Abstract
Trace amine-associated receptor 1 (TAAR1) is a G protein-coupled receptor activated by a broad range of monoamines and amphetamine-related psychostimulants. Recent studies demonstrated wide distribution of TAAR1 in brain, coexpression of TAAR1 with dopamine transporter (DAT) in a subset of dopamine neurons in both mouse and rhesus monkey substantia nigra, and monoamine transporter-modulated activation. This study explored whether TAAR1 could influence DAT-mediated dopamine uptake and efflux. Rhesus monkey TAAR1 expressed with DAT in human embryonic kidney 293 cells was dose-dependently activated by dopamine or (+)-methamphetamine. This activation resulted in large cAMP increases and a transient reduction in [3H]dopamine accumulation within the cells, which was similar to the effect of dopamine D1 receptor (D1) or forskolin treatment. In addition, TAAR1 effects on dopamine uptake could be blocked by a protein kinase A or protein kinase C (PKC) inhibitor. [3H]Dopamine efflux assays performed in Dulbecco's modified Eagle's medium displayed a TAAR1-dependent spontaneous [3H]dopamine efflux that was dose-dependently augmented by dopamine or (+)-methamphetamine and that was blocked by either methylphenidate or a PKC inhibitor. DAT cells in Krebs-HEPES buffer had an apparent spontaneous [3H]dopamine loss, but it could not be blocked by either methylphenidate or a PKC inhibitor. Taken together, this study provides evidence that TAAR1 is involved in functional regulation of DAT and suggests that TAAR1 is a potentially important target for therapeutics for methamphetamine addiction.
TAAR1 is a G protein-coupled receptor that is activated by a broad spectrum of amine compounds, including not only trace amines but also classic biogenic amines as well as amphetamine-related psychostimulants. Activation of TAAR1 by these agonists results in cAMP accumulation (Borowsky et al., 2001; Bunzow et al., 2001; Brancheck and Blackburn, 2003; Miller et al., 2005; Hart et al., 2006; Wainscott et al., 2007; Xie et al., 2007). There is substantial evidence that TAAR1 mRNA and protein are expressed in rodent and primate brain (Borowsky et al., 2001; Miller et al., 2005; Xie et al., 2007). In the substantia nigra, mRNA for TAAR1 is evident by in situ hybridization and real-time reverse transcription-polymerase chain reaction, and protein is detected by Western blot and immunocytochemistry (Borowsky et al., 2001; Miller et al., 2005; Xie et al., 2007). At the cellular level, a subset of dopamine neurons expresses TAAR1 along with dopamine transporter (DAT) in both the rhesus monkey and the mouse (Xie et al., 2007). Accordingly, the broad agonist spectrum for TAAR1 and reported coexpression of TAAR1 with DAT in dopamine neurons support the hypothesis that TAAR1 may be an important modulator of dopaminergic brain function and may contribute to physiological, pathological, and/or adaptive properties of psychostimulant drugs of abuse.
When TAAR1 is expressed heterologously in cell lines, it remains largely intracellular (Bunzow et al., 2001; Miller et al., 2005). We have pharmacologically characterized rhesus monkey TAAR1 in HEK293 cells using a highly sensitive CRE-Luc assay (Miller et al., 2005; Xie et al., 2007). On the basis of observing an intracellular distribution of the receptor in vitro, we studied TAAR1 activation in DAT-transfected HEK293 cells, reasoning that DAT would provide a conduit for monoamines and amphetamines to enter the intracellular space and that it would activate TAAR1 to a greater extent. Indeed, coexpression of DAT with TAAR1 greatly enhanced TAAR1 signaling in a DAT-dependent manner (Miller et al., 2005, Xie et al., 2007).
