoxymethyl-MTEP and MPEP to rats reduced the binding of [3H]methoxymethyl-MTEP with ID50 values of 0.8 and 2 mg/kg i.p., respectively, 1 h post-treatment. The mGlu5 agonist 2-chloro-5-hydroxyphenylglycine (CHPG) (0.3, 1, and 3 μmol) dose-dependently increased phosphoinositide (PI) hydrolysis in the hippocampus after i.c.v. administration in rats. CHPG-evoked increases in PI hydrolysis were blocked with MPEP at a dose (10 mg/kg i.p.) that markedly reduced [3H]methoxymethyl-MTEP binding in vivo. These results indicate that [3H]methoxymethyl-MTEP is a selective radioligand for labeling mGlu5 and is useful for studying the binding of mGlu5 receptors in rat brain in vitro and in vivo.
Metabotropic glutamate receptors (mGluRs) are G protein-coupled receptors that are activated by glutamate and can modulate fast excitatory responses evoked by glutamatergic stimulation of ionotropic receptors (Conn and Pin, 1997). There are eight mGluR subtypes, which are subdivided into three groups principally based on sequence homology, but also on signal transduction pathways and agonist selectivity (Nakanishi, 1992; Pin and Duvoisin, 1995). Group I mGluRs initiate cell responses through Gq/11 protein coupling to phospholipase C and stimulation of phosphoinositide hydrolysis. In contrast, group II and group III mGluRs are negatively coupled via Gi/Go to adenylyl cyclase and reduce forskolin-stimulated increases in cAMP in recombinant expression systems. Group I receptors are selectively activated by dihydroxyphenylglycine (DHPG), group II receptors can be stimulated by (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine and (+)-2-aminobicyclo[3.1.0]-hexane-2,6-dicarboxylate monohydrate (LY354740), and group III receptors are selectively stimulated by l-(+)-2-amino-4-phosphonobutyric acid and (R,S)-4-phosphonophenylglycine (Schoepp et al., 1999).
Group I mGluRs include the mGlu1 and mGlu5 subtypes. These two receptors exhibit a regional pattern of expression in the central nervous system, suggesting distinct, functional roles for each receptor (Spooren et al., 2001). For example, expression of mGlu5 receptor protein is high-to-moderate in frontal cortex, caudate putamen, nucleus accumbens, olfactory tubercle, hippocampus, and dorsal surface of the spinal cord, whereas low levels of expression are observed in cerebellum (Shigemoto et al., 1993; Romano et al., 1995). In contrast, mGlu1 receptors are present in high density in the cerebellum and low-to-moderate expression is found in frontal cortex, caudate putamen, nucleus accumbens, and olfactory tubercle (Martin et al., 1992).
Given the high level of expression of mGlu5 receptors in the limbic forebrain, these receptors are positioned to play key roles in emotional and behavioral processing. Selective and systemically available mGlu5 antagonists have recently been developed (Gasparini et al., 1999; Varney et al., 1999), and insight into the function of mGlu5 receptors in the brain is emerging. The selective mGlu5 antagonist MPEP produced anxiolytic effects in several rodent models of anxiety, including conditioned response tests (Vogel test, the four-plate test, fear-potentiated startle, and Geller-Seifter test), unconditioned response tests (social exploration, marble burying, and elevated plus-maze), and in stress-induced hyperthermia (Spooren et al., 2000,2001; Tatarczynska et al., 2001; Brodkin et al., 2002). MPEP also blocked both the acquisition and expression of fear in rats (Schulz et al., 2001). These findings suggest a role for mGlu5 receptors in the generation of fear and anxiety and point to the therapeutic potential of mGlu5 antagonists in the treatment of anxiety.
mGlu5 receptors may also be important in nociceptive processing because these receptors are localized to key points along the pain neuraxis (Jia et al., 1999; Walker et al., 2001b). In addition, intrathecal application of DHPG induces spontaneous nociceptive behaviors in rats (Fisher and Coderre, 1996) and mice (Karim et al., 2001) and also produces thermal hyperalgesia and allodynia in rats (Fisher and Coderre, 1998). mGlu5 antagonists have antinociceptive properties because MPEP blocked the responses of nociceptive neurons in the ventroposterolateral nucleus of the thalamus to pressure stimuli (Bordi and Ugolini, 2000) and reversed mechanical hyperalgesia in an inflammatory pain model (Walker et al., 2001a). Taken together, these results support a role for spinal and central mGlu5 receptors in modulating nociception and indicate that mGlu5 antagonists may be useful in the treatment of pain.
