Abstract
The purposes of this study were to characterize the subjective, psychomotor and physiological effects of pentazocine in non–drug-abusing volunteers and to compare and contrast the effects of pentazocine with those of morphine. Sixteen subjects without histories of opiate dependence were injected in an upper extremity vein with 0, 7.5, 15 or 30 mg/70 kg pentazocine or 10 mg/70 kg morphine, using a randomized, double-blind, crossover design. Pentazocine increased scores on the pentobarbital-chlorpromazine-alcohol group and lysergic acid diethylamide scales and decreased scores on the benzedrine group scale of the Addiction Research Center Inventory, increased adjective checklist ratings of “nodding,” “sweating” and “turning of stomach” and increased visual analog scale ratings of “difficulty concentrating,” “drunk” and “having unpleasant bodily sensations.” Pentazocine (30 mg) had a greater propensity to increase ratings associated with dysphoria than did 10 mg of morphine. Pentazocine produced impairment on four measures of psychomotor performance. Ten milligrams of morphine produced minimal psychomotor impairment. Both pentazocine and morphine induced miosis, but 10 mg of morphine had a greater magnitude of effect than 30 mg of pentazocine. The results of the present study demonstrate that 7.5 to 30 mg of pentazocine had orderly, dose-related effects on subjective, psychomotor and physiological variables. Further, a clinically relevant dose of pentazocine, 30 mg, produced a greater magnitude of dysphoric subjective effects than did 10 mg of morphine, which is consistent with the literature reporting that pentazocine has a greater likelihood of inducing psychotomimesis than do other opioids.
Pentazocine is an opioid that falls in the class of the mixed agonist-antagonists (or “mixed-action” opioids), of which nalbuphine and butorphanol are also members. Pentazocine is a benzomorphan derivative that has agonist effects at the kappa receptor and is either a partial agonist or a weak antagonist at the mu receptor (Reisine and Pasternak, 1996). Clinically, pentazocine is used for the treatment of moderate to severe pain. The psychopharmacology of this drug has been well characterized both in nonhumans and in opioid abusers, but less so in non–drug-abusing humans.
Pentazocine functions as a reinforcer in primates with a history of codeine or cocaine self-administration (e.g., Hoffmeister and Schlichting, 1972) and in rats with a history of morphine self-administration (Steinfels et al., 1982). The DS profile of pentazocine in nonhumans is complex and appears to depend on such factors as whether the drug serves as the training drug or the test drug, training drug dose and the species tested. In rats, pentazocine shares DS effects with lower doses of morphine and cyclazocine (Shannon and Holtzman, 1979) but not with higher doses (e.g.,Hirschhorn, 1977; Overton and Batta, 1979). It also appears that there may be asymmetrical generalization, morphine and cyclazocine being less likely to substitute for pentazocine when pentazocine is the training compound (Hirschhorn, 1977; Overton and Batta, 1979). In primates, although pentazocine does not substitute for morphine or cyclazocine (Schaefer and Holtzman, 1978, 1981), morphine and cyclazocine substitute for the pentazocine cue (White and Holtzman, 1982). The extent to which pentazocine shares DS effects with other mixed agonist-antagonists is equivocal in that incomplete substitution occurs among pentazocine, nalbuphine and butorphanol (White and Holtzman, 1982).
In opioid abusers, the reinforcing effects of pentazocine appear to be weak (Fraser and Rosenberg, 1964; Schuster et al., 1971). For example, in an inpatient study, non-dependent opioid abusers were given i.v. test doses of pentazocine (35 mg/70 kg) and morphine (30 mg/70 kg) during separate trials and then were given the option of enrolling in a 7-day study in which they would receive the same test drugs three times daily (Fraser and Rosenberg, 1964). All five subjects chose to enroll in the chronic morphine study, whereas only one enrolled in the pentazocine study (and he withdrew from the study after 2 days).
The subjective effects of pentazocine in opioid abusers have been examined in a number of studies (Fraser and Rosenberg, 1964; Jasinskiet al., 1970; Preston et al., 1987, 1989, 1992). In most of the studies, the SDQ or variants of it have been used, along with the 49-item ARCI, and morphine and hydromorphone have been included in the assay as comparator drugs. With low to moderate doses (7.5–45 mg), pentazocine administered parenterally produced increases in the MBG scale and increased ratings of “high,” “liking” and “good effects” on the SDQ. Subjects tended to identify pentazocine as “dope.” In short, the subjective effects profile resembled that of morphine. At higher doses (60 and 90 mg), however, additional effects were reported, including increases in the PCAG and LSD scales of the ARCI, decreases in scores on the MBG scale and increased ratings of “drunken,” “nervous” and “bad effects” on the SDQ (Jasinski et al., 1970; Preston et al., 1987). However, it should be noted that subjects still tended to report liking the drug effects. In addition, higher doses of pentazocine were not identified as “dope” but tended to be identified as other drugs (e.g., barbiturates) (Fraser and Rosenberg, 1964; Jasinskiet al., 1970; Preston et al., 1989). In the study that assessed the effects of 60 mg of pentazocine in opioid abusers (Jasinski et al., 1970), 10 mg/70 kg of nalorphine, a mixed agonist-antagonist, was also studied, and these compounds produced similar spectra of subjective effects. Thus, at doses that are currently recommended for postoperative pain relief (Physicians’ Desk Reference, 1996), pentazocine has morphine-like subjective effects, but it has at supratherapeutic doses has nalorphine-like effects. In addition, Jasinski et al., (1970) noted psychotomimetic effects in subjects who had received 120 to 140 mg/70 kg (s.c.) of pentazocine.
Pentazocine has been examined in several drug discrimination studies using nondependent opioid-abusing volunteers. In a two-choice discrimination, pentazocine engendered hydromorphone-appropriate responding in opioid abusers (Preston et al., 1992). In a three-choice discrimination (hydromorphone, pentazocine and saline), butorphanol substituted for the pentazocine cue, but nalbuphine did not completely substitute for either pentazocine or hydromorphone (Prestonet al., 1989). In a more recent three-choice discrimination (hydromorphone, butorphanol and saline), pentazocine did not substitute for either training drug (Preston and Bigelow, 1994). The fact that butorphanol, nalbuphine and pentazocine, three mixed agonist-antagonists, do not consistently substitute for each other indicates the heterogeneity of these compounds and is concordant with some of the nonhuman drug discrimination results (White and Holtzman, 1982).
