Abstract
Seizures and status epilepticus are among the neurological complications of cocaine overdose in humans. The aim of the present study was to evaluate the protective effectiveness and therapeutic index (separation between anticonvulsive and side effect profiles) of 14 newly approved and potential antiepileptic drugs using a murine model of acute cocaine toxicity and the inverted-screen test for behavioral side effect testing. Cocaine (75 mg/kg i.p.) produces clonic seizures (∼90% of mice), and conventional antiepileptic drugs have been reported to be either ineffective or only effective at doses producing significant sedative/ataxic effects. Clobazam, flunarizine, lamotrigine, topiramate, and zonisamide were ineffective against seizures up to doses producing significant motor impairment. In contrast, felbamate, gabapentin, loreclezole, losigamone, progabide, remacemide, stiripentol, tiagabine, and vigabatrin produced dose-dependent protection against cocaine-induced convulsions with varied separations between their anticonvulsant and side effect profiles: the protective index values (toxic TD50/anticonvulsive ED50) ranged from 1.26 (felbamate) to 7.67 (loreclezole), and gabapentin had the highest (protective index >152). Thus, several drugs were identified with greater protective efficacy and reduced motor impairment compared with classic antiepileptic drugs. Based on the proposed mechanism of action of these new anticonvulsants, it is noteworthy that 1) drugs that enhance γ-aminobutyric acid-mediated neuronal inhibition in a manner distinct from barbiturates and benzodiazepines offer the best protective/behavioral side effect profiles, and 2) functional antagonists of Na+ and Ca2+ channels are generally ineffective. Overall, this study provides the first description of the effectiveness of new antiepileptic drugs against experimentally induced cocaine seizures and points to several drugs that deserve clinical scrutiny for this indication.
Cocaine is a heavily abused psychomotor stimulant drug, with estimates of more than 30 million persons having used cocaine and 1.7 million regular users in the United States alone (NIDA, 1996). In addition to addiction potential, cocaine abuse bears a high risk of various medical complications (Schrank, 1992; Benowitz, 1993). There were, for example, approximately 150,000 cocaine-related emergency department visits in 1995 alone, accounting for about 27% of all drug-related emergency department visits in the United States (SAMHSA, 1997).
Generalized tonic-clonic seizures and status epilepticus capable of producing long-term neurological impairment and death are well documented neurological sequelae of cocaine abuse (Spivey and Euerle, 1990; Benowitz, 1993; Kunisaki and Augenstein, 1994). Seizures are considered a major determinant of cocaine-related lethality in humans (Spivey and Euerle, 1990). Current epidemiological data show that an estimated 2.3 to 8.4% of patients in emergency departments due to cocaine intoxication require anticonvulsive therapy (Lowenstein et al., 1987; Derlet and Albertson, 1989a). Seizures can be induced by cocaine after an accidental, massive overdose (“body packer syndrome” in individuals attempting to smuggle the drug in body cavities), as well as after the recreational use of relatively low doses of cocaine (Kramer et al., 1990; Dhuna et al., 1991; Schrank, 1992). The threshold for cocaine to precipitate seizures appears to decrease over time in cocaine abusers (Murray, 1986; Dhuna et al., 1991), suggesting the development of sensitization to the convulsive effects of cocaine. Data from clinical studies suggest that chronic cocaine use can initiate and facilitate the development of progressive epileptogenic changes, and individuals with a history of cocaine-unrelated seizures show increased sensitivity to the convulsive effects of cocaine (Kramer et al., 1990;Dhuna et al., 1991; Koppel et al., 1996).
Because there is no specific therapy for cocaine-induced seizures, the management of seizures is based on the general practices of emergency symptom control where standard antiepileptic drugs such as diazepam, phenytoin, and phenobarbital would typically be used (Johnson and Vocci, 1993; Kunisaki and Augenstein, 1994). Unfortunately, this treatment is not always effective, and the likelihood of cocaine-related long-term complications or death can markedly increase due to persistent seizure activity (Dhuna et al., 1991). The discovery of more effective antiepileptic drugs to specifically control cocaine-related seizures therefore is of clinical significance.
After a long period of stagnation, several new antiepileptic drugs have recently been approved across the world, and others are in various stages of clinical testing for the treatment of epilepsy and seizure disorders (Bazil and Pedley, 1998). These new antiepileptic drugs often differ from the “classic” antiepileptic drugs (e.g., carbamazepine, ethosuximide, phenytoin, valproic acid, benzodiazepines, and barbiturates) in terms of their mechanism of action, efficacy, pharmacokinetic properties, and safety profiles, and their advent is a long-awaited innovation in providing new therapeutic opportunities for patients with seizure disorders (Macdonald and Kelly, 1995; Bazil and Padley, 1998). However, the efficacy of these novel antiepileptic drugs against cocaine-induced seizures has not been tested.