Although the use of selective mGlu5 antagonists is becoming widespread in basic research, little is known about the in vitro binding affinities of these ligands (Gasparini et al., 2002) and the appropriate in vivo receptor occupancy required for efficacy in behavioral models. The present investigation describes the use of a novel radiolabeled mGlu5 antagonist, [3H]methoxymethyl-MTEP, for determining the binding of compounds to mGlu5 receptors in brain both in vitro and in vivo. In vivo phosphoinositide hydrolysis was also used to compare in vivo receptor occupancy with the functional efficacy of the mGlu5 antagonists.
Materials and Methods
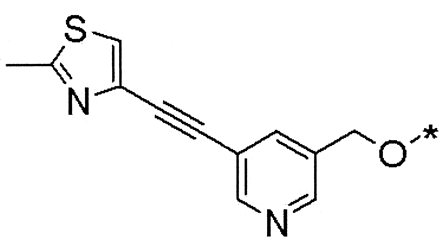
Male Sprague-Dawley rats (175–225 g) were purchased from Harlan (San Diego, CA) and mGlu5 knockout mice (20–25 g) (Lu et al., 1997) were purchased from Jackson Laboratories (Bar Harbor, ME). Wild-type littermates served as controls. All animals were group housed on a 12-h light/dark cycle with free access to food and water. All procedures were approved by the Institutional Animal Care and Use Committee in accordance with The Guide for the Care and Use of Laboratory Animals. [3H]Myo-inositol (17 Ci/mmol) was purchased from Amersham Biosciences (Piscataway, NJ). CHPG was obtained from Tocris Cookson (Ballwin, MO). All other compounds, including [3H]methoxymethyl-MTEP (Fig.1), were synthesized at Merck Research Laboratories (Cosford et al., 2002).
Structure of the tritiated radioligand methoxymethyl-MTEP. The radiolabeled position is indicated by the asterisk.
[3H]Methoxymethyl-MTEP Binding to Rodent Brain Membranes
Membrane Preparation.
Membranes were prepared as described previously (Ransom and Stec, 1988) using whole rat brain, or mGlu5+/+ or mGlu5−/−whole mouse brain.
In Vitro Binding with [3H]Methoxymethyl-MTEP.
Binding assays were performed at room temperature as described previously (Schaffhauser et al., 1998) with slight modifications. Briefly, membranes were thawed and washed once with assay buffer (50 mM HEPES and 2 mM MgCl2, pH 7.4), followed by centrifugation at 40,000g for 20 min. The pellet was resuspended in assay buffer and briefly homogenized with a Polytron.
For protein linearity experiments, increasing concentrations of membrane protein were added to 96-well plates in triplicate and binding was initiated by addition of 20 nM [3H]methoxymethyl-MTEP. The assay was incubated for 2 h and nonspecific binding was determined using 10 μM MPEP. The binding was terminated by rapid filtration through glass fiber filters (Unifilter-96 GF/B plate; Packard Instrument Company, Inc., Downer's Grove, IL) using a 96-well plate cell harvester (Brandel Inc., Gaithersburg, MD). Filters were washed three times with ice-cold assay buffer. After addition of scintillant, the radioactivity was determined by liquid scintillation spectrometry. Protein measurements were performed by DC Protein assay (Bio-Rad, Hercules, CA) using bovine serum albumin as the standard.
Saturation binding experiments were performed in triplicate with increasing concentrations of [3H]methoxymethyl-MTEP (1 pM–100 nM). The time course of association was measured by the addition of 10 nM [3H]methoxymethyl-MTEP to the membranes at different time points (0–240 min), followed by filtration. Dissociation was measured by the addition of 100 μM unlabeled methoxymethyl-MTEP at different time points to membranes previously incubated for 3 h with 10 nM [3H]methoxymethyl-MTEP. For competition experiments, 100 μg of membrane protein and 10 nM [3H]methoxymethyl-MTEP were added to wells containing increasing concentration of the test compound in duplicate (glutamate, methoxymethyl-MTEP, or MPEP).
Western Blot Analysis of mGlu5 Receptor Protein.
Brain hemispheres were homogenized in 20 volumes (w/v) of ice-cold homogenization buffer (PBS/0.1% CHAPS containing a protease inhibitor cocktail; Calbiochem, La Jolla, CA) using a Dounce homogenizer. Homogenates were incubated on a tube rotator at 4°C for 30 min and then centrifuged for 10 min at 10,000g. Supernatants were added 1:1 to 2× sample buffer (Laemmli, 1970) and boiled for 2 min. Proteins were separated using 4 to 12% Tris-glycine polyacrylamide gel electrophoresis-gold precast gels (BioWhittaker, Rockland, ME) and then transferred onto polyvinylidene difluoride membranes (Millipore Corporation, Bedford, MA). The membranes were blocked in PBS containing 10% nonfat dried milk and probed with the anti-mGlu5 antibody (Upstate Biotechnology, Lake Placid, NY) diluted 1:5000 in PBS containing 0.1% Tween 20. Anti-rabbit IgG-horseradish peroxidase (Amersham Biosciences) was used as the secondary antibody and diluted 1:5000 in PBS containing 0.1% Tween 20. The membranes were developed using enhanced chemiluminescence (Amersham Biosciences) followed by exposure to Kodak scientific imaging film (Eastman Kodak, Rochester, NY).