Non-drug abusers have been studied for their subjective responses to pentazocine; these people included both normal volunteers (Bellville and Green, 1965; Berkowitz et al., 1969; Belleville et al., 1979; Stacher et al., 1982, 1983; Saarialho-Kereet al., 1986; Boccuni et al., 1987; Bradley and Nicholson, 1987; Manner et al., 1987; Saarialho-Kereet al., 1988) and patients receiving the drug for pain relief (Keats and Telford, 1964; Kantor et al., 1966;Hamilton et al., 1967; Berkowitz et al., 1969;Boccuni et al., 1987). In most of the normal volunteer studies, variants of the VAS were used, although the descriptor adjectives varied from study to study. In the patient studies, patients either reported on their own or were asked about side effects they were experiencing from pentazocine. In the normal volunteer studies, there was considerable intrastudy variability on the ratings of euphoria, sedation, energy level and dysphoria. For example, some studies reported increases in euphoria or energy level (Saarialho-Kere et al., 1986; Manner et al., 1987), some studies reported dysphoria or decreases in energy level (Belleville et al., 1979; Stacher et al., 1982) and one study reported a lack of subjective effects from pentazocine (Bradley and Nicholson, 1987). Whether this variability was due to different routes of administration, different doses or different mood inventories is difficult to ascertain. Two studies reported instances of psychotomimetic effects: in one of the studies (N = 24), an i.v. infusion of 28 mg/70 kg over a 1-h period was used, and three subjects reported hallucinations (Stacher et al., 1982); in the other study (N = 24), a 100-mg p.o. dose of pentazocine resulted in extreme dysphoria in one subject (Stacher et al., 1983). In another study, anxiety was more frequently reported after 40 mg (i.m.) of pentazocine than after lower doses of pentazocine and 5 and 10 mg of morphine (Bellville and Green, 1965). In the patient studies, drowsiness was a frequently cited side effect, and dizziness and lightheadedness were also reported. Psychotomimetic effects were reported in some patients, its prevalence being greater with higher doses (Keats and Telford, 1964; Kantor et al., 1966;Hamilton et al., 1967; Berkowitz et al., 1969). One study compared the subjective effects of 15 mg of i.v. pentazocine in normal volunteers and patients suffering from migraine headaches; both groups of subjects reported lightheadedness, weakness and drowsiness, but only the migraine patients reported psychotomimetic effects (Bocccuni et al., 1987).
The effects of pentazocine on psychomotor performance have been examined in both opioid abusers and non-drug abusers. Parenterally administered pentazocine impaired DSST performance in opioid abusers in one study that tested up to 90 mg (i.m.) (Preston et al., 1987) but not in three other studies that tested lower doses (45–60 mg i.m.) (Preston et al., 1989, 1992; Preston and Bigelow, 1994). In non-drug abusers, there tends to be no or little impairment from p.o. pentazocine doses up to 75 mg (Saarialho-Kere et al., 1986; Bradley and Nicholson, 1987); in contrast, all of the studies that examined performance after parenteral administration of pentazocine (dose range: 21–45 mg) noted impairment, including performance decrements on such tests as the DSST, eye-hand coordination and choice reaction time (Belleville et al., 1979; Stacheret al., 1982; Manner et al., 1987; Saarialho-Kereet al., 1988).
The psychopharmacology of pentazocine has been extensively characterized in opioid abusers by testing a range of doses and using well-accepted subjective effects inventories sensitive to opioid effects. In addition, pentazocine has typically been compared to a fullmu agonist to determine whether qualitative or quantitative differences exist between drugs that have differential efficacies at the mu and kappa receptors. This sort of rigorous testing has been lacking in testing nonanalgesic pharmacodynamics of pentazocine in non-drug abusers. The present study is part of a series of studies (e.g., Zacny et al., 1994, 1997), in which we have employed an abuse liability testing methodology predominantly used to characterize opioid effects in abusers. The methodology includes testing a range of doses of the study drug, using a full mu agonist as a comparator drug and using well-established mood and psychomotor tests sensitive to opioid effects. In addition, because of recent studies demonstrating gender differences in analgesic effects of mixed-action opioids (e.g., Gear et al., 1996ab), including pentazocine, we used an equal number of females and males to determine whether gender modulated the nonanalgesic pharmacodynamics of pentazocine.
Materials and Methods
Subjects
Candidates were recruited via posters and local newspaper advertisements. Potential subjects who consumed, on average, at least one alcoholic drink per week and were between the ages of 21 and 39 were scheduled for a screening interview with one of our trained research personnel. During the interview, candidates completed the SCL-90, a questionnaire designed to assess psychiatric symptomatology (Derogatis et al., 1973), the Michigan Alcoholism Screening Test (Selzer, 1971) and a health questionnaire designed to determine their psychiatric and mental status. Candidates with any psychiatric problems, including drug- or alcohol-related problems or Diagnostic and Statistical Manual of Mental Disorders-IV Axis I psychiatric disorders (American Psychiatric Association, 1994), were excluded on the basis of a structured psychiatric interview.
Potential subjects participated in an orientation session before the start of the study. Before onset of the orientation session, subjects signed a written consent form that described the study in detail. In the consent form, subjects were told that the i.v. drugs to be used in the study were drugs commonly used in medical settings and that they might come from one of six classes: sedative, stimulant, opiate, general anesthetic (at subanesthetic doses), alcohol or placebo. Because of the psychotomimetic potential of pentazocine, even at clinical doses ( Physicians’ Desk Reference, 1996), we forewarned subjects of the possibility of this event by including the following statements, in bold print, in the consent form: “Hallucinations (usually visual), disorientation, confusion, and weird or uncontrollable thoughts may also occur with drugs that may be tested in this study. The risk has been estimated from clinical studies to be 10%. These effects subside within several hours, and no long-term adverse effects have been reported in the scientific literature.” Subjects as part of the screening process received a resting state electrocardiogram, and a physician performed an examination and obtained a medical history. Any participants who had experienced any adverse reactions to general anesthetics or pulmonary, renal, hepatic or cardiac problems were excluded from the study. Subjects were required to give a urine sample, upon which we performed the Cloned Enzyme Donor Immunoassay Technique (Boehringer Mannheim Corp.) toxicology screening for acetaminophen, alcohol, amphetamines, barbiturates, benzodiazepines, cocaine metabolites, opiates, phencyclidine and salicylate. None of the subjects tested positive for any of the above drugs or metabolites. Mood and psychomotor tests were practiced by volunteers during the orientation in order to acclimate them to the tests and to avoid any practice effects on psychomotor testing during experimental sessions. Payment for the study was made at the debriefing held once the study was completed. The study was approved by the local Institutional Review Board.