DAT is a phosphoprotein located on the cytoplasmic membrane, and it plays an important role in dopamine neurotransmission by mediating reuptake of released dopamine into presynaptic nerve terminals (Garris et al., 1994; Uhl and Johnson, 1994). It has been known for decades that the DAT is a direct target of methamphetamine and that efflux of dopamine occurs as a result of transport reversal (Azzaro and Rutledge, 1973; Fischer and Cho, 1979; Liang and Rutledge, 1982). The initial response of the DAT to methamphetamine is a rapid and reversible decrease in function due to a measurable reduction in Vmax that results as a consequence of phosphorylation events (Kitayama et al., 1994; Fleckenstein et al., 1997; Sandoval et al., 2001). DAT modulation by psychostimulant drugs influences extracellular dopamine level in brain, which plays a pivotal role in locomotor activity, motivation and reward, and cognition. However, it is unclear whether the DAT is directly modulated by drugs or the drugs affect the DAT through interaction with other cellular components such as G protein-coupled receptors.
This study examines whether activation of TAAR1 by either dopamine or methamphetamine can contribute to DAT-mediated dopamine uptake and efflux. Our results in transfected HEK293 cells demonstrate that TAAR1 affects dopamine uptake and efflux in a cAMP- and phosphorylation-dependent manner in vitro, and they provide evidence that TAAR1 activation by (+)-methamphetamine acts upon both dopamine uptake and efflux by the DAT.
Materials and Methods
Materials. Dopamine, (+)-methamphetamine, forskolin, methylphenidate, H89 and Ro32-0432 were purchased from Sigma-Aldrich (St. Louis, MO). Dulbecco's modified Eagle's medium (DMEM), 100× nonessential amino acids, fetal bovine serum, 100× penicillin/streptomycin, Geneticin (G418), and trypsin/EDTA were purchased from Invitrogen (Carlsbad, CA). Dual luciferase reporter assay system kit, pGL4.73, and passive lysis buffer (5×) were obtained from Promega (Madison, WI). [3H]Dopamine (60 Ci/mmol) was purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). ReadySafe cocktail was obtained from Beckman Coulter (Fullerton, CA). Calcium phosphate transfection reagents were prepared in our laboratory.
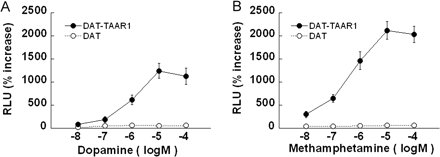
TAAR1 is activated by dopamine and methamphetamine. Stable DAT cells were transiently transfected with TAAR1 (DAT-TAAR1) or with pcDNA3.1 (DAT) along with the reporter vectors CRE-Luc and pGL4.73 for 12 h, and then they were treated with either dopamine (A) or (+)-methamphetamine (B) at a range of concentrations (10 nM–100 μM) for 18 h in serum-free media. Luciferase production resulting from CRE-Luc expression was measured by dual luciferase assay as an indicator of total cAMP accumulation and is reported as RLUs percentage of increase above baseline level (vehicle treatment). Both dopamine (A) and (+)-methamphetamine (B) dose-dependently increased CRE-Luc expression in a TAAR1-dependent manner. (+)-Methamphetamine was more efficacious than dopamine, eliciting a greater than 5-fold increase in CRE-Luc expression at 100 nM and a greater than 20-fold increase at 10 μM. Data shown are for three independent experiments performed in triplicate (means ± S.E.).
Cell Culture and Transfection. Cells were grown in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.1 mM nonessential amino acids at 5% CO2 in a 37°C water-jacketed incubator. G418 (250 μg/ml) was used for maintenance of selection of the stable cell lines. Calcium phosphate reagents [250 mM calcium chloride and 2× HBS buffer (280 nM NaCl, 10 mM KCl, 1.5 mM Na2HPO4, 12 mM d-glucose, and 50 mM HEPES, pH 7.05)] were prepared for transfection according to the procedures described previously (Xie et al., 2005). For cotransfection, the ratio for the constructs and the amount of total DNA were held constant with pcDNA3.1. The cells were exposed to the transfection media for an indicated period and then incubated for assay purposes.