Therefore, the aim of the present study was to comparatively evaluate efficacy and behavioral side effect profiles of 14 newly approved and potential antiepileptic drugs (Fig. 1) against cocaine-induced seizures in a murine model of acute cocaine toxicity. This model demonstrates important aspects of acute cocaine-related toxicity in humans, including seizures and resistance to the treatment with classic antiepileptic drugs (Witkin and Tortella, 1991; Gasior et al., 1997; Witkin et al., 1999). The present study provides the first description of the effectiveness of novel antiepileptic drugs against experimentally induced cocaine seizures and points to several drugs and some molecular targets that appear to be worthy of further experimental and clinical scrutiny.
Structures of antiepileptic drugs used in the present study. More information about these drugs can be found inMaterials andMethods.
Materials and Methods
Subjects.
Experimentally naive, male Swiss–Webster mice (Taconic Farms, Germantown, NY) between 10 and 12 weeks old and weighing 30 to 44 g were housed six per cage in an environmentally controlled vivarium (temperature, 22–26°C; humidity, 40–50%) with a 12-h light/dark cycle (7:00 AM to 7:00 PM lights on). Animals used in these studies were maintained in facilities fully accredited by the American Association for the Accreditation of Laboratory Animal Care, and all experimentation was conducted in accordance with the guidelines of the Institutional Care and Use Committee of the National Institute on Drug Abuse, National Institutes of Health, and the Guide for Care and Use of Laboratory Animals (National Research Council, 1996, National Academy Press, Washington, D.C.). All animals were acclimated to their home cages and to the light/dark cycle for at least 5 days before testing. Tap water and food pellets (NIH-07 diet; Zeigler Bros. Inc., Gardners, PA) were available ad libitum. Experiments were conducted between 9:00 AM and 3:00 PM in an experimental room. At least eight mice per group were used, and all mice were experimentally naive and used only once.
Drugs.
Clobazam (7-chloro-methyl-5-phenyl-1H-1,5-benzodiazepine-2,4-[3H,5H]-dione; mw = 300.7) and flunarizine (1-[bis(4-fluorophenyl)methyl]-4-(3-phenyl-2-propenyl)-piperazine dihydrochloride; mw = 477.4) were obtained from Sigma Chemical Co. (St. Louis, MO). Felbamate (Felbatol; 2-phenyl-1,3-propanediol dicarbamate; mw = 238.24) was obtained from Carter-Wallace, Inc. (Wallace Laboratories, Cranbury, NJ). Gabapentin [Neurontin; PD 87842, Cl-945; 1-(aminomethyl)cyclohexaneacetic acid; mw = 171.2] was obtained from Parke-Davis, Division of Warner-Lambert Company (Ann Arbor, MI). Lamotrigine (Lamictal; 3,5-diamino-6-[2,3-dichlorophenyl]-1,2,4-triazine; mw = 256.1) was obtained from GlaxoWellcome Inc. (Research Triangle Park, NC). Loreclezole [RO72063; (Z)-1-[2-chloro-2-(2,4-dichlorophenyl ethenyl]-1H-1,2,4-triazole; mw = 274.5] was obtained from Janssen Research Foundation, Division of Janssen Pharmaceutica N.V. (Beerse, Belgium). Losigamone (AO-033, ADD-137022; (±)-5(R,S), α-(S,R)-5-[(2-chlorophenyl)hydroxymethyl]-4-metoxy(5H)-furanone; mw = 254.7) was obtained from Dr. Willmar Schwabe GmbH & Co., (Karlsruhe, Germany). Progabide (Gabrene; SL 76.0002-00; 4-[{4-chlorophenyl)(5-fluoro-2-hydroxy-phenyl)methyleneamino]butanamide; mw = 334.8) was obtained from Synthélabo Recherche (Bagneux Cedex, France). Remacemide hydrochloride [AR-R 12924AA; (±)-2-amino-N-(1-methyl-1,2-diphenylethyl)acetamide hydrochloride; mw = 304.8] was obtained from Astra Charnwood (Loughborough, England). Stiripentol [4,4-dimethyl-1-[(3,4-methylenedioxy)phenyl]-1-penten-3-ol; mw = 234.0] was obtained from Laboratories Biocodex (Montrouge Cedex, France). Tiagabine (Gabitril; A-70569.0; (R)-(−)-1-[4,4-bis(3-methyl-2-thienyl)-3-butenyl]-3-piperidinecarboxylic acid, hydrochloride; mw = 412.0) was obtained from Abbott Laboratories (Abbott Park, IL). Topiramate [Topamax; RWJ-17021-000-DD; 2,3:4,5-bis-O-(1-methylethylidene)-(β)-d-fructo-pyranose-1-sulfamate; mw = 339.4] was obtained from The R. W. Johnson Pharmaceutical Research Institute (Spring House, PA). Vigabatrin [Sabril; γ-vinyl-γ-aminobutyric acid (GABA), MDL 71,754; (±)-4-amino-5-hexenoic acid; mw = 129.2] was obtained from Hoechst Marion Roussel Inc. (Cincinnati, OH). Zonisamide (Excegran; 1,2-benzisoxazole-3-methanesulfonamide; mw = 212.2) was obtained from Dainippon Pharmaceutical Company Ltd. (Osaka, Japan). Except for clobazam and flunarizine, all the antiepileptic drugs were graciously supplied by the drug companies as listed above.