Sixteen healthy volunteers, eight male and eight female, participated (mean age ± S.D. for males and females: 28.3 ± 5.7 and 24.6 ± 3.3; two-group t test, N.S.). No attempt was made to determine the phase of the menstrual cycle that female participants were in during the five-session study. All volunteers had some prior use of recreational drugs, but none had past histories indicative of dependence. Males’ and females’ self-reported number of alcohol drinks consumed per week (over the last 30 days), respectively, were 2.5 ± 1.2 and 4.2 ± 3.0 (two-group t test, N.S.). Two males and two females reported smoking tobacco cigarettes (<5/day). Three males and one female reported smoking marijuana in the past 30 days, and one of the males also reported using psilocibin mushrooms in this same time period. Regarding lifetime nonmedical drug use, four volunteers (two females) reported use of nitrous oxide, six volunteers (three females) reported use of cocaine, three volunteers (two females) reported use of benzodiazepines, seven volunteers (three females) reported use of hallucinogens (LSD, phencyclidine, mushrooms) and 13 volunteers (six females) reported use of cannabinoids. With the exception of cannabinoids, lifetime drug use of any one of these drugs was less than 50 times. Five males and six females reported having been prescribed opiates (reported as cocaine, acetaminophen with codeine, meperidine, acetaminophen with oxycodone, acetaminophen with propoxyphene or “prescribed painkillers”) in the past for pain relief. One of the six females also reported recreational use of opium. In all cases, use of any one opiate was reported as less than 50 times, and no subject reported using more than two opiates.
Procedure
Experimental design.
A placebo-controlled, double-blind, incomplete Latin square, crossover trial was conducted. Subjects participated in five sessions spaced at least 1 week apart. Sessions were approximately 360 min in duration. Subjects were injected in an upper-extremity vein with saline, 7.5, 15 or 30 mg/70 kg pentazocine or 10 mg/70 kg morphine over a 30-s interval. The drug was always delivered in a volume of 5 ml containing drug and/or saline. We chose not to test doses of pentazocine higher than 30 mg/70 kg because of clinical recommendations not to exceed acute i.v. injections of 30 mg ( Physicians’ Desk Reference, 1996). We chose to study 10 mg of morphine and to compare its effects to 30 mg of pentazocine because we have included this dose of morphine in our other opioid characterization studies, and because we wanted to compare two doses that are equieffective on analgesia. As to whether the two doses are equianalgesic is admittedly debatable: the morphine/pentazocine potency ratio in clinical studies (using analgesia as an endpoint) has been reported as ranging from 2:1 to 6:1 (Berkowitz et al., 1969;Brogden et al., 1973). Perhaps for this reason, in one classic pharmacology textbook, a dose range of 30 to 60 mg of pentazocine is cited as being equianalgesic to 10 mg of morphine (Reisine and Pasternak, 1996).
Experimental sessions.
The experiment took place in a departmental clinical laboratory. Subjects were instructed not to eat food or drink any nonclear liquids for 4 h, not to drink clear liquids for 2 h and not to use any drugs (including alcohol, but excluding normal amounts of caffeine and nicotine) 24 h before sessions. A toxicology screening was required before the start of each session for all participants, as was a pregnancy test for all female participants. Subjects were also given a breath-alcohol test to ensure that they had no alcohol in their system. An angiocatheter was inserted into one of the subject’s upper-extremity veins by an anesthetist, using proper sterile technique. Subjects then completed several subjective effects forms and psychomotor tests, and their respiratory rate, HR, noninvasive arterial oxygen saturation and blood pressure were monitored. Subjects then reclined in a semirecumbent position on a bed, and an anesthetist injected into the angiocatheter morphine, pentazocine or saline over 30 s. Before the injection the subject was told, “The injection you are about to receive may or may not contain a drug.” The drug had been previously drawn up by one anesthetist and was administered by another in order to preserve the double-blind nature of the study. However, the injecting anesthetist was aware of the drugs involved, so that if an adverse event occurred, appropriate measures could be taken to ensure the safety and well-being of the subject. The anesthetist remained in the laboratory for at least 15 min after the injection and was available to come back to the laboratory during the remainder of the session should an emergency arise. At periodic intervals after the injection (see below), the mood, psychomotor performance and physiological status of the subject were assessed. Drinking water was permitted 90 min after the injection, but eating was not allowed during the session. A snack was served to the subject after the session was completed. When not participating in tests, subjects were free to engage in sedentary recreational activities such as reading, listening to music, and watching TV, but studying was not permitted. Social interaction was possible in the study (for instance, the subject could converse with the research technician), but subjects generally engaged in solitary activities during sessions. After completion of sessions, subjects were transported home via a livery service with instructions not to engage in certain activities (such as cooking with a stove, driving an automobile, caring for children and drinking alcohol) for the next 12 h.
Dependent measures.
The following tests were completed before injection and 15, 60, 120, 180, 240 and 300 min after injection. On all of these measures, subjects did not have access to information on how they responded on previous tests from the same session. When subjects performed computerized tests, they had to move from the middle of the bed to the edge of the bed, still in a semirecumbent position. When subjects performed paper-and-pencil tests, they did not have to move at all. Thus subject movement during testing and between tests was minimal in the present study. The order in which dependent measures was assessed remained invariant across subjects and sessions; physiological measures were assessed first, then subjective measures and then psychomotor measures.
Subjective measures.
- 1.
- The ARCI is a true-false questionnaire designed to differentiate among different classes of psychoactive drugs (Haertzen, 1966). We used a computerized short form of the ARCI (Martin et al., 1971) that had 49 items and yielded scores for five different scales: PCAG, sensitive to sedative effects; BG and AMP, sensitive to amphetamine-like effects; LSD, sensitive to somatic and dysphoric changes; and MBG, often described as euphoria.
- 2.
- A locally developed adjective checklist included items from an opiate adjective checklist [derived from the SDQ (Fraser et al., 1961)] and a list reported as sensitive to the somatic and subjective effects of opiates from the mu and mixed agonist-antagonist classes (Preston et al., 1989). The checklist consisted of 12 items that the subject rated on a 5-point scale from 0 (“not at all”) to 4 (“extremely”). The items were as follows: “carefree,” “drive (motivated),” “dry mouth,” “flushing,” “good mood,” “headache,” “nodding,” “numb,” “skin itchy,” “sweating,” “turning of stomach” and “vomiting.”
- 3.
- A locally developed VAS consisted of twenty-three 100-mm lines, each labeled with the adjectives “coasting (‘spaced out’),” “confused,” “difficulty concentrating,” “dizzy,” “down (depressed),” “drunk,” “elated (’very happy’),” “feel bad,” “feel good,” “floating,” “having pleasant bodily sensations,” “having pleasant thoughts,” “having unpleasant bodily sensations,” “having unpleasant thoughts,” “heavy or sluggish feeling,” “high (‘drug’ high),” “hungry,” “lightheaded,” “nauseous,” “sedated (calm, tranquil),” “sleepy (drowsy, tired),” “stimulated (energetic)” and “tingling.” Subjects on this paper-and-pencil test were instructed to place a pencil mark on each line indicating how they felt at the moment; endpoints of the line were labeled “not at all” and “extremely.” In addition to the time-points listed above, the VAS was completed at 5, 45, 90, 105, 150 and 210 min after injection.
- 4.
