Abstract
Sutimlimab, a humanized monoclonal antibody targeting the classic complement pathway, is approved in the United States, Japan, and the European Union for the treatment of hemolytic anemia in adults with cold agglutinin disease. The objectives of this study were to support dose selection for phase 3 studies, assess dose recommendations, and establish the relationship between sutimlimab exposure and clinical outcome [hemoglobin (Hb) levels]. Clinically meaningful biomarkers were graphically analyzed and the exposure-response relationship was proposed. The pharmacokinetic (PK) characteristics of sutimlimab were best described by a two-compartment model with parallel linear and nonlinear clearance terms. Body weight was a significant covariate for the volume of distribution in the central compartment (Vc) and total body clearance of sutimlimab. Ethnicity (Japanese, non-Japanese) was a covariate on Vc and maximal nonlinear clearance. There were no PK differences between healthy participants and patients. After graphical exposure-response analysis for biomarkers, a pharmacokinetic-pharmacodynamic model was developed by integrating an indirect response/turnover model for Hb with a maximum effect (Emax) model, relating the Hb-elevating effect of sutimlimab to plasma exposure. Renal function and occurrence of blood transfusion were identified as covariates on Hb change from baseline. Simulations showed that Emax was attained with the approved dosing (6.5 g in patients <75 kg and 7.5 g in patients ≥75 kg), independent of covariate characteristics, and provided adequate sutimlimab exposure to maximize effects on Hb, bilirubin, and total complement component C4 levels. A change in Hb from baseline at steady state of 2.2 g/dl was projected, consistent with phase 3 study observations.
SIGNIFICANCE STATEMENT The final validated population pharmacokinetic (PK) and pharmacokinetic/pharmacodynamic (PK/PD) models confirm that the approved dosing regimen for sutimlimab (6.5 g in patients <75 kg and 7.5 g in patients ≥75 kg) is sufficient, without the need for further dose adjustments in populations of patients with cold agglutinin disease.
Introduction
Sutimlimab is a first-in-class, humanized immunoglobulin G 4 monoclonal antibody that targets the classic complement pathway (CP) by selectively inhibiting the complement component 1, s subcomponent (Bartko et al., 2018; Jäger et al., 2019; Röth et al., 2021). Sutimlimab has been approved by the US Food and Drug Administration (FDA), the European Medicines Agency, and in Japan for the treatment of hemolysis in adult patients with cold agglutinin disease (CAD) (https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761164s000lbl.pdf; https://www.ema.europa.eu/en/documents/product-information/enjaymo-epar-product-information_en.pdf).
CAD is a rare type of autoimmune hemolytic anemia, mediated by persistent complement activation via the classic CP leading to hemolysis (Berentsen, 2016; Röth et al., 2021), and an increased risk of thromboembolism (Chapin et al., 2016; Mullins et al., 2017; Bylsma et al., 2019; Broome et al., 2020; Jäger et al., 2020; Kamesaki et al., 2020; Delvasto-Nunez et al., 2021). Vascular symptoms such as acrocyanosis and Raynaud’s phenomenon are not present in all patients with CAD (Berentsen et al., 2015, 2020). Other symptoms of CAD including fatigue, dyspnea, weakness, dark urine, jaundice, and weight loss are nonspecific, which can complicate initial disease recognition and diagnosis (Berentsen, 2016; Röth et al., 2021).
The pharmacokinetics (PK) and pharmacodynamics (PD) of sutimlimab were first investigated in healthy participants in a phase 1 study (Bartko et al., 2018). In this study, the mean sutimlimab exposure increased in a greater than dose-proportional manner in the dose range of 3–30 mg/kg and in an approximately dose-proportional manner in the higher dose range of 60–100 mg/kg (Bartko et al., 2018). Complete inhibition of CP activity was achieved in all participants who received a sutimlimab dose of 3 mg/kg or higher. The duration of CP inhibition was dose related (Bartko et al., 2018).
The phase 3 CARDINAL (BIVV009-03; NCT03347396) and CADENZA (BIVV009-04; NCT03347422) trials examined the effect of sutimlimab in patients with CAD with or without a recent history of transfusion, respectively (Röth et al., 2021, 2022). In both trials, sutimlimab rapidly halted hemolysis and increased hemoglobin (Hb) levels. The activity of the classic CP was almost completely inhibited within 1 week after the initiation of sutimlimab treatment, with concomitant normalization of complement component 4 (C4) levels (Röth et al., 2021). The PK characteristics of sutimlimab in patients with CAD have not been published. The recommended dosage of sutimlimab for pivotal phase 3 studies was 6.5 g for patients weighing between 39 kg and less than 75 kg, and 7.5 g for patients weighing greater than or equal to 75 kg. This was administered by intravenous infusion over 1 to 2 hours once weekly for the first two doses, followed by every 14 days thereafter (https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761164s000lbl.pdf; https://www.ema.europa.eu/en/documents/product-information/enjaymo-epar-product-information_en.pdf).
The objectives of this study were to support dose selection for phase 3 studies, to assess the effectiveness of the approved dose recommendation, to determine whether any further dose adjustments may be required, and to establish the relationship of sutimlimab exposure and clinical outcome (i.e., Hb levels). Population PK (popPK) and PK/PD models were developed to account for intersubject variability and the effects of covariates on PK of sutimlimab and Hb, respectively. In addition, clinically meaningful biomarkers Hb, bilirubin and C4 values were graphically evaluated and the exposure-response relationship was proposed. Bilirubin is a recognized marker of extravascular hemolysis in CAD patients. C4 is the first soluble cleavable substrate of the C1 complex, the target of sutimlimab.