Dual Luciferase Reporter Assay. Cells were placed into wells of the 48-well plates (75,000 cells/well in 0.5 ml of DMEM) 2 days before transfection. The luciferase reporter construct CRE-Luc was transiently transfected into the cells to monitor cAMP variation, along with the assay reporter control pGL4.73 (a Renilla luciferase construct irresponsive to cAMP). Rhesus monkey TAAR1 was cotransfected into HEK293 or stable human DAT cells along with luciferase reporters, and the transfection was performed at 70 to 80% cell confluence. After 12-h incubation in transfection medium, the cells were exposed to drugs in serum-free DMEM. Dopamine and (+)-methamphetamine were dissolved in water (10 μM stock solution) and then diluted with serum-free DMEM into a series of concentrations (10–9–10–4 M) and added to triplicate wells at each dose within 0.5 h after the replacement of the transfection media. The culture plates were gently swirled and placed back into the incubator for 18 h. We prepared the 1× passive lysis buffer (PLB) and luciferase assay substrate reagents according to the manufacturer's protocol (Promega, Madison, WI). Cell lysates were prepared by addition of 100 μl of 1× PLB into each well. The samples were incubated on a shaking platform for 30 min at 25°C, and then the lysates (20 μl) were transferred into wells of opaque 96-well microplates (PerkinElmer Life and Analytical Sciences) for measurement of luciferase expression. Luciferase substrate reagents (50 μl) were injected into each well, and after a 2-s delay, luciferase levels were measured as relative light units (RLUs) for 12 s on a Wallac 1420 multilabel counter, Victor 3V (PerkinElmer Life and Analytical Sciences). RLUs represent the luciferase level (firefly and Renilla). The average ratio of firefly luciferase RLU to Renilla luciferase RLU was calculated for each triplicate. The ratio values were then divided by the ratio at baseline (vehicle treatment), and the results were converted into a percentage value. The percentage of increase of RLU above baseline, which represents the specific response to the agonist tested at each dose, was derived by subtracting the baseline from the percentage value at each dose.
[3H]Dopamine Uptake Assay. Cells were placed into wells of the 48-well plates (75,000 cells/well) 2 days before transfection in growth medium. HEK293 and stable cell lines expressing rhesus monkey TAAR1 or rhesus monkey D1 were generated in our laboratory and were transiently transfected with pcDNA3.1 or human DAT as indicated, at 70 to 80% cell confluence. The cells were incubated in transfection medium for 24 h and then further incubated in fresh complete medium overnight (12 h). The cells in three random wells on each plate were harvested for cell counting. [3H]Dopamine was diluted with serum-free DMEM and used to load the cells at the indicated concentrations at 25°C for time course studies. Nonspecific uptake was obtained by addition of 10 μM indatraline 15 min before and during radiation loading. The PKA inhibitor H89 (10 μM) or the PKC inhibitor Ro32-0432 (10 μM) was added 10 min before and remained during [3H]dopamine loading. Forskolin was mixed with [3H]dopamine for uptake at the 4-, 5-, 10-, 20-, and 30-min time points. All uptake assays were performed in serum-free DMEM, and the uptake was terminated by aspiration of the loading media. We prepared 1× PLB according to the manufacturer's protocol and added it into each test well (100 μl/well). The cell lysates were prepared by incubation of the sample on a rocking platform (VWR, West Chester, PA) for 30 min and totally transferred into scintillation vials containing 4 ml of ReadySafe scintillation cocktail each for radiation counting on an LS6500 multipurpose scintillation counter (Beckman Coulter). Each sample was counted for 1 min. Nonspecific uptake was subtracted from the total to yield specific accumulation of radiation. Each experiment was conducted in triplicate three times, and average dpm values were calculated for evaluation of the [3H]dopamine uptake.