- The Drug Effects/Liking questionnaire assessed the extent to which subjects currently felt a drug effect, on the basis of a scale of 1 to 5 (1 = “I feel no effect from it at all”; 2 = “I think I feel a mild effect, but I’m not sure”; 3 = “I feel an effect, but it is not real strong”; 4 = “I feel a strong effect”; 5 = “I feel a very strong effect”) and assessed the extent to which subjects currently liked the drug effect on a 100-mm line (0 = dislike a lot; 50 = neutral; 100 = like a lot). In addition to the time-points listed above, the Drug Effects/Liking Questionnaire was completed at 5, 45, 90, 105, 150 and 210 min after injection.
- 5.
- Subjects were given an adjective rating checklist to take home and were asked to complete it 24 h later, noting whether they had any of the symptoms listed on the checklist (“anxious,” “coasting (spaced out),” “clumsy,” “confused,” “difficulty concentrating,” “down,” “dry mouth,” “excessive hunger,” “excessive thirst,” “feel bad,” “feel good,” “headache,” “heavy or sluggish feeling,” “lightheaded,” “nausea,” “skin itchy,” and “vomiting”) during the 24 h after the session. Each symptom on this postsession questionnaire was rated on a 5-point scale ranging from “not at all” (0) to “extremely” (4).
Psychomotor/cognitive performance.
The following six tests were chosen because we have employed these tests in our prior opioid studies and because previous studies from other laboratories have indicated that the specific parameters of psychomotor/cognitive performance that the tests are designed to measure can be affected by opioids.
- 1.
- The Maddox Wing test measures relative position of the eyes in prism diopters. Some drugs cause extraocular muscles of the eye to diverge (exophoria), and this divergence is considered to be an indicator of psychomotor impairment (Hannington-Kiff, 1970).
- 2.
- An eye-hand coordination test required the subject to track a randomly moving target (a circle) on the computer screen using a computer mouse (Nuotto and Korttila, 1991). The object of this test was to keep a small cross, which was controlled by the mouse, inside the moving-target circle at all times as the circle moved randomly around the screen. The length of the test was 1 min. The dependent measure was the number of seconds that the cross was greater than 1 cm from the center of the target circle.
- 3.
- The DSST was a 1-min paper-and-pencil test that required the participant to replace digits with corresponding symbols according to a digit-symbol code listed on the top of the paper (Wechsler, 1958). The scores were the total number of symbols drawn and the correct number of symbols drawn by the participant. Different forms of the test (i.e., different symbol-digit codes) were used each time the test was presented to the subject. The DSST evaluates changes in information-processing performance and the ability to concentrate.
- 4.
- An auditory reaction test measured the time it took for subjects to react to an auditory stimulus (Nuotto and Korttila, 1991). Ten 50-dBA computer-generated tones were delivered at random time intervals (between 1 and 10 s) in a 1-min time period. The tone remained on until subjects depressed the computer keyboard spacebar or until 2 s had elapsed, whichever occurred first. The mean reaction time (in seconds) was the dependent measure.
- 5.
- A logical reasoning test measured higher mental processes such as reasoning, logic and verbal ability. This 1-min computerized test was similar to the Logical Reasoning Test developed by Baddeley (1968)except for test duration (1 min versus 3 min) and presentation medium (computer versus paper and pencil). The logical reasoning test employed five grammatical transformations (e.g., true vs. false statements, use of the verb precedes vs. the verbfollows) on statements about the relationship between two letters, A and B, which were displayed on the screen in one of two sequences, AB or BA. Below the letter pair was one of the statements (e.g., “A is preceded by B,” “B is not followed by A”). The subject’s task was to respond “True” or “False,” depending on the veracity of the statement, by depressing the 1 or 0 key on the number pad, which corresponded to true and false, respectively. The total number of statements answered and the number of statements answered correctly were the dependent measures.
- 6.
- A locally developed memory test measured short-term and long-term memory by presenting a sequential list of 15 words on the computer. These 15 words were presented in approximately 30 s. The subject was then given 120 s to write down as many words as he or she could remember. Different word lists were used for all sessions, including the practice session. To ensure comparability of words across sessions, the 15-word lists were equated on factors such as image-evoking ability of the words, degree of meaningfulness and frequency of usage (Paivio et al., 1968). The words in the lists had ratings of imagery and concreteness of greater than 5.0 and ratings of frequency of usage greater than 20 per million (Thorndike and Lorge, 1944). The list was presented 60 min after the injection. The subjects were asked to recall the list immediately after its presentation and at 300 min after injection.
Physiological measures.
Five physiological measures were assessed: HR, blood pressure, arterial oxygen saturation, respiration rate and miosis. HR, blood pressure and arterial oxygen saturation were measured noninvasively with a Merlin Model 54 monitor (Hewlett Packard, Andover, MA). Respiration rate was the number of breaths subjects took in 30 s (multiplied by 2 to get breaths/min). This was assessed by the experimenter counting the number of times the subject’s chest or stomach rose and fell. HR, blood pressure, arterial oxygen saturation and respiratory rate were assessed at the time-points listed above. Miosis, or pupil constriction, is a physiological marker of opiate effects. It was measured by photographing the subject’s right pupil in a dimly lit room. Miosis was measured before injection and 15, 60, 120, 180 and 300 min after injection.
Data analysis.
Three sets of repeated-measures analysis of variance (ANOVA) were used for statistical treatment of the data. The first analysis examined pentazocine effects: factors were Gender, Dose (0, 7.5, 15 and 30 mg/70 kg) and Time (2–13 levels). The second analysis compared peak and/or trough effects of saline, 30 mg of pentazocine and 10 mg of morphine; factors were Gender and Drug. Only postinjection values were included in this analysis, and values were determined for each subject independent of time-point. The third set compared effects of saline, 30 mg of pentazocine and 10 mg of morphine on several of the dependent measures (i.e., variables that were graphed): factors were Gender, Drug and Time. F values were considered significant for P ≤ .05 with adjustments of within-factors degrees of freedom (Huynh-Feldt) to protect against violations of symmetry. We performed Tukey post-hoc testing on the first and third sets of ANOVAs, comparing drug responses to saline at each time-point, and on the second set of ANOVAs, comparing each of the three conditions to each of the others. Variance measures that are reported adjacent to ratings and scores represent S.E.M.
Results
Subjective Effects
ARCI
Pentazocine.
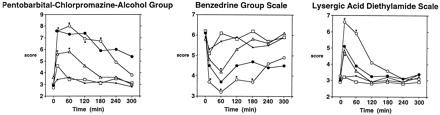
Significant Dose × Time effects were obtained on the PCAG (P < .001), BG (P < .001) and LSD (P < .001) scales. In a dose-related manner, PCAG and LSD scores increased, and BG scores decreased, after pentazocine injection (fig.1). Scores on the PCAG, BG and LSD scales reached their maximal peak (PCAG, LSD) and trough (BG) levels 15 to 60 min after injection. Duration of effect was evident for at least 3 h after the injection. For comparison purposes, figure 1 also shows scores from the 10 mg of morphine condition. PCAG and LSD scores were significantly increased, and BG scores were significantly decreased, after morphine administration. Magnitude and time course of effects were similar between morphine and 30 mg of pentazocine on the PCAG and BG scales, but LSD scores at the 15 min postinjection time-point were significantly greater in the 30 mg of pentazocine condition than in the morphine condition. There were no Gender × Drug interactions noted on any scale of the ARCI.