Materials and Methods
Study Design and Patient Population
Data from the studies listed in Table 1 were included in the analyses. Detailed information on study design, dosing, and inclusion/exclusion criteria have been published previously (Mühlbacher et al., 2017; Bartko et al., 2018; Jäger et al., 2019; Röth et al., 2021, 2022). These studies were conducted according to the International Conference on Harmonization Good Clinical Practice Guideline and the Declaration of Helsinki and were approved by local independent ethics committees or review boards. Participants provided written informed consent.
Clinical study design, dosing, and sampling for studies involved in the analyses
Model Development
The popPK model was developed with data from studies BIVV001-01, BIVV009-02, BIVV009-03, and BIVV009-05 and was further validated with a larger dataset as described in model validation. PK/PD model development included data from studies BIVV009-01 parts C and E, BIVV009-03 parts A and B, BIVV009-04 part A, and was evaluated later using final data from part B of studies BIVV009-03 and BIVV009-04.
(1) First structural and random models were identified including interindividual variability (IIV) assessment.
(2) After selection of the optimal structural PK or PK/PD model, trends in empirical Bayes estimates versus categorical and continuous covariates were graphically tested first and identified for covariate model development and covariate analysis. A list of all covariates can be found in Supplemental Methods (Population PK-PD Model Development; Population PK Model Development). Covariates were only considered on model parameters with identifiable IIV. The impact of identified covariates was then systematically evaluated using the forward-addition (P = 0.01) and backward-deletion (P = 0.001) methods. Evaluation of the quality of both the PK and PK/PD models was based on likelihood of the data (objective function value) goodness-of-fit plots, η-shrinkage, quality criteria, visual predictive check (VPC), and simulations.
(3) The selected covariate model was then qualified by prediction-corrected visual predictive checks (pcVPCs) and bootstrap analysis.
PopPK Model
The analysis population consisted of all evaluable participants, defined as those who had at least one postdose sutimlimab concentration sample greater than the lower limit of quantitation (5 ng/ml) and an associated dosing and blood sampling record for the postdose sample.
Structural and random models were identified first, followed by covariate model development. Relationship of the following covariates was evaluated on volume parameters: age, body weight, sex, race, Japanese ethnicity, renal function by creatinine clearance (CLCR), and estimated glomerular filtration rate (eGFR), hepatic function [serum aspartate transaminase (AST), albumin], and disease status. Relationship of the following covariates was evaluated on clearance parameters: age, body weight, sex, race, Japanese ethnicity, CLCR, eGFR, measures of hepatic function, and disease status (Supplemental Table 1). The impact of antidrug antibody (ADA), diluted infusion, and occurrence of blood transfusion was graphically evaluated after availability of the complete dataset, as part of model evaluation. The final popPK model was then used to simulate expected profiles of potential populations of interest and further used to estimate exposure parameters of sutimlimab [minimum concentration at steady state (Cmin,ss), maximum concentration at steady state (Cmax,ss), area under the curve at steady state (AUCss)] in patients with CAD. Further information on the PK model development methodology and evaluation is provided in Supplemental Methods (Model Evaluation; Population PK Model Development; Supplemental Tables 2 and 3).
Exposure-Response Analysis
Graphical Exploration of Biomarkers
Observed biomarker (Hb, bilirubin, and C4) and change in biomarker from baseline in patients with CAD were selected for the analysis. This comprised the creation of individual and dose group plots for observed and change (median, maximum, and 95th percentile) from baseline levels. An exploratory characterization of the exposure-response relationship was also attempted. A maximum effect (Emax) model was fitted to the change in Hb or C4 from baseline, and a maximum inhibitory effect (Imax) model was fitted to the change in bilirubin from baseline versus time-matched observed Cmin,ss.
PopPK/PD Model
A dynamic popPK/PD structural model for Hb was developed by linking the complete time profile of sutimlimab concentrations to the time profiles of Hb. The PK component consisted of the final popPK model; the PD component was based on an indirect response/turnover model for Hb dynamics over time, including a zero-order rate constant (kin) for Hb production and a first-order rate constant (kout) for elimination of Hb, which is inhibited by sutimlimab concentration in the central compartment (Supplemental Fig. 1). Structural and random models were initially identified, followed by covariate model development (Supplemental Methods: Population PK-PD Model Development). The covariates investigated were age, body weight, sex, race, ethnicity (Japanese/non-Japanese), blood transfusion, eGFR, CLCR, and measures of hepatic function (i.e., albumin, AST, serum alanine transaminase, and bilirubin) (Supplemental Table 4). The impact of ADA and dilution of infusion was graphically evaluated. Further information on the popPK/PD model is provided in Supplemental Methods (Model Evaluation; Population PK-PD Model Development).
Model Validation
Maximum a posteriori (MAP) probability Bayesian approach was applied to larger datasets to validate previously developed covariate models. The popPK and PK/PD full covariate models obtained in model development were applied to a dataset, including data from prior analysis used for model development and additional data from completed studies (mainly BIVV009-03 and BIVV009-04), with prior population parameter estimates for the assessment of individual parameters and concentration predictions.
As the larger datasets were submitted to a MAP Bayesian analysis, potential new covariates were not included in the model and tested for statistical significance again. Instead, individual random subject effect estimates (η) were plotted versus covariates already investigated in previous analysis, such as ADA status, occurrence of blood transfusion, and administration of diluted/undiluted infusion. The approach is justified because the η-shrinkage was within an acceptable range.