Gabapentin, tiagabine, remacemide, and vigabatrin were dissolved in sterile 0.9% NaCl. Losigamone, progabide, and topiramate were suspended in 0.1% (v/v) and flunarizine in 10% (v/v) Tween 80 (Sigma Chemical Co.). Clobazam and zonisamide were suspended in propylene glycol (Sigma Chemical Co.) and prepared in final concentrations of 30 and 40%, respectively, by the addition of sterile water (v/v). Felbamate, lamotrigine, loreclezole, and stiripentol were suspended in 40% (w/v) hydroxypropyl-γ-cyclodextrin (Research Biochemicals Inc., Natick, MA). Cocaine HCl (National Institute on Drug Abuse, Rockville, MD) was dissolved in sterile saline and administered i.p. When necessary, mild heat and sonication aided compounds into solution.
Routes of administration and pretreatment times of the antiepileptic drugs were based on information about their biological activity from the literature and as provided by drug companies and finally confirmed in pilot experiments: clobazam (i.p., 30 min), felbamate (s.c., 30 min), flunarizine (s.c., 30 min), gabapentin (i.p., 60 min), lamotrigine (i.p., 60 min), loreclezole (i.p., 60 min), losigamone (i.p., 30 min), progabide (i.p., 30 min), remacemide (i.p., 30 min), stiripentol (i.p., 60 min), tiagabine (i.p., 15 min), topiramate (s.c., 30 min), vigabatrin (i.p., 240 min), and zonisamide (i.p., 60 min). Longer pretreatment times of selected doses of topiramate and flunarizine were additionally tested using 2-fold longer pretreatment times than the times listed above. Injection volumes were 0.1 ml/10 g b.wt. Doses of drugs were expressed as milligrams of salt per kilogram of body weight with drug potencies also converted to mmol/kg free base to enable comparison of relative potencies.
Motor Toxicity.
Immediately before the administration of cocaine, mice were first tested on the inverted-screen test. The inverted-screen test was used to assess one form of behavioral toxicity induced by the antiepileptic drugs. In this test, compounds with sedative and/or ataxic properties produce dose-dependent increases in screen test failures, whereas other classes of drugs (e.g., psychomotor stimulants) do not (Ginski and Witkin, 1994). Experimentally naive mice were pretreated with either vehicle or test compound and returned to their home cage for the appropriate pretreatment interval. They were then individually placed on a horizontally positioned 14 × 14-cm wire mesh screen (0.8-cm screen mesh) elevated 38 cm above the ground. After slowly inverting the screen by 180 degrees, the mice were tested during a 2-min trial for their ability to climb to the upper surface. Mice unable to climb to the top (all four paws on the upper surface) within 2 min were scored as a failure. Results were treated qualitatively and were expressed as a toxic dose50 (TD50 value). Each TD50 value was calculated from a quantal dose-response curve of at least three data points and represented the dose of a drug (in mg/kg) predicted to produce screen failure in 50% of the mice tested.
Anticonvulsant Testing.
After the screen test, a convulsant dose of cocaine (75 mg/kg) was administered, and the mice were immediately placed in individual Plexiglas containers (14 × 25 × 36 cm high) for observation. The dose of 75 mg/kg cocaine was selected to be close to the convulsive ED90value of cocaine as determined during pilot experiments and from the literature (Witkin and Tortella, 1991; Gasior et al., 1997; Witkin et al., 1999). Control groups pretreated with an appropriate vehicle instead of an antiepileptic drug before cocaine injection were evaluated during testing of the antiepileptic drugs to confirm this value.
Cocaine-induced convulsions were defined as loss of the righting response lasting at least 5 s and the occurrence of clonic movements of all four limbs; tonic seizures were never observed. The presence or absence of convulsions was recorded for 30 min after cocaine injection; typically, seizures occurred within 15 min. Sudden locomotor activation with violent jumps and loss of the righting response often preceded clonic episodes in cocaine-challenged mice. Once seizures developed, the loss of the righting response often persisted over several minutes after cocaine injection; typically, mice would then recover and show normal behavior by the end of the 30-min observation period. Results were expressed as an ED50 value. Each ED50 value was calculated from a dose-response curve of at least three data points and represented the dose of a drug (in milligram per kilogram) predicted to protect 50% of the mice tested against cocaine-induced convulsions.
Although lethality was rarely observed after 75 mg/kg cocaine (3.3%,n = 60), mice were routinely left in the observation cages for an additional 30 min after completion of the seizure assessment to exclude any acute toxic interaction between the antiepileptic drugs and cocaine.
Data Presentation and Statistical Calculations.
Quantal dose(log)-response functions were constructed for each antiepileptic drug tested on the inverted-screen test and against cocaine-induced convulsions (Fig. 2). The TD50 and ED50 values with 95% confidence limits derived from these data were calculated according to the method described by Litchfield and Wilcoxon (1949) and are listed in Table 1.