[3H]Methoxymethyl-MTEP Binding in Vivo
Time Course of in Vivo Binding of [3H]Methoxymethyl-MTEP in Rats.
Rats were gently restrained in a plastic cone and the tail was warmed briefly to facilitate vessel dilation. [3H]Methoxymethyl-MTEP (50 μCi/kg; 1 ml/kg injection volume in isotonic saline) was then administered through a lateral tail vein. At the appropriate time, rats were euthanized and brain tissue was rapidly dissected on a cooled dissecting tray. Hippocampus and cerebellum were immediately weighed and homogenized in 10 volumes of ice-cold buffer (10 mM potassium phosphate and 100 mM KCl, pH 7.4) using a Polytron. Homogenates (400 μl) were then either placed directly into scintillation vials (total radioactivity) or filtered over GF/B membrane filters (Whatman, Maidstone, UK) and washed twice with 5 ml of ice-cold homogenization buffer to separate membrane-bound from free radioactivity (Atack et al., 1999). Filters and homogenates were then counted for radioactivity using a scintillation counter (Beckman Coulter, Inc., Fullerton, CA).
In Vivo Binding of [3H]Methoxymethyl-MTEP in mGlu5-Deficient Mice.
In vivo binding in mGlu5-deficient mice (and wild-type controls) was performed by administering [3H]methoxymethyl-MTEP (50 μCi/kg; 5 ml/kg injection volume in isotonic saline) through a lateral tail vein. Mice were euthanized 1 min later and forebrain and cerebellum were rapidly dissected, homogenized, and filtered as detailed above.
In Vivo Receptor Occupancy in Rats.
For studies to determine the in vivo receptor occupancy of unlabeled compounds, rats were dosed i.p. with unlabeled compound (dissolved in 50% polyethylene glycol 400; 2 ml/kg injection volume). One minute before tissue collection, [3H]methoxymethyl-MTEP was administered (50 μCi/kg) through a lateral tail vein. Animals were then euthanized and hippocampus was rapidly dissected, homogenized, and filtered as described above.
In Vivo Phosphoinositide Hydrolysis Assay
Surgery and Treatments.
Previously described methods (Patel and Freedman, 1994; Johnson et al., 1999) were followed with slight modifications. Rats were anesthetized with 2% isoflurane and implanted with intracerebral guide cannula (22-gauge; Plastics One, Roanoke, VA) targeted to the lateral ventricle. The stereotaxic coordinates used were −1.0 AP, −1.6 L, and −3.0 V based on bregma as a reference (Paxinos and Watson, 1986). After a 4- to 6-day surgical recovery period, rats were infused through a 28-gauge injection cannula (extending 1 mm past the tip of the guide cannula) with [3H]myo-inositol (2 μCi/8 μl/2 min). Twenty-four hours later, rats were injected with LiCl (10 mmol/kg s.c.). One hour after LiCl administration, the mGlu5 agonist CHPG was infused intracerebroventricularly (8 μl/2 min). CHPG was initially solubilized in 0.5 N NaOH and adjusted to pH 8.0 with 1 N HCl. In some experiments, MPEP was administered systemically 15 min after CHPG infusion. Animals were euthanized 0.5 to 2 h after i.c.v. infusion, and hippocampus and cerebellum were quickly dissected and stored at −70°C until assayed (see below).
Measurement of [3H]Inositol Phosphates.
Brain samples were homogenized in 1.25 ml of 10 mM LiCl using a probe sonicator. One aliquot was transferred to a scintillation vial for analysis of total 3H radioactivity. A second aliquot was centrifuged for 10 min at 10,000g. Supernatants were loaded onto ion exchange columns packed with AG 1-X8 anion exchange beads (Bio-Rad). Columns were washed with 10 ml of 60 mM formic acid/5 mM sodium tetraborate, and the [3H]inositol phosphates were eluted with 800 mM ammonium formate/100 mM formic acid. The amount of radioactivity was determined by liquid scintillation spectrometry. Results were expressed as a percentage of the total radioactivity per sample.