Time course of the effects of 0 (◊), 7.5 (□), 15 (▵) and 30 mg/70 kg (○) pentazocine on scores from the PCAG (left frame), BG (middle frame) and LSD (right frame) scales of the ARCI. For purposes of comparison with 30 mg of pentazocine, 10 mg of morphine (•) is also shown on the graph. Each pentazocine and morphine point represents the mean across 16 subjects. Time-point 0 refers to effects measured immediately before the injection. Asterisks on the graphs indicate that a pentazocine dose is significantly different from saline at a given time-point (Tukey post-hoc test; P < .05). Ranges of possible scores on the PCAG, BG, and LSD scales are 0 to 15, 0 to 9 and 0 to 14, respectively.
Peak and trough effects.
Table 1presents mean peak and trough effects of ARCI ratings that were sensitive to 30 mg of pentazocine and/or 10 mg of morphine. Significantly higher peak PCAG, AMP and LSD scores were obtained with 30 mg of pentazocine and 10 mg of morphine than with the saline condition. Further, peak LSD scores were significantly higher in the 30 mg of pentazocine condition than in the 10 mg of morphine condition. Trough BG scores in the 30 mg of pentazocine and 10 mg of morphine conditions were significantly lower than in the saline condition, but they did not differ from each other.
Mean peak or trough ratings (± S.E.M.) of ARCI scales sensitive to 30 mg of pentazocine and/or 10 mg of morphine (ratings correspond to saline, pentazocine and morphine, respectively)
Adjective Checklist
Pentazocine.
Significant increases were obtained on six adjectives from the adjective checklist: “dry mouth” (Dose × Time: P < .05), “flushing” (Dose × Time: P < .01), “nodding” (Dose × Time: P < .001), “skin itchy” (Dose: P < .05), “sweating” (Dose × Time: P < .05) and “turning of stomach (Dose × Time: P < .01). Effects were limited to the 30-mg dose and generally were present for only up to 60 min after the injection. The one exception was “nodding” ratings, which were significantly different from saline for up to 180 min after the injection of 30 mg of pentazocine. The rating, “drive (motivated),” was significantly decreased by 30 mg of pentazocine (Dose: P < .05). No Gender × Drug interactions were noted on any adjective from the checklist.
Peak and trough effects.
Table 2presents mean peak and trough effects of adjective checklist ratings that were sensitive to 30 mg of pentazocine and/or 10 mg of morphine. Pentazocine (30 mg) and morphine (10 mg) significantly increased peak ratings of “flushing” and “nodding” and decreased ratings of “drive.” Pentazocine increased peak ratings of “dry mouth,” “sweating” and “turning of stomach.” Morphine increased peak ratings of “skin itchy.”
Mean peak or trough ratings (± S.E.M.) of adjectives from the adjective rating checklist sensitive to 30 mg of pentazocine and/or 10 mg of morphine (ratings correspond to saline, pentazocine and morphine, respectively)
VAS
Pentazocine.
Significant Dose × Time effects (except where otherwise noted) were obtained on ratings of “coasting (‘spaced out’)” (P < .001), “confused” (P < .005), “difficulty concentrating” (P < .001) [fig.2, left frame], “dizzy” (P < .001), “drunk” (P < .05) [fig. 2, center frame], “feel good” (P < .001), “floating” (P < .001) [fig. 2, right frame], “having pleasant bodily sensations” (P < .01), “having unpleasant bodily sensations” (P < .05), “heavy or sluggish feeling” (P < .05), “high” (P < .001), “hungry” (Dose: P < .005), “lightheaded” (P < .001), “nauseous” (Dose: P < .05), “sedated” (Dose: P < .05), “sleepy (‘drowsy, tired’)” (P < .001) and “tingling” (P < .001). All of these VAS ratings except “feel good” and “hungry” increased after drug injection. For 11 of these 17 VAS ratings, effects significantly different from placebo occurred only with the 30-mg dose. Dose-related effects occurred with the ratings “coasting (spaced out),” “dizzy,” “floating,” “high,” “hungry” and “lightheaded.” For most of the ratings that achieved statistical significance, post-hoc testing revealed that latency to peak effects was generally 5 to 15 min and that duration of effects was from 120 to 180 min. The exceptions were as follows: ratings of “having pleasant bodily sensations” and “tingling” lasted for only 15 min. Decreased ratings of “feel good” were not apparent until 105 min after injection, but they remained at significantly lower levels for the remainder of the session. For comparison purposes, figure 2 also shows ratings from the 10 mg of morphine condition. Ratings of “difficulty concentrating” and “drunk” in the morphine condition did not differ significantly from such ratings in the saline condition. Ratings of “floating” tended to be higher for the first hour after injection in the 30 mg of pentazocine condition than in the morphine condition, but the differences were not statistically significant. No Gender × Drug interactions were noted on any of the VAS scales.
Time course of the effects of 0 (◊), 7.5 (□), 15 (▵) and 30 mg/70 kg (○) pentazocine on “difficulty concentrating” (left frame), “drunk” (center frame) and “floating” (right frame) ratings from the VAS (range: 0–100 mm). For purposes of comparison with 30 mg of pentazocine, 10 mg of morphine (•) is also shown on the graph. Each pentazocine and morphine point represents the mean across 16 subjects. Time-point 0 refers to effects measured immediately before the injection. Asterisks on the graphs indicate that a pentazocine dose is significantly different from saline at a given time-point (Tukey post-hoc) test; P < .05).
Peak and trough effects.
Table 3presents mean peak and trough effects of VAS ratings that were sensitive to 30 mg of pentazocine and/or 10 mg of morphine. Significantly higher peak “coasting (spaced out),” “confused,” “difficulty concentrating,” “dizzy,” “floating,” “heavy or sluggish feeling,” “high,” “lightheaded,” “nauseous,” “sedated,” “sleepy” and “tingling” ratings were obtained with doses of pentazocine (30 mg) and morphine (10 mg) when compared with the saline condition. Further, peak “difficulty concentrating” and “floating” ratings were significantly higher in the 30 mg of pentazocine condition than in the 10 mg of morphine condition. Pentazocine (30 mg), but not morphine (10 mg), significantly increased peak ratings of “drunk,” “feel bad,” “having pleasant bodily sensations” and “having unpleasant bodily sensations,” relative to saline. Trough ratings of “hungry” were significantly lower in the 30 mg of pentazocine condition than in the saline condition.