Model evaluation was performed by standard goodness-of-fit plots, investigation of covariates, prediction-corrected VPC, and quality criteria such as mean prediction error (MPE), root mean squared error (RMSE), and absolute average fold error (AAFE). Further information on the model evaluation is provided in Supplemental Methods (Model Evaluation). Trends in empirical Bayes estimates versus categorical and continuous covariates were graphically tested (box and scatter plots). Absence of covariate effect was concluded if there was no major trend.
Dose Selection
Concentration versus CP activity inhibition relationship was evaluated using data from BIVV001 Parts A–C and BIVV009-02 studies in healthy participants and patients. Individual serum concentrations of sutimlimab and CP activity were time-matched, and the PK/PD relationship was modeled using an inhibitory Emax model as described below:
 where E0 is the baseline, Imax is the maximum inhibition, C is the concentration of sutimlimab, IC50 is the concentration associated to 50% of the maximum effect, and H is the Hill factor (also referred as gamma, a parameter used to describe sigmoidicity). It was assumed that a similar relationship existed in healthy participants and patients. The 90% inhibition (IC90) was identified graphically and was estimated from IC50 of PK/PD relationship. The IC90 was used as threshold concentration for dose selection.
where E0 is the baseline, Imax is the maximum inhibition, C is the concentration of sutimlimab, IC50 is the concentration associated to 50% of the maximum effect, and H is the Hill factor (also referred as gamma, a parameter used to describe sigmoidicity). It was assumed that a similar relationship existed in healthy participants and patients. The 90% inhibition (IC90) was identified graphically and was estimated from IC50 of PK/PD relationship. The IC90 was used as threshold concentration for dose selection.
The popPK model was used to simulate the dose required in 97 participants (66 healthy subjects and 31 patients from two phase 1 studies) to maintain concentrations above threshold concentration when administered once weekly for 1 week and biweekly thereafter. The weekly regimen for the first few doses was proposed to achieve rapid increase in concentrations due to the expected steep nature of the PD response and rapidly saturating processes that cause nonlinearities in PK. The threshold concentration in participants was justified based on most of the observed concentrations displaying nonlinearities below these levels, exacerbating risk for breakthroughs occurring rapidly given likely dosing deviations. As such, maintaining levels above threshold concentration would be expected to provide an additional concentration buffer for dosing deviations or unexpected variability in PK. Percentage of participants with Cmin,ss above threshold concentration was estimated. The sutimlimab dose for pivotal phase 3 studies was selected based on maximum percentage of participants with estimated Cmin,ss above the threshold concentration.
Simulations were performed representing populations (including the various sources of IIV). For the stochastic simulations, dose and covariates were sampled from the observed data considering potential correlations among them. In the deterministic simulations, covariates were kept at median values except for the covariate under evaluation. Covariates were evaluated using the minimum and maximum values from the observed data. Each participant in the dataset was simulated 100 times. In total, 17,600 virtual participants for PK and 7200 for Hb were simulated. The simulations were statistically summarized using median (defined by the 50th percentile of the simulated values) and 90% prediction interval (defined by the 5th and 95th percentiles of the simulated values).
Exposure-Safety Analysis
Individual empirical Bayesian PK parameter estimates obtained from the popPK analysis were used to simulate PK profiles and to derive individual sutimlimab PK exposure metrics contingent on each participant’s actual dosing records (Supplemental Methods: Exposure Parameters). Systemic sutimlimab-exposure metrics were obtained, including AUCss and Cmax,ss. Sutimlimab PK exposure metrics were categorized by quartiles, and the rates of selected safety events (infections and infestations, gastrointestinal disorders, vascular disorders, skin and subcutaneous tissue disorders, general disorders and administration site conditions, musculoskeletal and connective tissue disorders, nervous system disorders, injury, poisoning and procedural complications, respiratory disorders) were compared across the quartile categories. These analyses were performed for all participants who took part in BIVV009-03 and BIVV009-04 studies.
Dose-Safety Analysis
Sutimlimab doses per kg body weight were categorized by quartiles, and the rates of safety events described above were compared across the quartile categories. The same analysis was repeated with body weight quartiles.
Software and Computational Approach
Nonlinear mixed-effects modeling software (NONMEM) (version 7.4; ICON, Hanover, MD) was used for popPK and PK/PD modeling of Hb data. The first-order conditional estimation method of NONMEM with IIV and residual variability interaction (FOCE INTER) was used for model development. For MAP Bayesian analysis, the estimation step was omitted using the NONMEM option MAXEVAL = 0 to compute the individual estimates based on the population estimates of θ, ω, and σ obtained in the full covariate model.
SAS (version 9.4) or R (version 3.6) was used for data preparation, model diagnostics, statistical summaries, and simulations. Graphical exposure-response analysis was conducted using rstanemax package (version 0.1.3) in R. Information on handling of missing or erroneous data is provided in Supplemental Methods (Handling of Missing or Erroneous Data).