Data Analysis and Statistics
In vitro binding curves were fitted using the Prism GraphPad program (GraphPad Software, San Diego, CA). Nonlinear regression analysis was used to calculate IC50 values for in vitro displacement studies and to obtain ID50values for in vivo experiments. The on- and off-rates were calculated from association-dissociation curves using the one-phase exponential association and decay equations in Prism. The on-rate (K on) was calculated by subtracting the off-rate (K off) from the observed on-rate (K ob) and dividing by the radioligand concentration. Values expressed are the arithmetic means ± S.E.M. or the geometric mean (lower, upper standard error). Differences between treatment groups were assessed by analysis of variance followed by either Dunnett's t test or Student-Newman-Keuls test to identify specific group differences. Pearson product moment correlation analysis was conducted to determine the degree of dose dependence in the PI hydrolysis experiments.
Results
In Vitro Binding Studies.
[3H]Methoxymethyl-MTEP showed high specific binding in membranes isolated from whole rat brain, with good linearity between 10 and 400 μg of protein (Fig.2A). Specific binding was defined with 10 μM MPEP as cold displacer and was greater than 90% of total binding in rat brain membranes. In contrast, in membranes prepared from rat cerebellum, specific binding of [3H]methoxymethyl-MTEP was less than 10% of total binding (data not shown). Membranes from mGlu5+/+ mouse brain also showed good linearity with increasing protein concentrations (Fig. 2B). No specific binding was observed in membranes from the mGlu5−/− mice. Western blot analysis of brain extracts from mGlu5+/+ mice revealed a dense immunoreactive band of approximately 150 kDa corresponding to the known molecular mass of the mGlu5 receptor protein (Fig.3). In contrast, the brain extracts from the mGlu5−/− mice showed a relative lack of specific mGlu5 immunoreactivity. Similarly, in rat brain, the 150-kDa mGlu5 protein was present in high concentrations in hippocampal homogenates and low concentrations in cerebellar extracts, consistent with the known distribution of these receptors in these two regions (Fig. 3).
A, specific binding with 20 nM [3H]methoxymethyl-MTEP is linear with increasing protein concentrations in membranes from rat brain. Nonspecific binding was defined with 10 μM MPEP and subtracted from total counts to obtain specific binding. Data represent the mean ± S.E.M. from two experiments performed in triplicate. B, specific binding with 20 nM [3H]methoxymethyl-MTEP is linear with increasing protein concentrations in membranes from wild-type mGlu5+/+ mouse brain. Nonspecific binding was defined with 10 μM MPEP and subtracted from total counts to obtain specific binding. No specific binding was obtained with membranes from brain tissue obtained from mGlu5−/− mice. Data represent the mean ± S.E.M. from two experiments performed in triplicate.
Western blot analysis of mGlu5 receptor protein in brain homogenates. Extracts from forebrain tissue from mGlu5+/+ and mGlu5−/− mice were run on SDS-polyacrylamide gel electrophoresis. Extracts from rat hippocampus (H) and cerebellum (C) were also tested. Proteins were transferred to polyvinylidene difluoride membranes and probed with the anti-mGlu5 antibody (1:5000 dilution). Membranes were developed using enhanced chemiluminescence.
Unlabeled methoxymethyl-MTEP (10 μM) was also tested for binding to a large panel of receptors including adenosine, adrenergic, dopamine, GABA, NMDA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, kainate, histamine, muscarinic, nicotinic, and 5-hydroxytryptamine receptors and exhibited negligible binding at each (data not shown).
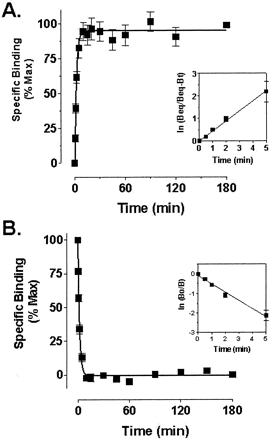
Analysis of [3H]methoxymethyl-MTEP binding in rat brain membranes revealed a single binding site that was saturable (B max = 487 ± 48 fmol/mg protein, n = 3) and of high affinity (K d = 20 ± 2.7 nM,n = 3) (Fig. 4). Association experiments demonstrated that [3H]methoxymethyl-MTEP binding reached equilibrium within 10 min (Fig. 5A). [3H]Methoxymethyl-MTEP dissociated rapidly in the presence of 100 μM unlabeled methoxymethyl-MTEP with complete dissociation occurring within 5 min (Fig. 5B). Analysis of the association/dissociation curves yielded on- and off-rates of 0.0211 nM−1 min−1 and 0.4847 nM−1 min−1, respectively. The K d calculated from these values (K off/K on) was 23 nM.