Mean peak and trough ratings (± S.E.M.) of VAS adjectives sensitive to 30 mg of pentazocine and/or 10 mg of morphine (ratings correspond to saline, pentazocine and morphine, respectively)
Drug Effects and Drug Liking
Pentazocine.
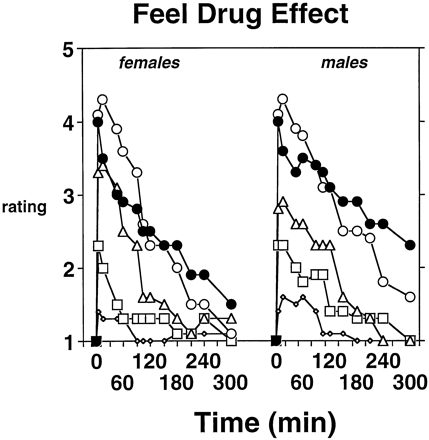
Significant Dose × Time increases were obtained on the “Feel Drug Effect” question (P < .001). Gender did not play a modulatory role on this measure. This lack of a gender effect is apparent in figure 3, which shows that pentazocine effects were dose-related and approximately of the same magnitude and duration in both males and females. There did appear to be a slightly longer duration of action with the 30 mg of pentazocine dose in males than in females, but the differences were relatively small and not statistically significant. “Like Drug Effect” ratings approached significance (Dose × Time: P = 0.06); visual inspection of the data indicated that the largest change from baseline in the four drug conditions was in the 30 mg of pentazocine condition, in which liking ratings increased by 12 mm from baseline (48.3 mm) to 5 min after injection (60.5 mm).
Time course of the effects of 0 (◊), 7.5 (□), 15 (▵) and 30 mg/70 kg (○) pentazocine on the “Feel Drug Effect” question from the Drug Effects/Liking questionnaire in females and males. For purposes of comparison with 30 mg of pentazocine, 10 mg of morphine (•) is also shown on the graph. Each pentazocine and morphine point represents the mean across eight subjects. Time point 0 refers to effects measured immediately before the injection. For the sake of clarity, asterisks indicating which pentazocine doses are different from saline at which time-points are omitted. The 30 mg of pentazocine dose was significantly different from saline for up to 180 min and 210 min after injection, respectively, in females and males.
For comparison purposes figure 3 also shows scores from the 10 mg of morphine condition. For both females and males, the morphine dose produced a slightly lower magnitude of effect than the 30 mg of pentazocine dose early in the session. However, with both sexes, the duration of effect tended to be longer with 10 mg of morphine. Males showed a slightly higher magnitude of effect with morphine at the end of the session, but differences between the sexes did not achieve statistical significance at any postinjection time-point.
Peak and trough effects.
Significantly higher peak “feel drug effect” ratings were obtained with 30 mg of pentazocine (4.3 ± 0.1) and 10 mg of morphine (4.0 ± 0.2), relative to the saline condition (1.6 ± 0.2). Peak drug effect ratings in the 30 mg of pentazocine condition and the 10 mg of morphine condition did not differ significantly from each other. Because of the bipolar nature of the drug liking question (i.e., 50 = neutral and 0 and 100 are representative of extreme dislike and extreme liking, respectively), separate peak and trough effect analyses were performed on this measure. Peak liking ratings were significantly higher in the 30 mg of pentazocine condition (70.5 ± 3.5) than in the saline condition (53.1 ± 1.7). However, trough liking ratings were also significantly lower in the 30 mg of pentazocine condition (31.8 ± 4.2) than in the saline condition (43.4 ± 1.7). Peak and trough morphine ratings did not differ significantly from those of saline.
Postsession Questionnaire
Pentazocine.
On the questionnaire that assessed residual effects of the drug, significant Dose effects were obtained with pentazocine on four ratings: “feel good” (P < .05), “heavy or sluggish feeling” (P < .005), “nausea” (P < .01) and “lightheaded” (P < .01). With the latter three ratings, only the 30-mg pentazocine dose differed from base line. On “feel good” ratings, post-hoc testing revealed no differences among the three active drug conditions and the saline condition. A Gender × Dose effect (P < .05) was obtained with the rating “confused,” and post-hoc testing revealed that females, but not males, reported higher ratings in the 30 mg of pentazocine condition relative to the saline condition.
Pentazocine vs. morphine.
Significant Drug effects were obtained on ratings of “heavy or sluggish feeling” (P < .05) and “lightheaded” (P < .05), and morphine manifested higher ratings than saline. A Gender × Drug effect (P < .05) was obtained with the rating “anxious,” butpost-hoc testing revealed no significant differences, with either males or females, among the three conditions.
Psychomotor Performance
Pentazocine.
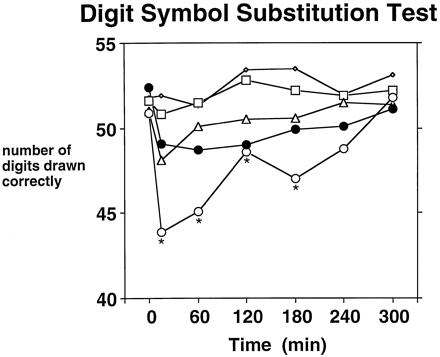
Pentazocine impaired performance on the Maddox Wing test (Dose × Time: P < .001), DSST (number completed, Dose × Time: P < .001; number correct, Dose × Time: P < .005 [fig. 4]), logical reasoning test (number completed, Dose × Time: P < .05) and memory test (Dose: P < .01). Impairment was generally limited to the 30-mg dose and occurred from 15 to 180 min after injection. For comparison purposes, figure 4 shows DSST performance in the morphine condition. Post-hoc testing revealed that morphine impaired DSST performance at the 120-min postinjection time-point. On the memory test, immediate as well as delayed free recall was impaired by pentazocine; an average of two fewer words was recalled in the 30 mg of pentazocine condition (6.1 ± 0.5) than in the saline condition (8.2 ± 0.5). There was a Gender × Dose effect (P < .05) on the eye-hand coordination test: females, but not males, performed significantly worse in the 30-mg condition, relative to the saline condition. Finally, there were Gender effects on the DSST (number correct and number completed) (P < .05) and on the logical reasoning test (number correct and number completed) (P < .05), females performing better than males.
Time course of the effects of 0 (◊), 7.5 (□), 15 (▵) and 30 mg/70 kg (○) pentazocine on number of symbols correctly drawn on the DSST. For purposes of comparison with 30 mg of pentazocine, 10 mg of morphine (•) is also shown on the graph. Each pentazocine and morphine point represents the mean across 16 subjects. Time point 0 refers to effects measured immediately before the injection. Asterisks indicate that a pentazocine dose is significantly different from saline at a given time-point (Tukey post-hoctest; P < .05).