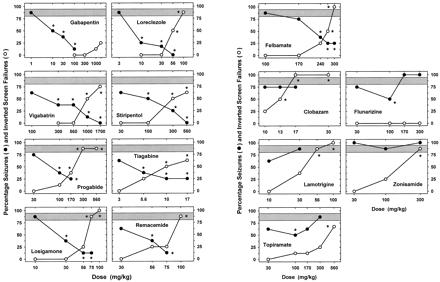
Effect of antiepileptic drugs on the inverted-screen test (○) and against 75 mg/kg cocaine-induced convulsions (●). Mice pretreated with a drug were first tested on the inverted-screen test and then they immediately received 75 mg/kg cocaine and were observed for the occurrence of clonic seizures within 30 min after cocaine injection. Each data point reflects the percentage of mice falling off the screen (○) and showing clonic seizures after cocaine injection (●). Eight to 16 mice were used for each data point. All vehicle-treated mice correctly performed the inverted-screen test (0% failures), and 87.5 ± 7.2% mice developed clonic seizures after 75 mg/kg cocaine; the latter effect is reflected by the shaded region. See Table 1 for TD50 and ED50 values and Table2 for protective indices calculated from these dose-response functions. *p < .05; compared with control treatments on the inverted-screen test (0 of 16 failed) and against cocaine-induced seizures (21 of 24 developed seizures; Fisher’s exact test).
Potencies of antiepileptic drugs against 75 mg/kg cocaine-induced convulsions (ED50 values) and potencies of these drugs to produce behavioral toxicity on the inverted-screen test (TD50values)
The rank order of relative potencies of the antiepileptic drugs (mmol/kg) to produce motor toxicity and protection were constructed relative to the most potent drug (lowest TD50 and ED50 values). Tiagabine was the most potent drug in both tests (see Results), its potency was denoted as unity, and relative potencies of the remaining drugs were calculated be dividing their potencies by the potency of tiagabine.
Correlations between the potencies of antiepileptic drugs to produce motor toxicity in the inverted-screen test and the protective potencies against cocaine-induced seizures were calculated using the Pearson product moment correlation method (Fig.3).
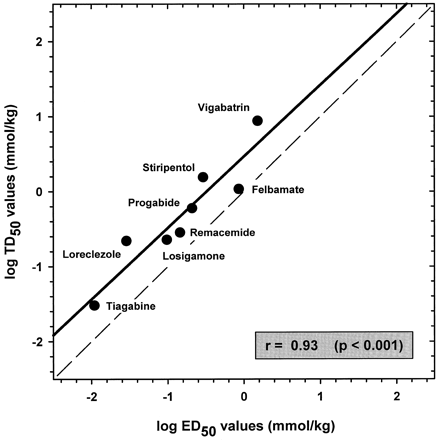
Correlation between the potencies of antiepileptic drugs to produce motor toxicity on the inverted-screen test (TD50 values on ordinate) and to protect against cocaine-induced seizures (ED50 values on abscissa). The dashed line represents a hypothetical 1:1 relationship between TD50 and ED50 values. The solid line represents the computed linear regression line of the observed relationship between TD50 and ED50 values. TD50and ED50 values were positively correlated (r = 0.93, p < .001; Pearson product moment correlation).
The protective index (PI) for each antiepileptic drug was calculated by dividing the respective toxic TD50 value by the corresponding anticonvulsant ED50 value. The protective index is a quantitative measure of the separation between anticonvulsant potency and behavioral side effect potency (Löscher and Nolting, 1991).
Fisher’s exact probability test was used for specific comparisons between each dose-treatment and the vehicle control group. To make these statistical comparisons as conservative as possible, the effect of each dose of each antiepileptic drug was compared with a control group of 16 mice (0 of 16 failures) in the inverted-screen test and with a control group of 24 mice (21 of 24 developed seizures) in the cocaine-seizure test. The outcomes of additional control treatments were recorded but were not used for statistical comparisons. The control group values selected for comparisons (Fig. 2) were representative of the outcomes of behavioral and anticonvulsive testing. None of the vehicles alone at the concentrations used to prepare solutions of the antiepileptic drugs affected the motor performance or had any anticonvulsant/proconvulsant effect when tested against 75 mg/kg cocaine (p > .05, Fisher’s exact test). Differences were considered statistically significant when the statistical probability of error was <.05.
Results
Motor Toxicity.
Except for flunarizine (30–300 mg/kg) and gabapentin (10–1700 mg/kg), the remaining antiepileptic drugs dose-dependently increased the percentage of mice falling off the screen (Fig. 2). Higher doses of flunarizine and gabapentin were not tested due to solubility limitation. Table 1 lists the TD50 values and the 95% confidence limits for the antiepileptic drugs in the inverted-screen test.
Anticonvulsant Effects against Cocaine-Induced Convulsions.