Saturation curve and Scatchard plots of [3H]methoxymethyl-MTEP binding in rat brain membranes. Membranes were incubated for 2 h at room temperature with increasing concentrations of [3H]methoxymethyl-MTEP in assay buffer (50 mM HEPES and 2 mM MgCl2, pH 7.4). Nonspecific binding was defined with 10 μM MPEP. Assay was terminated by rapid filtration with ice-cold buffer. Data represent the mean ± S.E.M. of three separate experiments performed in triplicate.
Association and dissociation time course curves for [3H]methoxymethyl-MTEP binding to rat brain membranes. Association of binding (A) was determined by incubating membranes with 10 nM [3H]methoxymethyl-MTEP at room temperature for the time indicated and then terminated by rapid filtration. Dissociation of binding (B) was initiated by addition of excess unlabeled methoxymethyl-MTEP (100 μM), followed by rapid filtration at the indicated time points. Insets show a semilogarithmic transformation of the same data. Values represent the mean ± S.E.M. of three separate experiments performed in triplicate.
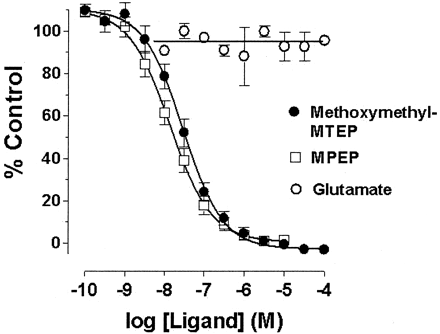
The ability of two mGlu5 antagonists to displace [3H]methoxymethyl-MTEP binding in rat brain membranes is shown in Fig. 6. Unlabeled methoxymethyl-MTEP and MPEP both displaced [3H]methoxymethyl-MTEP binding with geometric mean IC50 values of 30 (20, 40) nM and 15 (8, 20) nM, n = 4, respectively, in rat brain membranes. Glutamate, up to 1 mM, did not displace [3H]methoxymethyl-MTEP binding, confirming that the radioligand binds noncompetitively to mGlu5 receptors.
Displacement of [3H]methoxymethyl-MTEP binding from rat brain membranes by mGlu5 receptor antagonists. Experiments were performed in the presence of 10 nM [3H]methoxymethyl-MTEP and increasing concentrations of displacing ligand or glutamate. Data represent the mean ± S.E.M. of four separate experiments performed in triplicate.
In Vivo Binding Studies.
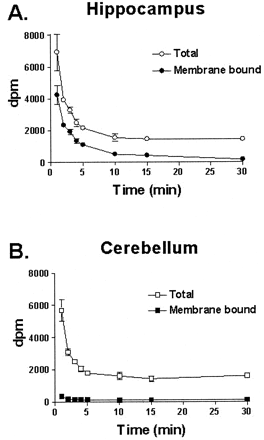
Fig. 7shows the time course of distribution of [3H]methoxymethyl-MTEP in hippocampus and cerebellum after intravenous administration in rats. Radioactivity was counted in both unfiltered homogenates (total counts) and in filtered and washed homogenates (membrane-bound counts). In the hippocampus, the time course of total and membrane-bound radioactivity were similar, with [3H]methoxymethyl-MTEP rapidly entering the brain (maximal levels at 1 min) and then distributing rapidly. Approximately 65% of the radioactivity in the hippocampus was membrane-bound (membrane-bound dpm to total dpm ratio). Although the total counts in cerebellum were comparable with hippocampus, the cerebellum had very little specific [3H]methoxymethyl-MTEP binding (membrane-bound counts) compared with hippocampus. The binding of [3H]methoxymethyl-MTEP was approximately 12-fold higher in hippocampus relative to cerebellum. Because maximal binding in hippocampus was observed at 1 min, in all subsequent experiments, [3H]methoxymethyl-MTEP was administered 1 min before tissue collection.
Time course of the distribution of [3H]methoxymethyl-MTEP in rat hippocampus (A) and cerebellum (B) after intravenous administration of radioligand. Total counts represent radioactivity in unfiltered homogenates, whereas membrane-bound counts represent radioactivity in homogenates that have been filtered over GF/B membranes. Values shown represent the mean ± S.E.M. (n = 3–4 rats/time point).
The binding of [3H]methoxymethyl-MTEP was also examined in mGlu5+/+ and mGlu5−/− mice 1 min after intravenous administration. Whole forebrain binding was compared with cerebellum binding because the amount of mouse hippocampus was insufficient for determination of radioactivity. As shown in Fig.8, binding of [3H]methoxymethyl-MTEP was 17-fold greater in forebrain than in cerebellum in the wild-type mGlu5+/+ mice. In mice lacking the mGlu5 receptor, however, forebrain binding of [3H]methoxymethyl-MTEP was comparable with the background levels of binding observed in the cerebellum (Fig. 8).