Results
Data Accounting and Baseline Demographics
The final PK model parameters were estimated during model development using 2470 PK observations from 154 participants. The percentage of postdose PK observations below the quantification limit (BLQ) was 7%. The final PK analysis dataset for model evaluation using the Bayesian approach included 4140 evaluable observations from 196 participants (healthy subjects and patients). The percentage of postdose BLQ observations was 6%. The final PK/PD model parameters were estimated during model development using 2368 Hb concentrations from 72 patients with CAD. The final PK/PD analysis dataset for model evaluation using the Bayesian approach included 4269 Hb observations from 72 patients with CAD (all participants from studies BIVV009-03 and BIVV009-04 and six from study BIVV009-01 part C with four of them progressing into part E). The baselines characteristics for both datasets are shown in Table 2. In total, eight participants in studies BIVV009-03 and BIVV009-04 were ADA positive, and 22 participants in studies BIVV009-03 and BIVV009-04 received blood transfusion.
Baseline characteristics by study
The final PK analysis dataset included 196 participants (healthy participants and patients from all five studies). The number of participants from each study/cohort can be found in Table 1.
PopPK Model
Several potential structural models were investigated [one or two compartments, mixed-linear and nonlinear (Michaelis-Menten) elimination]. A two-compartment model with Michaelis-Menten elimination best described the sutimlimab data. IIV terms were included on clearance (CL), volume of distribution of the central compartment (Vc), volume of distribution of the peripheral compartment (Vp), and maximum nonlinear clearance (Vmax). The residual error model includes additive and proportional error terms for the BIVV009-03 study and separate additive and proportional error terms for non–BIVV009-03 studies. Parameter estimates from the final popPK model are summarized in Table 3.
Parameter estimates of the full covariate popPK model
Body weight and Japanese ethnicity were found to be statistically significant covariates for CL and Vc, and Japanese ethnicity was found to be a significant covariate for Vc and Vmax. In addition, age had a significant effect on Vc (Table 3). After including age, body weight, and Japanese participant covariates, the following covariates did not have an additional effect on the PK of sutimlimab: sex, race, albumin, hepatic function, renal function, and disease status. The effect of ADA was graphically evaluated.
Evaluation of the PK model using all data showed good agreement between predictions and observations in the goodness-of-fit plots in general (Supplemental Fig. 2). Quality criteria and pcVPC showed an acceptable performance of the model to describe the data (Supplemental Figs. 3 and 4; Supplemental Table 5). Simulation-based diagnostics using mean normalized prediction distribution errors (NPDE) do support the results of goodness-of-fit plots. Overall, the mean NPDE was 0.0255 (P = 0.21) with a variance of 0.866 (P < 0.001), indicating no bias and an ability of the model to reasonably capture the underlying variability (Supplemental Fig. 5). The MPE of the population predictions was small, with −1.18% of the observations with zero of the absolute MPE included in the 95% confidence interval (CI). The relative MPE of individual predicted data based on individual empirical Bayes parameter estimates (IPRED) indicated a minor underprediction of −1.58% of the observations. The precision of the model was reasonable, with an RMSE of 45.2% and 27.1% for predicted data based on population parameter estimates (PRED) and IPRED, respectively. AAFE was 1.37 and 1.17 for PRED and IPRED, respectively, and thus near the optimal value of 1 (Supplemental Table 5).
Box and scatter plots of the individual post hoc estimates of random effects versus body weight, age, ethnicity, race, sex, ADA status, disease status, occurrence of blood transfusion, infusion dilution, and measures of renal and hepatic function did not reveal marked correlation that would require correction of the covariate model (data not shown).
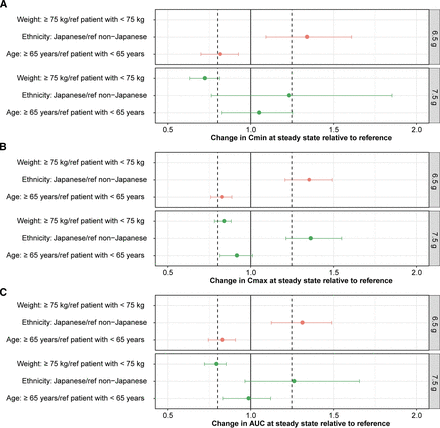
The mean changes of post hoc Cmin,ss Cmax,ss, and AUCss at steady state ranged from 0.7 to 0.8 in patients weighing ≥75 kg, from 1.2 to 1.4 in Japanese patients, and from 0.8 to 1.0 in elderly patients compared with the reference populations. The calculated 95% CIs overlap 1 in many instances (Fig. 1).
Change in Cmin,ss (A), Cmax,ss (B), and AUCss (C) at steady state relative to the reference population. Individual post hoc estimates of Cmin,ss, Cmax,ss, and AUCss at steady state were calculated using the approved sutimlimab dosing regimen (6.5 g in red, 7.5 g in green). The solid circles represent the mean values, with error bars representing the 95% CI.
Graphical Exploration of Biomarkers
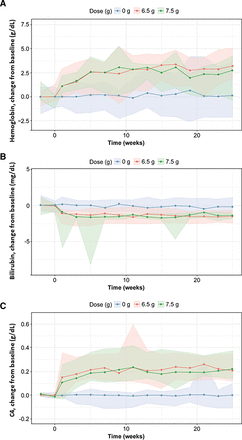
Hemoglobin and C4 levels increased and bilirubin decreased after administration of sutimlimab in patients with CAD. After placebo treatment, there were no visible changes over time for Hb, bilirubin, and C4 (Fig. 2). The median observed changes from baseline at steady state following the approved regimen were 2.6 g/dl for Hb, 0.23 g/l for C4, and −1.7 mg/dl for bilirubin; and the median biomarker levels at steady state were 11.8 g/dl for Hb, 0.28 g/l for C4, and 0.801 mg/dl for bilirubin after 6.5 or 7.5 g sutimlimab.