Of the 14 antiepileptic drugs tested, 9 (felbamate, gabapentin, loreclezole, losigamone, progabide, remacemide, stiripentol, tiagabine, and vigabatrin) protected against cocaine-induced seizures (Fig. 2). The protection was dose dependent for these 9 drugs, and full protection was achieved with loreclezole, stiripentol, and vigabatrin. The protective ED50 values are listed in Table 1. Marked sedation or ataxia at the highest doses of felbamate (300 mg/kg), losigamone (100 mg/kg), progabide (300 and 560 mg/kg), and tiagabine (17 mg/kg) potentiated by cocaine discouraged evaluation of higher doses. These behavioral side effects were especially evident in the case of losigamone (100 mg/kg) and progabide (300 and 560 mg/kg) and made quantification of seizure occurrence impossible after the treatment with these doses. Although gabapentin administered in a dose range from 1.0 to 100 mg/kg produced dose-dependent and almost full protection against cocaine-induced seizures (Fig. 2), higher doses of gabapentin (300–1700 mg/kg) appeared to partially lose protective efficacy. Specifically, 37.5, 25, and 50% of mice pretreated with 300, 1000, and 1700 mg/kg gabapentin, respectively, developed clonic seizures after cocaine injection. Additionally, there was significant locomotor activity depression after cocaine injection in the mice pretreated with 1000 and 1700 mg/kg gabapentin; however, mice recovered from this effect within 30 and 60 min after the cocaine injection, respectively. Different consequences appeared with a combination of the highest dose of remacemide and cocaine: six of eight mice pretreated with 100 mg/kg remacemide died within 5 to 10 min after the cocaine injection without developing seizures. None of the mice treated at doses up to 100 mg/kg died within 24 h when remacemide was given alone.
Five other antiepileptic drugs (clobazam, flunarizine, lamotrigine, topiramate, and zonisamide) generally failed to protect against cocaine-induced convulsions (Fig. 2). Significant protection was afforded after one intermediate dose of flunarizine and topiramate (100 mg/kg in both cases), but higher doses of these drugs were ineffective. The highest doses of clobazam (30 mg/kg) and topiramate (560 mg/kg) in combination with 75 mg/kg cocaine produced significant sedation that made quantification of seizure occurrence impossible. Evaluation of higher doses of zonisamide was discouraged by a marked locomotor depression observed after cocaine injection in the group pretreated with 100 mg/kg. In the case of flunarizine, doses higher than 300 mg/kg were not tested due to solubility limitations. Higher doses of lamotrigine (56 and 100 mg/kg) produced ataxia in mice that was markedly potentiated after cocaine injection. Moreover, four of eight mice pretreated with 56 mg/kg lamotrigine developed status epilepticus (seizures lasting continuously for several minutes) within 30 min after cocaine injection, and three additional mice developed status epilepticus within the next 30 min (cumulative percentage: 87.5% mice with status epilepticus within 60 min after cocaine injection). This effect was dose dependent because 100% of mice treated with 100 mg/kg lamotrigine and 75 mg/kg cocaine developed status epilepticus within 30 min after cocaine injection. Furthermore, five of eight mice died without recovering from the status epilepticus within 60 min after cocaine injection; the remaining three mice continued to be in status epilepticus and were euthanized approximately 75 min after cocaine injection. Except for lamotrigine (100 mg/kg) and remacemide (100 mg/kg), none of the remaining antiepileptic drugs potentiated the lethal effects of cocaine (75 mg/kg).
Selected doses (300 mg/kg) of flunarizine and topiramate were additionally evaluated against cocaine-induce seizures using 2-fold longer pretreatment times (60 min). Flunarizine and topiramate were ineffective when administered 60 min before cocaine; the outcome of this appraisal did not differ from the effects of the same doses administered 30 min before cocaine (p > .05, Fisher’s Exact Test). Behavioral effects on the inverted-screen test also did not differ as a function of pretreatment time.
Rank Order of Potencies and Correlation between TD50and ED50 Values.
Tiagabine was the most potent antiepileptic drug both in the inverted-screen test and against cocaine-induced convulsions. The potencies of tiagabine in both tests were assigned as one.
The following rank order of potency (molar comparison) was revealed in the inverted-screen test relative to tiagabine: clobazam (1.37-fold), lamotrigine (4.30-fold), loreclezole (7.33-fold), losigamone (7.60-fold), zonisamide (7.63-fold), remacemide (9.47-fold), progabide (19.9-fold), felbamate (36.0-fold), topiramate (46.0-fold), stiripentol (51.8-fold), vigabatrin (292-fold).
The following rank order of potency (molar comparison) was revealed against cocaine-induced convulsions relative to tiagabine: loreclezole (2.64-fold), gabapentin (5.91-fold), losigamone (8.82-fold), remacemide (13.2-fold), progabide (19.0-fold), stiripentol (26.5-fold), felbamate (78.2-fold), vigabatrin (138-fold).