In vivo binding of [3H]methoxymethyl-MTEP in mGlu5+/+ and mGlu5−/− mice. Mice were administered 50 μCi/kg [3H]methoxymethyl-MTEP intravenously and euthanized 1 min later. The membrane-bound counts in forebrain and cerebellum were determined. Values indicate the mean ± S.E.M. (n = 8–10 mice/group). ∗, p< 0.05 versus forebrain by analysis of variance and Dunnett'st test.
Binding of [3H]methoxymethyl-MTEP in rats was reduced in hippocampus by pretreatment with the unlabeled compounds methoxymethyl-MTEP and MPEP (Fig. 9). The dose required to produce a 50% reduction in the in vivo binding of [3H]methoxymethyl-MTEP (receptor occupancy) was 0.7 mg/kg methoxymethyl-MTEP and 2 mg/kg MPEP 1 h postadministration. The highest dose tested for each compound, 50 mg/kg, reduced binding by 92 and 93%, respectively. The remaining nondisplaceable counts (approximately 450 dpm) were defined as nonspecific binding. The time course of receptor occupancy of MPEP was also examined. As shown in Fig. 10, within 5 min of an i.p. injection of MPEP, the specific binding of [3H]methoxymethyl-MTEP was decreased by 79% and remained reduced by approximately 75% for 60 min. By 2 h postinjection, the binding was still decreased by 60% and by 4 h, the binding was reduced by only 27%. Hence, substantial receptor occupancy lasting at least 2 h is associated with an i.p. injection of 10 mg/kg MPEP.
Reductions in binding of [3H]methoxymethyl-MTEP by systemically administered mGlu5 antagonists in rats. Unlabeled methoxymethyl-MTEP (A) or MPEP (B) was dosed i.p. (1 h before tissue collection) and 50 μCi/kg [3H]methoxymethyl-MTEP was administered intravenously 1 min before tissue collection. Hippocampal tissue was dissected, homogenized, and filtered. The values represent the mean ± S.E.M. (n = 4–6 rats/group). ∗, p< 0.05 versus vehicle by analysis of variance and Dunnett'st test.
Time course of the decrease in [3H]methoxymethyl-MTEP binding by systemically dosed MPEP in rats. Animals were injected with MPEP (10 mg/kg i.p.) and received an i.v. injection of [3H]methoxymethyl-MTEP 1 min before tissue collection at the indicated times. Hippocampal tissue was dissected, homogenized, and filtered. Values represent the mean ± S.E.M. (n = 4–6 rats/group). ∗,p < 0.05 versus time 0 by analysis of variance and Dunnett's t test.
In Vivo PI Hydrolysis.
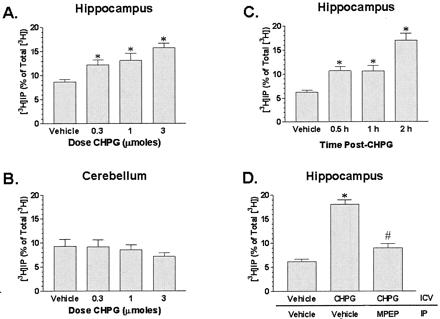
Intracerebroventricular infusion of the mGlu5 agonist CHPG elicited dose-dependent (0.3–3.0 μmol) increases in PI hydrolysis in hippocampus, but not in cerebellum in rats (Fig. 11, A and B). Correlation analysis of the CHPG-evoked increases in PI hydrolysis in the hippocampus (pairing dose and [3H]IP as a percentage of total radioactivity) revealed a significant positive correlation (r = 0.66, p < 0.05). The time course experiment indicated that the maximal increase in PI hydrolysis (approximately 2.5-fold above basal) in hippocampus occurred 2 h post-i.c.v. administration (Fig. 11C). Systemic administration of MPEP (10 mg/kg i.p.) blocked the increase in PI hydrolysis elicited by CHPG (Fig. 11D). In a separate experiment, MPEP (10 mg/kg i.p.) alone did not effect PI hydrolysis (vehicle i.c.v./vehicle i.p. = 6.81 ± 0.5% versus vehicle i.c.v./MPEP i.p. = 6.49 ± 0.33%; n = 5/group).
mGlu5 agonist CHPG induced increases in phosphoinositide hydrolysis in vivo. A and B, rats were infused i.c.v. with vehicle or CHPG (0.3, 1, or 3 μmol) 1 h after receiving an injection of LiCl (10 mmol/kg s.c.). Hippocampus and cerebellum were harvested 2 h later and assayed for [3H]inositol phosphate accumulation. C, CHPG (3 μmol) or vehicle was infused i.c.v. and hippocampus was collected 0.5, 1, or 2 h later. D, CHPG (3 μmol) or vehicle was infused i.c.v. and vehicle or MPEP (10 mg/kg) was injected i.p. Hippocampus was collected 2 h after i.c.v. infusion. Values are the mean ± S.E.M. (n = 5–6 rats/group). ∗, p < 0.05 versus vehicle control by analysis of variance and Newman-Keuls test. #,p < 0.05 versus CHPG group by analysis of variance and Newman-Keuls test.