Median change from baseline in hemoglobin (A), bilirubin (B), and C4 (C) over time by dose in patients with CAD (study BIVV009-04); 0 g sutimlimab (placebo) shown in blue, 6.5 g in red, and 7.5 g in green. The shaded areas represent the 2.5%–97.5% percentiles of the respective doses.
Cmin,ss and change in biomarkers were time matched for characterization of exploratory PK/PD relationship. The estimated Emax (Hb and C4) or Imax (bilirubin) captured maximum observed effect on biomarker. The estimated Cmin,ss for half-maximum effect on Hb and bilirubin (ECmin for Hb, ICmin for bilirubin) were 50.7 to 298 μg/ml with all observed Cmin,ss (Supplemental Fig. 6).
PK/PD Model
The time course of Hb was modeled using a turnover model, including kin for Hb production and kout for elimination of Hb, which is inhibited by sutimlimab concentration in central compartment. IIV was identified for Hb baseline (Ebase) and Emax. Significant covariates were CLCR on Ebase and blood transfusion status (binary covariate) on Emax. After including CLCR and blood transfusion as covariates, the following covariates did not reveal marked correlation with model parameters: body weight, age, ethnicity, race, sex, ADA status, disease status, infusion dilution, and measures of renal and hepatic function. The parameter estimates for the final PK/PD model are shown in Table 4. All parameters were estimated with reasonable precision and IIV was described with acceptable η-shrinkage.
Parameter estimates for the final PK/PD model
Goodness-of-fit plots generally showed a good agreement between predicted and observed Hb levels, as they form a relatively close distribution around the identity line. The population predictions at lower Hb levels were slightly underpredicted (Supplemental Fig. 7).
Evaluation of the Hb PK/PD model showed good agreement between predictions and observations in the goodness-of-fit plots (Supplemental Fig. 8). Quality criteria and VPC also showed an acceptable performance of the model to describe the data (Supplemental Figs. 8 and 9; Supplemental Table 6). Simulation-based diagnostics using mean NPDE do support the results of goodness-of-fit plots. Overall, the mean NPDE was 0.00261 (P = 0.058) with a variance of 1.21 (P < 0.001), indicating no bias and a tendency of the model to overpredict the underlying variability (Supplemental Fig. 10). However, the NPDE versus time and PRED show no trend, indicating an adequate performance of the model to describe the Hb observations. The MPE of the population predictions was small with −1.76% of the observations (zero of absolute MPE not included in 95% CI). The MPE of IPRED was negligible with 0.013% of the observations (zero of absolute MPE included in 95% CI). The precision of the model was good, with an RMSE of 14.4% and 9.2% for PRED and IPRED, respectively. AAFE was 1.12 and 1.07 for PRED and IPRED, respectively, and thus near the optimal value of 1 (Supplemental Table 6).
Box and scatter plots of the individual post hoc estimates of random effects versus body weight, age, ethnicity, race, sex, ADA status, disease status, occurrence of blood transfusion, infusion dilution, and measures of renal and hepatic function did not reveal marked correlation that would require correction of the covariate model (data not shown).
Dose Selection
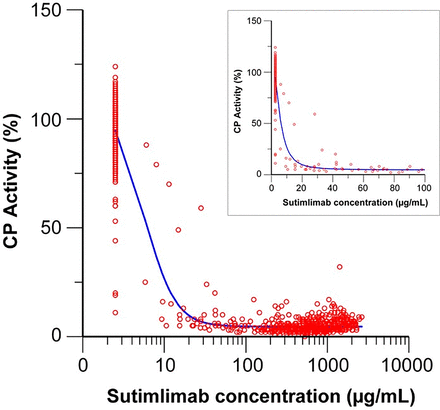
Near-maximal classic CP activity inhibition was observed for sutimlimab concentrations >20 μg/ml (Fig. 3). A steep concentration-effect relationship was observed for the inhibition of CP activity. Based on the inhibitory Emax model, a 50% inhibition of CP activity was predicted at 6.2 μg/ml sutimlimab, and that of an IC90 was 15.5 μg/ml. The very low IC50 combined with a Hill parameter of 2.4 suggests a very steep concentration-effect relationship and that sutimlimab concentrations above 100 μg/ml would be sufficient to maintain a near-maximal knockdown of CP activity and avoid nonlinear PK. The final popPK model was used to simulate steady-state concentration in patients with CAD. Three dose levels were simulated for comparison: 5.5 g, 75 mg/kg, and the approved regimen of 6.5 g for body weight <75 kg and 7.5 g for body weight ≥75 kg. The approved regimen of 6.5 g for body weight <75 kg and 7.5 g for body weight ≥75 kg (6.5 g/7.5 g) was selected based on the predicted exposure being above 100 μg/ml in the maximum number of patients. The cutoff value of 75 kg was selected to coincide with the median body weight of 631 participants extracted from a US electronic medical record and claims database. This 6.5-g/7.5-g tiered dosing regimen was predicted to maintain target trough concentrations >100 μg/ml throughout the dosing period in approximately 94% of patients with CAD (Supplemental Table 7) to minimize the risk of breakthrough hemolysis while providing sufficient safety margins. The data from BIVV009-03 and BIVV009-04 studies showed only seven patients (7/66 = 10.6%) with trough levels below 100 μg/ml during the treatment period without dose interruption, which was consistent with the predicted values based on the popPK model, 6.2% (90% CI: 2.0%–12.0%).