Peak and trough effects.
Pentazocine (30 mg) had significantly greater trough effects on the DSST (number completed [p < 0.01] and number correct [p < 0.005]) when compared to the saline condition. The effects of 10 mg morphine were not significantly different from saline on these measures. Both pentazocine and morphine induced a greater degree of exophoria on the Maddox Wing test than did saline (p < 0.001), and the two active drug conditions did not differ from each other. A significant Dose (p < 0.01), but not Dose × Time, effect was obtained on the memory test; post hoc testing revealed significantly poorer performance in the 30 mg pentazocine condition, relative to saline.
Physiological Effects
Pentazocine.
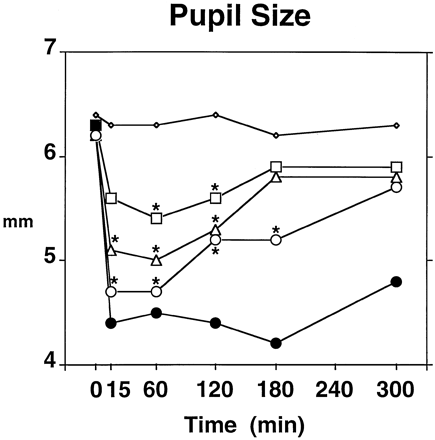
Significant effects were obtained on systolic blood pressure (Dose × Time: P < .05), diastolic blood pressure (Dose × Time: P < .05), arterial oxygen saturation (Dose: P < .01), respiration rate (Dose: P < .05) and miosis (Dose × Time: P < .001). Post-hoc testing revealed that systolic blood pressure was significantly elevated at the 5-min postinjection time-point in the 30 mg of pentazocine condition, relative to the saline condition (126.1 mm Hg vs. 115.6 mm Hg, respectively). Arterial oxygen saturation rate was significantly lower in the 15 (98.5%) and 30 mg (98.5%) pentazocine conditions, relative to the saline condition (98.9%), but the differences were not clinically significant. Post-hoc testing revealed no significant differences between saline and any of the pentazocine doses on diastolic blood pressure or respiration rate. Pentazocine decreased pupil size in a dose-related fashion (fig.5). The miotic response to morphine is also shown in figure 5: post-hoc testing revealed that morphine induced miosis during the entire postinjection period and induced a greater degree of miosis than did 30 mg of pentazocine at the 120- and 180-min postinjection time-points. There were Gender effects in systolic blood pressure (females 109.8 mmHg, males 124.9 mmHg; P < .005), arterial oxygen saturation rate (females 99.0%, males 98.2%; P < .005) and respiration rate (females 15.3 breaths/min, males 12.7 breaths/min; p < 0.05).
Time course of the effects of 0 (◊), 7.5 (□), 15 (▵) and 30 mg/70 kg (○) pentazocine on pupil size of the right eye. For purposes of comparison with 30 mg of pentazocine, 10 mg of morphine (•) is also shown on the graph. Because of technical difficulties in obtaining pupil photographs on some of the sessions in two subjects, each pentazocine point represents the mean across 15 subjects, and each morphine point represents the mean across 14 subjects. Time point 0 refers to effects measured immediately before the injection. Asterisks indicate that a pentazocine dose is significantly different from saline at a given time-point (Tukey post-hoc test; P < .05).
Peak and trough effects.
No significant differences in peak effects were obtained on systolic or diastolic blood pressure. Trough arterial oxygen saturation rates achieved statistical significance (P < .05), but post-hoc testing revealed no differences among the three drug conditions. Trough miosis values were significantly lower in the two active drug conditions than in the saline condition (30 mg of pentazocine, 4.6 mm; 10 mg morphine, 4.0 mm; saline, 5.8 mm), and further, trough miosis values were significantly lower in the morphine condition than in the pentazocine condition. There was a Gender × Drug effect on trough respiration rate: females showed lower trough respiration rates in the morphine condition (10.8 breaths/min), relative to saline (13.2 breaths/min), whereas males did not (morphine, 10.7 breaths/min; saline, 10 breaths/min). Neither females nor males showed lower trough respiration values in the 30 mg of pentazocine condition, relative to the saline condition.
Adverse Effects
Four subjects vomited during or after one or more of the sessions. All four subjects vomited during or after the morphine session. One and three subjects, respectively, vomited during or after the 15 and 30 mg of pentazocine sessions. One subject reported auditory and visual disturbances after the session in which she had received 15 mg of pentazocine and reported visual disturbances during the session in which she received 30 mg of pentazocine. These effects were less than 24 h in duration, and the subject, when contacted about a month after completion of the study, reported no further psychotomimetic effects during the 30 days after completion of the study.
Discussion
Pentazocine produced orderly, dose-related changes in subjective, psychomotor, and physiological effects. In many cases, though, only the 30-mg dose differed significantly from saline. When 30 mg of pentazocine was compared with morphine, a number of similarities and differences emerged. In terms of similarities, the doses produced a similar magnitude of effect on many subjective effects variables, as measured by the ARCI, VAS and opiate adjective checklist. In terms of differences, 30 mg of pentazocine produced a profile of subjective effects that was more dysphoric than that of morphine and, unlike morphine, impaired psychomotor performance. Peak and trough effects indicative of greater dysphoria from 30 mg of pentazocine relative to 10 mg of morphine included ARCI LSD scores and VAS ratings of “difficulty concentrating,” “like drug effects,” “drunk,” “having unpleasant bodily sensations” and “feel bad” and the adjective checklist ratings of “sweating” and “turning of stomach.”
Our results can be compared with studies that have assessed the effects of pentazocine in opioid abusers because of the similarity in methodologies used (e.g., ARCI). In opioid abusers, lower doses of pentazocine (e.g., 30–45 mg) increased MBG scores and drug liking and did not cause psychomotor impairment (Jasinskiet al., 1970; Preston et al., 1989, 1992, 1994). In the present study, no such increase in MBG scores was found with any dose of pentazocine tested, and subjects did not report liking the drug effects. In addition, the highest dose of pentazocine tested impaired performance on the DSST and memory and logical reasoning tests. In opioid abusers, higher doses of pentazocine (e.g., 60–90 mg) increased PCAG and LSD scores, increased “drunken,” “nervous” and “bad effects” responses on the SDQ and impaired DSST performance (Jasinski et al., 1970; Preston et al., 1987). These effects were found in our non–opioid-abusing volunteers, but at lower doses. It appears, then, that there is a difference in populations in sensitivity to the dysphoric effects of pentazocine, non-drug abusers being more sensitive to lower doses.