Discussion
Recent evidence points to the importance of mGlu5 receptors in brain function and as a potential therapeutic target for a number of central nervous system disorders, including anxiety, pain, and Parkinson's disease (Spooren et al., 2001). The present investigation demonstrates that [3H]methoxymethyl-MTEP is a novel, high-affinity mGlu5 antagonist and is useful for studying mGlu5 receptors in brain both in vitro and in vivo. We have shown that [3H]methoxymethyl-MTEP binding to rat brain membranes is saturable, reversible, and to a single site. [3H]Methoxymethyl-MTEP also exhibited low nonspecific binding in rat brain membranes (using 10 μM MPEP as displacer) and no specific binding in membranes from mGlu5-deficient mice. The lack of specific binding of [3H]methoxymethyl-MTEP in brain membranes from mice lacking mGlu5 receptors as well as the lack of affinity of unlabeled methoxymethyl-MTEP to a large panel of brain receptors suggests that this radioligand is highly selective for mGlu5 receptors. We've also demonstrated that [3H]methoxymethyl-MTEP binds noncompetitively to mGlu5 receptor sites, because high concentrations of glutamate did not displace [3H]methoxymethyl-MTEP binding. Finally, the mGlu5 antagonists MPEP and unlabeled methoxymethyl-MTEP displaced [3H]methoxymethyl-MTEP binding in vitro, with IC50 values of 15 and 30 nM, respectively. MPEP has previously been shown to inhibit quisqualate-stimulated PI hydrolysis in mGlu5-expressing cells with an IC50 of 36 nM, and DHPG-induced PI hydrolysis in rat hippocampal slices with an IC50 of 8 nM (Gasparini et al., 1999). Hence, the binding results obtained herein with MPEP are in agreement with the previously published functional data.
To date, two other radioligands have been used to examine group I mGluR binding in vitro. [3H]Quisqualate was shown to bind with high affinity to both mGlu1 and mGlu5 receptors in transfected cells, and under certain conditions to native mGlu1 and mGlu5 receptors in rat and human brain sections (Mutel et al., 2000). More recently, the subtype-selective ligand [3H]M-MPEP, which is structurally similar to [3H]methoxymethyl-MTEP, was found to bind with high affinity to human recombinant mGlu5 receptors in transfected cells (Gasparini et al., 2002). The latter investigation reported an IC50 of 20 nM for MPEP in displacement assays, in agreement with the 15 nM value obtained herein against native mGlu5 receptors in rat brain membranes.
In vivo studies revealed that [3H]methoxymethyl-MTEP penetrated the brain rapidly with peak radioactivity achieved 1 min after intravenous administration in rats. The majority (65%) of the radioactivity was membrane-bound, suggesting that most of the [3H]methoxymethyl-MTEP that reaches the brain binds to the receptor. In addition, membrane-bound [3H]methoxymethyl-MTEP was nearly 12-fold higher in hippocampus compared with cerebellum, which is consistent with the known distribution of mGlu5 receptors in these two brain regions (Romano et al., 1995). Similarly, in mGlu5-deficient mice, intravenous administration of [3H]methoxymethyl-MTEP produced very low binding in forebrain (levels similar to cerebellum), whereas in wild-type control mice forebrain binding was 17-fold greater than in cerebellum. These in vivo results are consistent with the low nonspecific binding obtained in vitro and further emphasize the high selectivity of [3H]methoxymethyl-MTEP. Furthermore, these findings indicate that [3H]methoxymethyl-MTEP is a useful radioligand for studying mGlu5 receptors in the whole, living animal.
Systemic administration of the selective mGlu5 antagonists MPEP and methoxymethyl-MTEP reduced [3H]methoxymethyl-MTEP binding in vivo, with ID50 values of 2 and 0.7 mg/kg, respectively, 1 h postadministration. These results suggest that MPEP and methoxymethyl-MTEP are highly brain-penetrant and able to occupy brain mGlu5 receptors at relatively low doses. Maximal inhibition of binding occurred at 30 mg/kg for each compound at which the radioligand binding was decreased by 92 and 93%, respectively. The 50-mg/kg dose did not reduce binding beyond that of the 30-mg/kg dose and is therefore suitable for defining nonspecific binding. The dose range for receptor occupancy of MPEP determined herein is in line with the doses (1–30 mg/kg i.p., 1 h) reported to produce anxiolytic effects in rats (Tatarczynska et al., 2001; Brodkin et al., 2002).