Potencies of the antiepileptic drugs to produce motor toxicity in the inverted-screen test were positively correlated with the potencies to inhibit cocaine-induced seizures (r = 0.93,p < .001; Fig. 3). Although the regression line was parallel to one described by a perfect 1:1 relationship, the leftward shift of the line from one going through the origin reflects the greater potencies of the drugs to transduce anticonvulsant effects over behavioral side effects.
PIs.
The protective effects of those antiepileptic drugs that demonstrated dose-dependent protection against cocaine-induced seizures were favorably separated from the potencies of these drugs to induce motor toxicity in the inverted-screen test. This separation is reflected in PI values greater than unity. PI values ranged from 1.26 for felbamate to 7.67 for loreclezole to that of gabapentin, which was greater than 152 (Table 2).
Novel antiepileptic drugs: PIs against cocaine-induced seizures and proposed mechanisms of action
Discussion
The present study provides the first information about the effectiveness of newly approved and potential antiepileptic drugs against experimentally induced cocaine seizures in mice. There are four major findings of this study: 1) some, but not all, of the new antiepileptic drugs conferred a dose-dependent protection against cocaine-induced seizures; 2) there was generally a positive separation between anticonvulsive and behavioral side effect potencies of the drugs effective against cocaine-induced seizures, and there were some antiepileptic drugs with an exceptionally favorable therapeutic window; 3) new antiepileptic drugs generally showed both better efficacy and more favorable separation between protective and behavioral side effect potencies than classic antiepileptic drugs; and 4) based on the proposed mechanisms of action of the drugs tested, it can be concluded that drugs that enhance GABA-mediated neuronal inhibition in a manner distinct from barbiturates and benzodiazepines offer the best protective/behavioral side effect profiles against cocaine-induced seizures, whereas functional antagonists of Na+ and Ca2+ channels are generally ineffective.
Of the 14 antiepileptic drugs tested, 9 drugs dose-dependently protected against cocaine-induced seizures: felbamate, gabapentin, loreclezole, losigamone, progabide, remacemide, stiripentol, tiagabine, and vigabatrin (Tables 1 and 2). The remaining five antiepileptic drugs (clobazam, flunarizine, lamotrigine, topiramate, and zonisamide) appeared generally ineffective even at doses that produced significant impairment of locomotor coordination. Except for flunarizine and gabapentin, each of the remaining 12 antiepileptic drugs affected normal behavior of mice by impairing in a dose-dependent manner locomotor coordination in the inverted-screen test. The drugs produced behavioral side effects regardless of their ability to protect against seizures and demonstrated a wide range of potencies to produce these effects. Nevertheless, the anticonvulsive potencies of the effective antiepileptic drugs were positively correlated with potencies to produce behavioral impairment, suggesting similar mechanisms of actions involved in the mediation of these two effects (Fig. 3).
Despite a strong positive correlation between potencies of the drugs to protect against seizures and to produce side effects, these two effects were favorably separated as evidenced by PI values greater than unity (Table 2). The PI values ranged from 1.26 for felbamate to 7.67 for loreclezole and to more than 152 for gabapentin. However, doses of gabapentin of 300 mg/kg and higher, although ineffective per se in the inverted-screen test, produced a marked sedation with episodes of seizure activity after cocaine administration, warning against overestimation of the therapeutic window of gabapentin against cocaine-induced seizures. Some indications of a possible toxic interaction between cocaine and the high doses of two other antiepileptic drugs, lamotrigine (development of status epilepticus and increased lethality) and remacemide (increased lethality), were also uncovered in the present study and after high doses of phenytoin (Witkin et al., 1999). Noteworthy, the blockade of Na+ channels has been implicated in the action of phenytoin, lamotrigine, and, at least to some extent, remacemide (Rogawski and Porter, 1990; Meldrum, 1994; Clark et al., 1995), thus implicating the involvement of Na+ channels in the initiation and/or expression of toxic effects of these drugs in combination with cocaine.
Novel antiepileptic drugs clearly differed from classic antiepileptic drugs in terms of their efficacies and behavioral side effect profiles against cocaine-induced seizures. A host of classic antiepileptic drugs (Table 3) are ineffective against cocaine-induced seizures in mice (Witkin and Tortella, 1991; Gasior et al., 1997; Witkin et al., 1999). Moreover, the drugs that afforded significant attenuation of cocaine-induced seizures did so only at doses producing behavioral side effects (Witkin et al., 1999), resulting in PI values less than or slightly greater than unity (Table3), in contrast to the novel antiepileptic drugs (Table 2). The preclinical data on the efficacy and side effect profile of the conventional antiepileptic drugs obtained by means of the murine model of cocaine-induced seizures (Witkin and Tortella, 1991; Witkin et al., 1999) are generally in accord with clinical observations on the limited effectiveness and narrow therapeutic window of these drugs in the treatment of cocaine intoxication in humans (Dhuna et al., 1991). From this perspective, the superior efficacy with favorable separation from behavioral toxicity of some novel antiepileptic drugs uncovered in the present study may be of clinical importance.