A number of studies have examined subjective effects of pentazocine in non–drug-abusing volunteers. One study compared and contrasted subjective effects of i.m. morphine (5 and 10 mg) and i.m. pentazocine (10, 20 and 40 mg), using a signs and symptoms checklist (Bellville and Green, 1965). There were a number of similarities between the two drugs (e.g., increases in “sleepy,” “lightheaded” and “weak”), but there were also some differences. With increasing doses of pentazocine, but not with morphine, subjects reported more shakiness, less happiness and more anxiety. These dysphoric symptoms, along with symptoms of pentazocine that did not differ from those of morphine, are largely consistent with the pattern of subjective effects of morphine and pentazocine in our study. Other studies, too, have reported dysphoric effects of pentazocine in normal volunteers (Belleville et al., 1979;Stacher et al., 1983). However, another study examined subjective effects of 21 and 42 mg/70 kg (i.v.) pentazocine and found increases in VAS ratings of euphoria for up to 20 min after injection and no changes in VAS ratings of dysphoria over the 3-h session (Manneret al., 1987). Two other volunteer studies examining p.o. and injected pentazocine have also found positive subjective effects with pentazocine (Saarialho-Kere et al., 1986, 1988). It is unclear what could account for the marked differences in subjective effects between the studies with normal volunteers, although such variables as sample size (some studies used as few as six subjects), subject’s past history of drug use (not reported in other studies), type of subjective effects testing methodology and dose and route of pentazocine administration may serve as factors for the interstudy differences.
Psychotomimesis is a term that encompasses a number of symptoms associated with unpleasantness and fear in most people: derealization, depersonalization, visual and auditory disturbances (including hallucinations) and uncontrollable and/or unpleasant thoughts. It is reported that even at clinically used doses of pentazocine, 10% of patients experience one or more of these effects (Wood et al., 1974). Because this risk of psychotomimesis is relatively high compared with other marketed opioids, including other mixed agonist-antagonists, we included a warning of risk of psychotomimetic effects in the consent form. Indeed, one of our subjects did report unpleasant auditory and visual disturbances of a transient nature. It is probable that if we had tested higher doses of pentazocine, such as 60 mg, the incidence of psychotomimesis would have been higher. Our findings that pentazocine had a constellation of subjective effects that included dysphoria (e.g., increased scores on the LSD scale of the ARCI, increased VAS ratings of “having unpleasant bodily sensations”) is consistent with the clinical studies that have found dysphoric and psychotomimetic effects with this drug (cf.Brogden et al., 1973).
In the present study, pentazocine impaired psychomotor and cognitive performance, which is in accordance with other normal volunteer studies (e.g., Stacher et al., 1982; Saarialho-Kereet al., 1988). The minimal effect of morphine on psychomotor performance has also been found in a number of other studies (e.g., Zacny et al., 1997). Another difference between pentazocine and morphine in the present study was that only pentazocine induced sweating; several clinical studies have noted a higher incidence of sweating with pentazocine than with morphine (Forrest et al., 1969; Paddock et al., 1969). Thirty milligrams of pentazocine induced a lesser degree, and shorter duration, of miosis than did 10 mg of morphine; a similar finding was obtained in a patient study in which a 40-mg infusion of pentazocine induced a greater degree of miosis than did a 15-mg infusion of morphine (Barker et al., 1972).
As part of our series of opioid drug characterizations, we have examined the effects of two other mixed-action opioids: butorphanol (Zacny et al., 1994) and nalbuphine (Zacny et al., 1997). Butorphanol appears to be more sedating than pentazocine and nalbuphine, as measured by PCAG scores and “sleepy” ratings, and to impair psychomotor performance to a greater extent, as measured by the DSST. Of these three mixed-action opioids, nalbuphine appears to resemble morphine the most in terms of its subjective effects profile. Pentazocine has a profile of subjective effects that tends to be more dysphoric in nature than those of the other two opioids. Pentazocine, unlike the other mixed-action opioids, increased systolic blood pressure, a result that is consistent with clinical studies showing pentazocine to be the only opioid among the three that reliably increases blood pressure (cf.Bailey and Stanley, 1994). Our series of mixed-action opioid studies provides results that appear to be consistent with both nonhuman and human studies that have detected differential DS effects of the three opioids (White and Holtzman, 1982; Preston et al., 1989; Preston and Bigelow, 1994) and with clinical studies that have noted differences in incidences of side effects of the opioids (Reisine and Pasternak, 1996).
We included equal numbers of males and females in the present study to determine whether gender played a modulatory role in the nonanalgesic pharmacodynamics of pentazocine. This rationale was based on several laboratory and clinical studies in which the analgesic effects of pentazocine, butorphanol and nalbuphine were of greater magnitude (in terms of either peak effect or duration) in females (Gear et al., 1996ab). We found little evidence of gender playing a modulatory role in the present study (see fig. 3). The apparent lack of a gender effect should be interpreted with some caution, because we did not analyze pentazocine effects as a function of menstrual cycle, which has been shown to influence drug effects, and because of the small sample sizes employed in the study. One gender effect that was found was with trough respiration rate: females showed respiratory depression with morphine, but males did not. Because drug dose was adjusted for body weight, the heavier weights of males in the present study cannot account for this gender difference. In a previous study conducted with butorphanol (Zacny et al., 1994), morphine was also used as a comparator drug, and there were seven male and five female participants. A retrospective analysis on trough respiration rate in that study revealed that there was a marginal Drug effect with no apparent differences between males and females on morphine-induced respiratory depression. This retrospective analysis, combined with the apparent lack of any literature reporting gender effects on morphine-induced respiratory depression, suggests that the gender differences in the present study were a chance occurrence. It would certainly be of clinical interest to replicate the present study systematically, using both analgesic and nonanalgesic variables to determine whether gender has a selective effect on pentazocine-induced analgesia.
Acknowledgments
We thank Dr. Jerome M. Klafta, Dr. Christopher J. Young, Dr. P. Allan Klock, Mary Maurer, C.R.N.A., Nada Williamson, C.R.N.A., and Robert Shaughnessy, C.R.N.A., for their assistance in administering the drugs and monitoring the physiological status of the subjects. We also thank Karin Kirulis for screening potential subjects and conducting the structured interviews.
Footnotes
-
Send reprint requests to: James P. Zacny, Department of Anesthesia and Critical Care/MC4028, University of Chicago, 5841 S. Maryland Avenue, Chicago, IL 60637.
-
↵1 This research was supported in part by Grant DA-08573 from the National Institute on Drug Abuse.
- Abbreviations:
- ARCI
- Addiction Research Center Inventory: PCAG: pentobarbital-chlorpromazine-alcohol group
- BG
- benzedrine group
- LSD
- lysergic acid diethylamide
- MBG
- morphine-benzedrine group
- AMP
- amphetamine
- DS
- discriminative stimulus
- DSST
- digit symbol substitution test
- SDQ
- single dose questionnaire
- VAS
- visual analog scale
- Received December 30, 1997.
- Accepted May 4, 1998.
- The American Society for Pharmacology and Experimental Therapeutics