As expected, MPEP exhibited receptor occupancy that was time-dependent after systemic administration. Maximal occupancy, after 10-mg/kg dose of MPEP, occurred within 5 min of administration, suggesting rapid absorption, brain penetration, and onset of receptor occupancy. The receptor occupancy was comparable between 5 and 60 min, but decreased from 60 to 27% between 2 and 4 h. The results from both the time-course and dose-response experiments provide some guidelines to follow in terms of dosage selection and behavioral assessment times for MPEP. For example, doses in excess of 50 mg/kg i.p. should be avoided because these doses are in excess of those needed to produce 100% occupancy and may elicit off-target, non-mGlu5-mediated effects, such as blockade of NMDA receptors (O'Leary et al., 2000). Likewise, at time points beyond 4 h, MPEP exhibits low receptor occupancy and will likely be less effective than at earlier times.
Intracerebroventricular administration of the mGlu5 agonist CHPG dose-dependently stimulated PI hydrolysis in hippocampus, but not cerebellum, in rats. This is consistent with a previous report (Johnson et al., 1999), with the known distribution of mGlu5 receptors, and with the in vivo radioligand binding results obtained in this study. The stimulation of PI hydrolysis produced by CHPG was detected at 30 min postinfusion, but was maximal at 2 h. This latency is likely due to the time required to adequately penetrate the hippocampal tissue, stimulate the receptors, and accumulate the [3H]inositol phosphates. MPEP blocked the increases in PI hydrolysis induced by CHPG at a dose (10 mg/kg) that also reduced [3H]methoxymethyl-MTEP binding by 79% and at a dose that is effective in reducing anxiety-like behaviors (Tatarczynska et al., 2001; Brodkin et al., 2002). Hence, the dose range of MPEP required to produce substantial receptor occupancy is in line with the dose required to block an mGlu5-mediated functional response.
Although in vivo or ex vivo receptor occupancy assays using radioligands have previously been established for dopamine (Sumiyoshi et al., 1994), serotonin (Laporte et al., 1994), muscarinic (Freedman et al., 1989), NMDA (Murray et al., 2000), and benzodiazepine (Goeders and Kuhar, 1985) receptors, this is the first report to describe an in vivo receptor occupancy assay for any receptors in the mGluR family. Despite the existence of radiolabeled ligands for group I mGluRs ([3H]quisqualate; Mutel et al., 2000) and for group II mGluRs ([3H](2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine IV, [3H]LY354740, and 2S-2-amino-2-(1S,2S-2-carboxycyclopropan-1-yl)-3-(xanth-9-yl)propionic acid ([3H]LY341495); Mutel et al., 1998;Schaffhauser et al., 1998; Wright et al., 2001), there are several advantages to [3H]methoxymethyl-MTEP. In addition to a high degree of specificity and selectivity for mGlu5 receptors, [3H]methoxymethyl-MTEP provides excellent brain penetration that allows for in vivo administration. Methoxymethyl-MTEP, like MPEP, is among a series of mGlu5 antagonists that bind noncompetitively to the receptor. The binding site of competitive agonists or antagonists resides in the large amino-terminal extracellular domain, whereas the binding site of the noncompetitive antagonists is in the seven transmembrane domain (Pagano et al., 2000). Hence, endogenous glutamate, which is present in relatively high concentrations in brain homogenates, does not compete with [3H]methoxymethyl-MTEP, allowing for a high degree of displaceable binding for this radioligand.
In summary, we described the use of the radiolabeled mGlu5 antagonist [3H]methoxymethyl-MTEP for determining the binding of compounds to mGlu5 receptors in rat brain in vitro and in vivo. This radioligand will be useful for further study of mGlu5 receptors and in the development of additional potent and selective mGlu5 antagonists that may have therapeutic potential in a number of important neurological and psychiatric disorders.
Footnotes
- Received June 20, 2002.
- Accepted August 9, 2002.
DOI: 10.1124/jpet.102.040618
Abbreviations
- mGluR
- metabotropic glutamate receptor
- DHPG
- 3,5-dihydroxyphenylglycine
- MPEP
- 2-methyl-6-(phenylethynyl)-pyridine
- MTEP
- 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine
- CHPG
- 2-chloro-5-hydroxyphenylglycine
- PBS
- phosphate-buffered saline
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]propanesulfonate
- PI
- phosphoinositide
- The American Society for Pharmacology and Experimental Therapeutics