Relationship between sutimlimab concentration and CP activity (parts A and B of study BIVV009-01). Circles represent the observed values, and the solid line shows the prediction. The x-axis on linear scale in the inset limits sutimlimab concentrations up to 100 μg/ml.
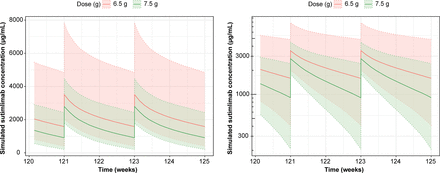
The final popPK and PK/PD models were used to simulate exposures (Fig. 4) and Hb profiles (Fig. 5) at the approved dosing regimen for adult patients (6.5-g dose in patients who weigh <75 kg and 7.5-g dose in patients who weigh ≥75 kg). Doses were administered on day 0, day 7, and then every 14 days thereafter through week 125.
Simulated concentration versus time profiles by dose after administration of 6.5 g sutimlimab to patients weighing <75 kg and 7.5 g sutimlimab to patients weighing ≥75 kg. Doses were administered on day 0, day 7, and then every 14 days thereafter through week 125. Left panel: linear scale; right panel: semilogarithmic scale; 6.5-g dose shown in red and 7.5-g dose in green. Lines represent the median, and the respective shaded areas represent the range between 5th and 95th percentiles of the simulated values. Concentration and Hb profiles at 6.5 and 7.5 g are similar and overlap.
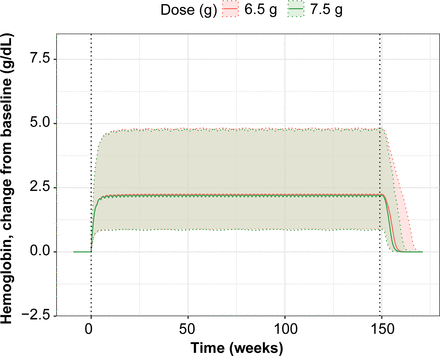
Simulated Hb change from baseline versus time by dose after administration of 6.5 g sutimlimab to patients weighing <75 kg and 7.5 g sutimlimab to patients weighing ≥75 kg. Doses were administered on day 0, day 7, and then every 14 days thereafter through week 149. Figure illustrates the complete treatment period on a linear scale; 6.5-g dose shown in red and 7.5-g dose in green. The solid lines represent the median, and the respective shaded areas represent the range between 5th and 95th percentiles of the simulated values. The dotted vertical lines represent the start or end of treatment period.
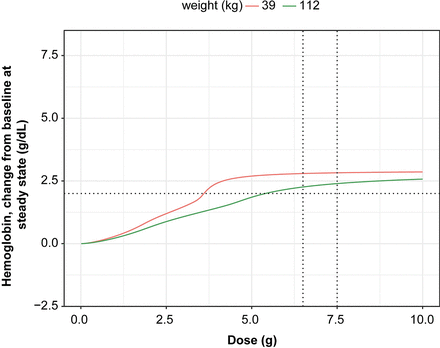
Stochastic simulations of the evaluation of the impact of dose (6.5 g in patients <75 kg and 7.5 g in patients ≥75 kg) stratified by minimum (39 kg) and maximum (112 kg) body weights showed the Hb change from baseline time profiles to be very similar (Fig. 6).
Relationship between Hb change from baseline at steady state by weight (minimum and maximum weight in the population). The vertical dotted lines indicate the approved dosing: 6.5 and 7.5 g; the horizontal line represents 2 g/dl change in Hb from baseline.
The impact of statistically significant PK and PD covariates on the typical changes in Hb from baseline at steady state with the approved dosing regimen was shown to be of 1.2-fold change for a patient weighing 39 kg compared with 112 kg, of 1.3-fold change increase for a patient with CLCR value of 174 ml/min compared with a patient with CLCR value of 24 ml/min, and of 1.9-fold change for a patient without occurrence of transfusion compared with a patient with occurrence of transfusion. No impact of ethnicity or age was observed (Supplemental Fig. 11).
Exposure-Safety Analysis
The proportions of patients with selected safety events by serum sutimlimab-exposure quartiles are provided in Supplemental Results (Supplemental Figs. 12 and 13) for the AUCss and Cmax,ss exposure metrics. Each quartile includes 16 to 17 participants. A trend is observed toward higher rates of gastrointestinal (GI) disorders with an increased Cmax,ss and general disorders and administration site reactions for AUCss and Cmax,ss. The difference in percentage of patients experiencing adverse effects is between 6% and 7% between the lowest and highest AUCss or Cmax,ss quartiles for GI disorders, general disorders, and administration site discomfort. This difference is considered not relevant. In general, there does not appear to be a clear trend between the other adverse events and high exposure.
Dose-Safety Analysis
The proportions of patients with selected safety events by dose and body weight quartiles are provided in Supplemental Results (Supplemental Figs. 14 and 15). For GI disorders, the difference in percentage of patients experiencing adverse effects is about 5% between the lowest and highest dose quartiles. For vascular disorders, the difference in percentage of patients experiencing adverse effects is about 7% between the lowest and highest weight quartiles. In general, there does not appear to be a clear trend between the other adverse events and dose or weight.
Discussion
The PK of sutimlimab was best described by a two-compartment model with parallel linear and nonlinear clearance terms (Michaelis-Menten elimination kinetics). This model adequately described the nonlinearity of sutimlimab PK especially visible at lower doses (0.3–60 mg/kg). This model also adequately described the PK of sutimlimab in patients with CAD and healthy participants.