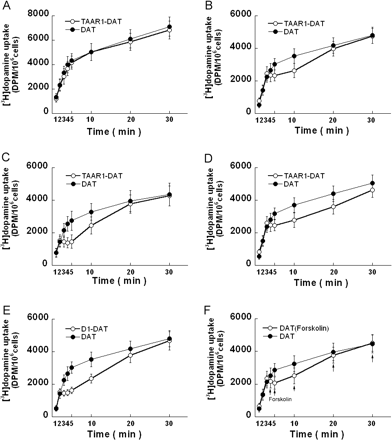
TAAR1 effects on [3H]dopamine uptake. Stable TAAR1, stable D1 or HEK293 cells were transiently transfected with DAT for 24 h, resulting in three different cell lines, TAAR1-DAT, D1-DAT, and DAT, respectively. After incubation in growth medium for 12 h, the cells were exposed to [3H]dopamine in serum-free media at 25°C. A, uptake at 10 nM [3H]dopamine was similar in TAAR1-DAT cells and DAT cells. B, uptake at 100 nM [3H]dopamine ([3H]dopamine/dopamine; 1:10 dilution) occurred similarly in TAAR1-DAT cells and DAT cells during the first 3 min but then abruptly halted in the TAAR1-DAT cells. After 20 min, total [3H]dopamine uptake was similar in both cell lines. C, uptake at 1 μM [3H]dopamine ([3H]dopamine/dopamine; 1:50 dilution) occurred similarly in TAAR1-DAT cells and DAT cells during the first 2 min but then abruptly halted in the TAAR1-DAT cells. After 20 min, total [3H]dopamine uptake was similar in both cell lines. D, uptake at 10 nM [3H]dopamine combined with 90 nM (+)-methamphetamine (parallel to uptake at 10 nM [3H]dopamine + 90 nM dopamine shown in B) in TAAR1-DAT cells and DAT cells was similar during the first 3 min. After 3 min, there was an abrupt halt in uptake in the TAAR1-DAT cells that was prolonged in comparison with the effect of dopamine shown in B. E, uptake at 100 nM [3H]dopamine ([3H]dopamine/dopamine; 1:10 dilution) occurred similarly in D1-DAT cells and DAT cells during the first 2 min but then abruptly halted in the D1-DAT cells. After 20 min, total [3H]dopamine uptake was similar in both cell lines. Note that D1-dependent effects occurred after 2 min, whereas TAAR1-dependent effects occurred after 3 min (data in B). F, comparison of uptake at 100 nM [3H]dopamine ([3H]dopamine/dopamine; 1:10 dilution) in DAT cells (untreated) and DAT cells treated with 10 μM forskolin added along with the [3H]dopamine only at the 4-, 5-, 10-, 20-, and 30-min time points (arrows). Data shown are for three independent experiments performed in triplicate (means ± S.E.).
[3H]Dopamine Efflux Assay. Cells were prepared and counted as described above in [3H]Dopamine Uptake Assay. [3H]Dopamine was diluted with serum-free DMEM and used at 20 μM to load the cells at 25°C for a period of 20 min, at which time the intracellular [3H]dopamine concentration had reached steady state. Nonspecific uptake is obtained by addition of 10 μM indatraline 15 min before and during radiation loading. The radiation loading was performed in serum-free DMEM or in modified Krebs-HEPES buffer (25 mM HEPES, 120 mM NaCl, 5 mM KC1, 2.5 mM CaCl2, 1.2 mM MgSO4, 1 μM pargyline, 2 mg/ml glucose, and 0.2 mg/ml ascorbic acid, pH 7.5). The uptake was terminated by aspiration of the loading media or buffer. The cells were quickly washed with fresh ice-cold serum-free DMEM or efflux assay buffer (25 mM HEPES, 120 mM NaCl, 5 mM KC1, 1.2 mM MgSO4, 1 μM pargyline, 2 mg/ml glucose, and 0.2 mg/ml ascorbic acid, pH 7.5), and drugs in either serum-free DMEM or efflux buffer were added. Efflux was initiated by placing the tissue culture plate in a 37°C water bath and terminated by aspiration of the medium or buffer. The PKA inhibitor H89 (10 μM), the PKC inhibitor Ro32-0432 (10 μM), or the DAT inhibitor methylphenidate (10 μM) was mixed with the indicated drugs in either serum-free DMEM or efflux buffer. The cell lysates was prepared and counted as described above in [3H]Dopamine Uptake Assay. Nonspecific uptake was subtracted from the total to yield specific accumulation of radiolabeled substrate. In time course efflux assays, the dopamine level at time of 0 min was taken as control. In dose-response efflux assays, all cells were incubated with drugs or vehicle for 30 min immediately following 20 nM [3H]dopamine loading, and the values obtained were compared with the [3H]dopamine level at time of 0 min following 20 nM [3H]dopamine loading. All experiments were conducted in triplicate three times and average dpm values were calculated for evaluation of the [3H]dopamine efflux.