Classic antiepileptic drugs: PIs against cocaine-induced seizures and proposed mechanisms of action
Pharmacological evidence has suggested the involvement of multiple receptor systems, including glutamatergic, GABAergic, serotoninergic, and cholinergic, in cocaine-induced experimental seizures (Ikeda et al., 1983; Itzhak and Stein, 1992; Ritz and George, 1997; Ye et al., 1997; Witkin et al., 1999). Although a precise sequelae of the neurochemical events triggered by cocaine that lead to seizure activity remains unknown, of note is that cocaine-induced convulsions can be suppressed by agents such asN-methyl-d-aspartate (NMDA) receptor antagonists, GABAergic drugs, and some antiepileptic drugs (e.g.,Witkin et al., 1999; present study) that are also effective in suppressing seizure activity induced by “classic” convulsive stimuli (Rogawski and Porter, 1990; Dalby and Nielsen, 1997). The involvement of enhanced excitatory (particularly glutamatergic) and decreased inhibitory (particularly GABAergic) neurotransmission in the generation of sustained local epileptic activity followed by initiation and spread of seizures has been well documented (McNamara et al., 1993;Bradford, 1995). Enhanced dopaminergic neurotransmission can enhance the release of glutamate (Reid et al., 1997) and decrease the release of endogenous GABA (Melis and Gale, 1983; Lindefors, 1993). A direct inhibitory effect of cocaine on the GABAAreceptor-mediated Cl− current in hippocampal neurons has been documented (Ye et al., 1997). Pharmacologically induced increases in levels of endogenous GABA have also been shown to attenuate cocaine-induced increases in extracellular dopamine in the striatum and nucleus accumbens (Dewey et al., 1997; Kushner et al., 1997; Morgan and Dewey, 1998). Although limited, this mechanistic understanding points to the GABAergic and glutamatergic neurotransmitter systems as potential pharmacological targets for drugs to protect against cocaine-induced convulsions.
In general, the rational design of antiepileptic drugs against epilepsy is directed toward drugs that potentiate GABAergic neurotransmission and/or attenuate glutamatergic neurotransmission (Rogawski and Porter, 1990; Löscher and Schmidt, 1994). Another potential strategy for termination of the development of seizure activity is through the pharmacological modification of Na+, K+, and Ca2+ channel conduction (Rogawski and Porter, 1990). The antiepileptic drugs tested in the present study have different molecular structures (Fig. 1) and differ in the mechanisms of action that are thought to underlie their anticonvulsive effects (Table 2). Based on the proposed mechanism of action of these drugs (Table 2), it is noteworthy that the drugs that increase levels of endogenous GABA (gabapentin, vigabatrin, stiripentol, and tiagabine) and some drugs directly acting at the GABAA receptor complex (loreclezole and progabide) offer a better protective/behavioral side effect profile relative to those directly acting at the NMDA receptor complex (remacemide and felbamate). Furthermore, the drugs that block T-type Ca2+ and/or Na+ channels (flunarizine, lamotrigine, zonisamide, and topiramate) are generally ineffective against cocaine-induced seizures (Table 2). This trend also generally holds true for the classic antiepileptic drugs (Table 3). Of the classic antiepileptic drugs (Table 3), the drugs that can increase GABA levels (valproic acid) or directly potentiate function of the GABAA receptor complex (e.g., clonazepam) show efficacy against cocaine-induced seizures, whereas T-type Ca2+ channel blockers (e.g., trimethadione) and Na+ channel blockers (e.g., phenytoin) are generally ineffective, with ethosuximide being an exception (Table 3). The ineffectiveness of various classes of Ca2+channel blockers against cocaine-induced seizures despite the ability to ameliorate cardiovascular complications associated with cocaine intoxication has been reported (Derlet and Albertson, 1989b; Tella et al., 1992).
Although positive, allosteric modulators of the GABAA receptor complex acting at the benzodiazepine- and barbiturate-binding sites show generally limited efficacy and narrow therapeutic windows against cocaine-induced convulsions (Witkin and Tortella, 1991; Witkin et al., 1999; present study), other positive modulators of the GABAAreceptor complex, such as neuroactive steroids, have been reported to be fully efficacious at behaviorally inactive doses and showed PI values ranging from 3.2 to 7.3 (Gasior et al., 1997). Neuroactive steroids are thought to transduce their action through a specific binding site on the GABAA receptor complex that is structurally and functionally distinct from binding sites for clinically useful benzodiazepines and barbiturates (for a review, seeGee et al., 1995). Likewise, loreclezole’s anticonvulsive action has been ascribed to sites distinct from the benzodiazepine and barbiturate sites at the GABAA receptor complex (Wafford et al., 1994; Green et al., 1996), and loreclezole was fully effective (Fig. 2) and displayed a PI value of 7.67 against cocaine-induced seizures (Table 3). Specifically, pharmacological properties of loreclezole have been attributed to its specificity for GABAA receptors containing β2/β3 subunits (Wafford et al., 1994; Wingrove et al., 1994), whereas these subunits do not significantly affect the pharmacological properties of benzodiazepines and barbiturates (Hadingham et al., 1993). Furthermore, two other benzodiazepine-unrelated GABAergic drugs, the competitive agonist progabide (Morselli et al., 1995) and the noncompetitive agonist losigamone (Chatterjee and Nöldner, 1997), both dose-dependently inhibited cocaine-induced seizures with PI values of 2.87 and 2.35, respectively. Taken together, the results with different modulators of GABAergic neurotransmission tested against cocaine-induced seizures suggest new pharmacological approaches targeting this neurotransmitter system that extend beyond the “classic” benzodiazepine- and barbiturate-like drugs.