Body weight and ethnicity (Japanese) were identified as covariates on the PK of sutimlimab, signifying the importance of demographic factors in sutimlimab PK. However, other covariates such as sex, race, albumin, hepatic function, renal function, and disease status did not have an additional impact on sutimlimab PK.
Separate residual error models were used for study BIVV009-03 due to the large difference in observed concentration between steady-state dosing (BIVV009-03) and single- or multiple-dose studies. Goodness-of-fit plots indicate population predictions to deviate from observed concentrations at very low (<30 μg/ml) and very high (>5000 μg/ml) concentrations only. This is thought to be of no clinical relevance considering that maximal effects (complete CP inhibition) occur at sutimlimab levels above 20 μg/ml. Overall, there was no indication of any major model misspecification. Also, the pcVPCs confirmed concordance between observed and model-based simulations. Taken together, these suggest that the final popPK model is adequate for simulation of sutimlimab-exposure estimates in adults.
The changes of exposure parameters (i.e., Cmin,ss, Cmax,ss, and AUCss) at steady state in patients weighing ≥75 kg, elderly patients (age >65 years), and non-Japanese patients relative to the reference populations respectively ranged from 0.7 to 0.8, 0.8 to 1.0, and 1.2 to 1.4, respectively, altogether indicating a relatively limited impact of the previously identified covariates. Simulated concentration-time profiles using the final model showed an overlap in exposure after the 6.5-g and 7.5-g doses, indicating the that cutoff was appropriately selected. Additional simulations also showed that exposures decreased with increasing body weight and that exposures were greater in Japanese participants versus non-Japanese participants. However, minimum sutimlimab concentration at steady state was greater than the target of 100 μg/ml to prevent hemolysis, suggesting that the approved dosing regimen (6.5 g in patients <75 kg and 7.5 g in patients ≥75 kg) and dose levels provide adequate exposure in all patients-with-CAD groups. Overall, a good predictability trend of the popPK model was shown consistently and successful external validation of the model was demonstrated.
The present exposure-response analysis was performed in two steps: graphical exploratory exposure-response analysis and development of a popPK/PD model for Hb. The popPK/PD model should support characterization of IIV in clinical response (Hb increase) and its explanation by covariates. Hemoglobin and C4 increased, and bilirubin was shown to decrease after administration of sutimlimab in patients with CAD in all studies. At dose of 6.5 or 7.5 g sutimlimab, the responses for Hb, C4, and bilirubin are markedly higher than those of the placebo group. Exploratory exposure-response relationship was characterized by observed Cmin,ss versus observed biomarker change. The estimated Emax (Hb and C4) or Imax (bilirubin) captured maximum observed effect on biomarkers. The estimated Cmin,ss for half-maximum effect on Hb and bilirubin (ECmin for Hb, ICmin for bilirubin) was 50.7 to 298 μg/ml with all observed Cmin,ss. The result is in the same order of magnitude as the EC50 of the turnover model for Hb (155 μg/ml). Thus, EC50 is confirmed to be safely beyond 1.5–3 times the target sutimlimab Cmin,ss (100 μg/ml) to avoid breakthrough bleeds. In addition, these values are comparable to those observed in PK analyses following approved doses.
The dynamic PK/PD model was an indirect response model with sutimlimab concentration inhibition of Hb turnover rate. Selection of an indirect response model was supported by hysteresis plots for time-matched observed Hb versus observed sutimlimab concentrations, which are suggestive of a time delay between sutimlimab exposure and changes in Hb response (data not shown). Integrating the turnover model with an Emax model to capture the drug effect is consistent with the results of the graphical exploratory analysis. The magnitude of ETA shrinkage on the IIVs was ≤30% for all PD parameters with IIV terms. In general, observations are randomly distributed around the identity line in the goodness-of-fit plots. However, low Hb measures are not captured on a population level by the model, which is reflected in the VPC, where the lower prediction interval does not capture all observations over time. The model appears to adequately predict the median change in Hb with and without treatment (i.e., change in Hb over time with placebo treatment).
The model estimated the turnover time of 188 hours with a derived median kout of 0.0053 hours−1, which corresponds to a half-life of 5.4 days. This half-life is lower than the lifetime of Hb (∼120 days) but is supposed to reflect higher degradation rate of Hb in patients with CAD. The drug effect was estimated to have an Emax of 25% from baseline, which is in line with the projected effect of ∼2 g/dl and consistent with the observations in phase 3 studies. The EC50 was 155 μg/ml, which is approximately 1.5 times the target sutimlimab Cmin,ss (100 μg/ml) to avoid hemolysis. The model does not contain a placebo effect (assumed as 0), which is in line with the lack of time-dependent changes observed during the exploratory analysis. Interindividual variability was identified for Ebase and Emax. After stepwise covariate modeling, only CLCR and blood transfusion were kept in the model as covariates of Ebase and Emax, respectively. The introduction of covariates on Ebase and Emax reduced the IIV of these parameters by 12.2% and 16.5%, respectively. Patients receiving at least one blood transfusion during the study showed a lowering of Emax of 40%. The blood transfusion was modeled as a binary covariate (i.e., the time of the blood transfusion was not taken into account). This could contribute to the oscillations in Hb levels observed in some patients. Baseline CLCR was positively correlated with baseline Hb levels, which is consistent with current knowledge of anemia and chronic kidney disease (Hsu et al., 2002). The additive residual unknown variability was 0.92 g/dl and also reflects circadian variation of Hb levels throughout the study. This was not specifically accounted for in the model and probably at least partially explains the oscillations observed in some patients. The residual error also remained unchanged compared with the base model.