Results
Dose-Dependent TAAR1 Activation by Dopamine and (+)-Methamphetamine. To illustrate that TAAR1 is activated by either dopamine or (+)-methamphetamine, we used a dual luciferase assay to evaluate luciferase production resulting from CRE-Luc expression, as an indicator of total cAMP accumulation. We evaluated the CRE-Luc response to a range of doses (10–8–10–4 M) of dopamine or (+)-methamphetamine in stable DAT cells transiently transfected with TAAR1 (DAT-TAAR1) or pcDNA3.1 (DAT), along with the reporter vectors CRE-Luc and pGL4.73. In DAT-TAAR1 cells, either dopamine (Fig. 1A) or (+)-methamphetamine (Fig. 1B) dose-dependently induced cAMP accumulation. EC50 values were 0.95 and 0.32 μM, respectively, and the maximal response was reached at the concentration of 10 μM in both cases. (+)-Methamphetamine was more efficacious than dopamine. However, in DAT cells, neither dopamine (Fig. 1A) nor (+)-methamphetamine (Fig. 1B) induced dose-dependent cAMP accumulation. Accordingly, the dopamine or (+)-methamphetamine response in DAT-TAAR1 cells is TAAR1-dependent, and the TAAR1 activation leads to increases in cAMP accumulation.
TAAR1 Activation by Dopamine or (+)-Methamphetamine Regulates [3H]Dopamine Uptake. To determine whether TAAR1 activation by dopamine or (+)-methamphetamine can regulate DAT function, DAT was transiently transfected into stable TAAR1 cells and HEK293 cells, resulting in TAAR1-DAT and DAT cells, respectively, and dopamine uptake assays were assessed. At 10 nM [3H]dopamine, TAAR1-DAT and DAT cells displayed similar time-dependent [3H]dopamine uptake (Fig. 2A). Uptake at 100 nM [3H]dopamine ([3H]dopamine/dopamine; 1:10 dilution) occurred similarly in TAAR1-DAT cells and DAT cells during the first 3 min, but then it abruptly halted in the TAAR1-DAT cells (Fig. 2B). After 20 min, total [3H]dopamine uptake was similar in both cell lines. Uptake at 1 μM[3H]dopamine ([3H]dopamine/dopamine; 1:50 dilution) resulted in a similar but more rapid effect, in which uptake was similar in TAAR1-DAT cells and DAT cells during the first 2 min; thereafter, there was an abrupt halt, or reduction in [3H]dopamine accumulation in the TAAR1-DAT cells. Next, there was an apparent increased rate (slope) of [3H]dopamine uptake observed, suggesting a potential of achieving greater uptake after 30 min, compared with DAT-only cells. Nevertheless, total [3H]dopamine uptake was similar in both cell lines after 20 min (Fig. 2C). In parallel to Fig. 2B, uptake at 10 nM [3H]dopamine combined with 90 nM (+)-methamphetamine displayed a similar TAAR1 effect but with a more prolonged halt in uptake in the TAAR1-DAT cells (Fig. 2D).
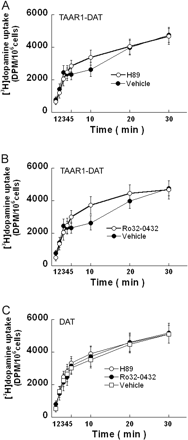
Effects of TAAR1 on [3H]dopamine uptake are phosphorylation-dependent. HEK293 and stable TAAR1 cells were transiently transfected with DAT for 24 h. The PKA inhibitor H89 (10 μM) or the PKC inhibitor Ro32-0432 (10 μM) was added 10 min before 100 nM [3H]dopamine ([3H]dopamine/dopamine; 1:10 dilution) loading at 25°C in serum-free DMEM. In DAT-transfected stable TAAR1 cells, either H89 (A) or Ro32-0432 (B) prevented the abrupt halt in 100 nM [3H]dopamine uptake. In DAT-transfected HEK293 cells, neither H89 nor Ro32-0432 had an effect on dopamine uptake (C). Data shown are for three independent experiments performed in triplicate (means ± S.E.).