NMDA blockade is also a viable mechanism by which seizures (Rogawski and Porter, 1990; Löscher and Schmidt, 1994), including cocaine-induced seizures (Rockhold et al., 1991; Witkin and Tortella, 1991; Itzhak and Stein, 1992; Witkin et al., 1999), can be suppressed. Suppression of cocaine-induced seizures has been reported after pretreatment with both competitive and noncompetitive NMDA receptor antagonists, and there was a positive correlation between anticonvulsive potencies and affinities of these compounds for specific binding sites on the NMDA receptor (Witkin et al., 1999), suggestive of the involvement of the NMDA receptor complex in initiation and/or expression of cocaine-induced convulsions. Itzhak and Stein (1992)demonstrated an increase in the number of NMDA receptors in cortical membranes of cocaine seizure-kindled mice. In the present study, there were two antiepileptic drugs tested, remacemide and felbamate, for which blockade of the NMDA receptor complex has been implicated as one of the mechanisms responsible for their anticonvulsive effectiveness (Clark et al., 1995; Sofia, 1995). Although remacemide and felbamate significantly suppressed cocaine-induced convulsions, there was less than 2-fold separation between protective and side effect profiles. This range of separation was below the PI values of other effective, new antiepileptic drugs studied here (Table 2), but it was higher than that of classic antiepileptic drugs (Table 3; Witkin et al., 1999). Although the same receptors are responsible for the anticonvulsive and behavioral side effects of many NMDA receptor antagonists (Witkin et al., 1999), there sometimes is sufficient dissociation in these two effects to generate a reasonable therapeutic window (e.g., glycine-site ligands, some competitive antagonists).
Given the paucity of effective treatments for cocaine-related seizures in humans and an alarming increase in cocaine-related emergency department visits (SAMHSA, 1997), the present study documenting the efficacy and behavioral side effect profiles of novel antiepileptic drugs against cocaine-induced convulsions can guide clinical testing of these drugs for cocaine-related seizures and status epilepticus. Importantly, the drugs tested in the present study have already been either approved for human use or tested in humans for other than cocaine-related indications. Additionally, this study provides the first evidence that new anticonvulsants that facilitate GABA-mediated neuronal inhibition in a manner distinct from barbiturates and benzodiazepines offer a robust protective/behavioral side effect profile against cocaine-induced seizures in mice. The latter finding and recent reports on the effectiveness of vigabatrin against the reinforcing effects of cocaine in experimental animals (Kushner et al., 1997; Dewey et al., 1998) and in vitro studies showing that vigabatrin can attenuate cocaine-induced increases in extracellular dopamine concentrations in the striatum and nucleus accumbens (Kushner et al., 1997; Morgan and Dewey, 1998) point to a novel pharmacological strategy for the treatment of cocaine addiction and toxic effects. Finally, because cocaine typically is abused chronically and often in escalating doses by humans, the effectiveness of the new antiepileptic drugs remains to be studied against chronic models of cocaine-induced seizures (e.g., a “kindling” model). Additional testing of the new antiepileptic drugs against chronic models of cocaine-induced seizures should add to our understanding of the seizure-generating properties of cocaine.
Acknowledgments
We thank all those involved in facilitating our effort to obtain drug samples. The antiepileptic drugs were generously provided by the drug companies noted in Materials and Methods.
Footnotes
-
Send reprint requests to: Maciej Gasior, M.D., Ph.D., Drug Development Group, Behavioral Neuroscience Branch, National Institute on Drug Abuse, Intramural Research Program, National Institutes of Health, 5500 Nathan Shock Dr., Baltimore, MD 21224. E-mail:mgasior{at}intra.nida.nih.gov
-
↵1 Preliminary findings of this study were presented at the Experimental Biology 1999 Meeting, Washington, D.C., April 17–21, 1999 [Gasior M, Ungard JT and Witkin JW (1999) Evaluation of novel anticonvulsants as potential blockers of cocaine-induced convulsions.FASEB J A475].
-
↵2 A Visiting Fellow in the National Institutes of Health granted from the Fogarty International Center, Bethesda, MD. Permanent affiliation: Department of Pharmacology, Medical University School, Lublin, Poland.
- Abbreviations:
- GABA
- γ-aminobutyric acid
- NMDA
- N-methyl-d-aspartate
- PI
- protective index
- Received February 9, 1999.
- Accepted April 29, 1999.
- U.S. Government