Model predictability, evaluated by means of a Bayesian analysis using predefined criteria, demonstrated that the final popPK/PD model is suitable to describe Hb data obtained from sutimlimab studies. A successful external validation of the model was also demonstrated. Deterministic simulations showed that Emax was attained independent of the PK (i.e., body weight, ethnicity, and age) and PD (i.e., renal function and occurrence of blood transfusion) covariate characteristics.
Given the overall low incidence of anti-sutimlimab antibodies in the BIV009-03 and BIV009-04 clinical studies (eight participants of 66 in the phase 3 studies) the effect of ADAs on sutimlimab PK and PD is considered limited, as was also confirmed by comparing plasma concentration-time profiles or PD profiles of sutimlimab for each patient. Further, no participants with confirmed treatment-emergent positive ADAs (treatment boosted or treatment induced) had treatment-emergent adverse events concerning hypersensitivity or anaphylactic reactions associated with sutimlimab.
The approved dose regimen (6.5 g for body weight <75 kg and 7.5 g for body weight ≥75 kg) was selected to maintain concentrations above 100 μg/ml in the maximum number of patients for near-maximal suppression of CP activity. The observed data from BIV009-03 and BIV009-04 studies showed that trough levels were maintained above 100 μg/ml during the treatment period without dose interruption in 90% of patients. Predicted change in Hb based on population PK/PD was maximum at the therapeutic weight tiered dose regimen (maximum Hb is more than 2 g/dl) including extreme body weight patients (39 and 112 kg).
The majority of the patients (51 of 66) from BIV009-03 and BIV009-04 studies were in the 6.5-g treated group. The dose level mean in mg/kg of the 6.5-g treatment group is 108.6 ± 18.1 mg/kg, and that of the 7.5-g treatment group is 89.7 ± 10.1 mg/kg. The mean of the dose in mg/kg overlaps between the two dose levels. The adverse events from BIV009-03 and BIV009-04 were graphically evaluated for correlation with dose (in mg/kg) or weight quartiles. No major trend was observed in adverse events with dose or body weight. In conclusion, no effect of dose (in mg/kg) or body weight on safety was observed.
In summary, the present analysis showed that the PK and PK/PD of sutimlimab can be accurately predicted using the respective models. This rigorous assessment of PK and PD data confirms the approved dosing regimen of sutimlimab, without the need for further dose adjustments in specific populations. Similar changes of Hb, bilirubin, and total C4 levels from baseline after 6.5 g or 7.5 g sutimlimab and negligible changes in the placebo group were observed. Exploratory dose/exposure-safety analyses indicate well tolerated administration with the approved dosing regimen.
Acknowledgments
The authors would like to thank the investigators, healthcare providers, research staff, volunteers, and patients who participated in the studies. Medical writing and editing support were provided by Emma East, Alex Goonesinghe, and Liz Jennings of Lucid Group Communications Ltd. This support was funded by Sanofi.
Data Availability
Qualified researchers may request access to patient-level data and related study documents, including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.vivli.org/.
Authorship Contributions
Participated in research design: Frank, Kovar, Strougo, Vage, Wong.
Conducted experiments: Frank, Strougo, Vage.
Performed data analysis: Frank, Kovar, Strougo, Vage, Teuscher, Wong.
Wrote or contributed to the writing of the manuscript: Frank, Kovar, Strougo, Vage, Teuscher, Wong.
Footnotes
- Received November 16, 2022.
- Accepted April 28, 2023.
This study was funded by Sanofi.
The authors have declared a conflict of interest. T.F. is an employee of a Sanofi company and holds company stock shares. A.K. is an employee of a Sanofi company and holds company stock shares. A.S. is an employee of a Sanofi company. C.V. is an employee of a Sanofi company. N.W. is an employee of a Sanofi company and holds company stock shares. N.T. works as a consultant for Sanofi.
↵1Current affiliation: Oncology Development, Sanofi, Amsterdam, Netherlands.
↵
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
Abbreviations
- AAFE
- absolute average fold error
- ADA
- antidrug antibody
- AUCss
- area under the curve at steady state
- C4
- complement component 4
- CAD
- cold agglutinin disease
- CI
- confidence interval
- CL
- clearance
- Cmax,ss
- maximum concentration at steady state
- Cmin,ss
- minimum concentration at steady state
- CLCR
- creatinine clearance
- CP
- complement pathway
- Ebase
- Hb baseline
- eGFR
- estimated glomerular filtration rate
- Emax
- maximum effect
- GI
- gastrointestinal
- Hb
- hemoglobin
- IC90
- concentration of drug required for 90% inhibition
- IIV
- interindividual variability
- IPRED
- individual predicted data based on individual empirical Bayes parameter estimates
- kout
- first-order dissipation rate constant
- MAP
- maximum a posteriori
- MPE
- mean prediction error
- NONMEM
- nonlinear mixed-effects modeling
- NPDE
- normalized prediction distribution errors
- pcVPC
- prediction-corrected visual predictive check
- PD
- pharmacodynamic(s)
- PK
- pharmacokinetic(s)
- popPK
- population pharmacokinetics
- PRED
- predicted data based on population parameter estimates
- RMSE
- root mean squared error
- Vc
- volume of distribution of the central compartment
- VPC
- visual predictive check
- Copyright © 2023 by The Author(s)
This is an open access article distributed under the CC BY Attribution 4.0 International license.