TAAR1 effects on [3H]dopamine efflux. Stable TAAR1 cells and HEK293 cells were transiently transfected with DAT for 24 h, to generate TAAR1-DAT and DAT cells, respectively. After incubation in growth medium for 12 h, the cells were exposed to [3H]dopamine (20 nM) at 25°C in serum-free DMEM for 20 min. Uptake was terminated by aspiration of the loading medium. The cells were quickly washed and exposed to 10 μM dopamine (A, TAAR1-DAT cells; B, DAT cells) or 10 μM (+)-methamphetamine (C, TAAR1-DAT cells; D, DAT cells) in serum-free DMEM for different time or to varied concentrations (1 nM–100 μM) of dopamine (E) or methamphetamine (F) for 30 min. In the dose-response efflux assays, values obtained were compared with the [3H]dopamine level at time of 0 min following 20 nM [3H]dopamine loading. Both dopamine and (+)-methamphetamine time-dependently (A and C) or dose-dependently (E and F) induced [3H]dopamine efflux in TAAR1-DAT cells, which also showed spontaneous [3H]dopamine efflux over time (A and C). In DAT cells, neither 10 μM dopamine (B) nor 10 μM (+)-methamphetamine (D) induced [3H]dopamine efflux, and there was no spontaneous [3H]dopamine efflux. Data shown are for three independent experiments performed in triplicate (means ± S.E.).
D1 Receptor Activation or Forskolin Stimulation Mimic the Effect of TAAR1 on [3H]Dopamine Uptake. To clarify whether the effect of TAAR1 on dopamine uptake is associated with cAMP signaling, we evaluated the effect of dopamine D1 receptor and forskolin on the dopamine uptake. DAT was transiently transfected into stable D1 cells and HEK293 cells, resulting in D1-DAT and DAT cells, respectively, and dopamine uptake was assessed. Uptake at 100 nM [3H]dopamine ([3H]dopamine/dopamine; 1:10 dilution) occurred similarly in D1-DAT cells and DAT cells during the first 2 min, but then it abruptly halted in the D1-DAT cells (Fig. 2E). After 20 min, total [3H]dopamine uptake was similar in both cell lines. The uptake halt induced by D1 activation seemed more rapidly relative to the effect of TAAR1 (compare with Fig. 2B). To explore whether forskolin stimulation could mimic the effect of TAAR1 or D1 on dopamine uptake, we assessed dopamine uptake in DAT cells in the presence or absence of forskolin. Forskolin (10 μM) added along with the [3H]dopamine at the 4-, 5-, 10-, 20-, and 30-min time points resulted in a similar effect on dopamine uptake (Fig. 2F).
The Effect of TAAR1 on [3H]Dopamine Uptake Is Both PKA- and PKC-Dependent. To further characterize the effect of TAAR1 on dopamine uptake, we compared the effects of PKA and PKC inhibitors on dopamine uptake in TAAR1-DAT and DAT cells. The PKA inhibitor H89 (10 μM) or the PKC inhibitor Ro32-0432 (10 μM) was added to the cells 10 min before and remained during dopamine uptake. Either inhibitor prevented the TAAR1-dependent effect on [3H]dopamine uptake at 100 nM (Fig. 3, A and B). However, in DAT cells, neither H89 nor Ro32-0432 altered [3H]dopamine uptake (Fig. 3C). Accordingly, TAAR1-mediated dopamine uptake inhibition is associated with both PKA- and PKC-dependent phosphorylation.
TAAR1 Activation Induces [3H]Dopamine Efflux. To determine whether TAAR1 activation by dopamine or (+)-methamphetamine can regulate DAT function, we further evaluated the effect of TAAR1 on the dopamine efflux. TAAR1-DAT and DAT cells were loaded with 20 nM [3H]dopamine for 20 min and then exposed to 10 μM dopamine (Fig. 4, A and B) or 10 μM(+)-methamphetamine (Fig. 4, C and D) in serum-free DMEM for different times, or to varied concentrations (1 nM–100 μM) of dopamine (Fig. 4E) or (+)-methamphetamine (Fig. 4F) for 30 min. In TAAR1-DAT cells, a spontaneous [3H]dopamine efflux is apparent, and 10 μM dopamine (Fig. 4A) or 10 μM(+)-methamphetamine (Fig. 4B) promoted [3H]dopamine efflux. However in DAT cells, there was no [3H]dopamine efflux, either spontaneous or in the presence of dopamine or (+)-methamphetamine (Fig. 4, B and D). Notable is a sharp increase at 3 to 5 min in dopamineand (+)-methamphetamine-induced [3H]dopamine efflux (Fig. 4, A and C), parallel to the timing of the “halting” effect in [3H]dopamine uptake experiments (Fig. 2, B–D). Apparently, both the spontaneous efflux and the efflux induced by dopamine or (+)-methamphetamine are TAAR1-dependent in TAAR1-DAT cells. We further compared [3H]dopamine efflux in TAAR1-DAT cells and DAT cells in response to a range of concentrations (10–9–10–4 M) of dopamine or (+)-methamphetamine. In TAAR1-DAT cells, both dopamine and (+)-methamphetamine dose-dependently induced [3H]dopamine efflux, with maximal efflux at 100 μM for dopamine (49%; Fig. 4E) and (+)-methamphetamine (59%; Fig. 4F). However in DAT cells, neither dopamine nor (+)-methamphetamine induced [3H]dopamine efflux.
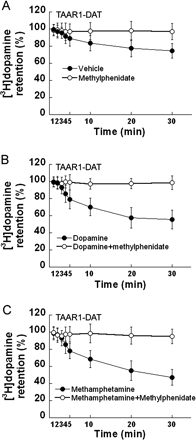
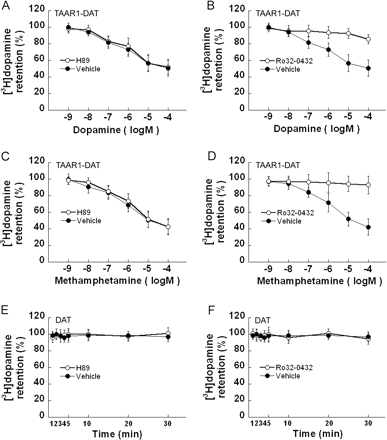
[3H]Dopamine Efflux in TAAR1-DAT Cells Is Blocked by Methylphenidate or a PKC Inhibitor. To determine whether [3H]dopamine efflux was mediated via DAT or was phosphorylation-dependent, we evaluated the effects of DAT blockade and phosphorylation inhibition on [3H]dopamine efflux from TAAR1-DAT cells. TAAR1-DAT cells were loaded with 20 nM [3H]dopamine for 20 min. Methylphenidate blocked spontaneous [3H]dopamine efflux (Fig. 5A) as well as [3H]dopamine efflux in the presence of 10 μM dopamine (Fig. 5B) or 10 μM (+)-methamphetamine (Fig. 5C). Other TAAR1-DAT cells were loaded with 20 nM [3H]dopamine for 20 min and exposed to a range of concentrations (10–9–10–4) of dopamine or (+)-methamphetamine in the presence or absence of 10 μM H89 or 10 μM Ro32-0432 (Fig. 6, A–D). H89 did not affect [3H]dopamine efflux induced by either dopamine (Fig. 6A) or (+)-methamphetamine (Fig. 6C), but Ro32-0432 attenuated [3H]dopamine efflux induced by dopamine (Fig. 6B) or (+)-methamphetamine (Fig. 6D). In DAT cells loaded with 20 nM [3H]dopamine for 20 min, neither H89 (Fig. 6E) nor Ro32-0432 (Fig. 6F) had an effect on [3H]dopamine levels. Accordingly, both the spontaneous efflux and the efflux induced by dopamine or (+)-methamphetamine are DAT-mediated and PKC-dependent.
[3H]Dopamine Efflux in DAT Cells Occurs in Assay Buffer but Is Not Affected by Methylphenidate or Ro32-0432. [3H]Dopamine efflux has been reported in DAT-expressing cells in vitro in assays using assay buffer (Eshleman et al., 1994). Our results from efflux assays performed in serum-free DMEM did not show spontaneous dopamine efflux or ability of dopamine or (+)-methamphetamine to induce dopamine efflux in the absence of TAAR1. Accordingly, we evaluated dopamine efflux using identical assay buffers as in Eshleman et al. (1994). Stable DAT cells were loaded with 20 nM [3H]dopamine in modified Krebs-HEPES buffer and then exposed to 10 μM(+)-methamphetamine or vehicle in efflux assay buffer. Vehicle caused an apparent loss of [3H]dopamine, and 10 μM (+)-methamphetamine slightly augmented this loss (Fig. 7A). However, the [3H]dopamine loss in the presence of vehicle (Fig. 7B) or (+)-methamphetamine (Fig. 7C) was not affected by 10 μM methylphenidate or 10 μM Ro32-0432. Accordingly, the dopamine loss in the assay buffer was independent of DAT and PKC.
Methylphenidate blocks TAAR1-dependent [3H]dopamine efflux. Stable TAAR1 cells were transiently transfected with DAT (TAAR1-DAT) for 24 h. After incubation in growth medium for 12 h, cells were exposed to [3H]dopamine (20 nM) at 25°C in serum-free DMEM for 20 min. Uptake was terminated by aspiration of the loading medium. The cells were quickly washed and exposed to vehicle (A), 10 μM dopamine (B), or 10 μM(+)-methamphetamine (C) in the presence or absence of 10 μM methylphenidate, in serum-free DMEM for different times. Methylphenidate inhibited spontaneous [3H]dopamine efflux (A) as well as efflux induced by dopamine (B) or (+)-methamphetamine (C). Data shown are for three independent experiments performed in triplicate (means ± S.E.).
Discussion
TAAR1 activation has been evaluated in different studies that provide substantial evidence that TAAR1 is a G protein-coupled receptor associated with cAMP signaling (Borowsky et al., 2001; Bunzow et al., 2001; Miller et al., 2005; Hart et al., 2006; Wainscott et al., 2007, Xie et al., 2007). TAAR1 is expressed in brain monoaminergic regions, and its coexpression with DAT in substantia nigra and monoamine transporter-modulated activation suggests that TAAR1 may be functionally related with monoamine transporters (Borowsky et al., 2001; Miller et al., 2005; Xie et al., 2007). The current study provides evidence that TAAR1 modulates DAT function in vitro.
[3H]Dopamine efflux in TAAR1-DAT cells is PKC-dependent. Stable TAAR1 cells and HEK293 cells were transiently transfected with DAT for 24 h to generate TAAR1-DAT and DAT cells, respectively. After incubation in growth medium for 12 h, the cells were exposed to 20 nM [3H]dopamine at 25°C in serum-free DMEM for 20 min. Uptake was terminated by aspiration of the loading medium. The cells were quickly washed and exposed to different doses (10–9–10–4) of dopamine (A and B) or (+)-methamphetamine (C and D) in the presence or absence of 10 μM H89 or 10 μM Ro32-0432, or to 10 μM H89 or 10 μM Ro32-0432 only for different time (E and F), in serum-free DMEM. In DAT-TAAR1 cells, H89 did not affect [3H]dopamine efflux induced by either dopamine (A) or (+)-methamphetamine (C), but Ro32-0432 attenuated [3H]dopamine efflux induced by dopamine (B) or (+)-methamphetamine (D). In DAT cells, neither H89 (E) nor Ro32-0432 (F) had an effect on [3H]dopamine levels in the cells. Data shown are for three independent experiments performed in triplicate (means ± S.E.).
TAAR1 coexpressed with DAT was strongly activated by dopamine or (+)-methamphetamine measured by the CRE-Luc assay, with EC50 values of 950 and 320 nM, respectively. We then assessed the effect of TAAR1 activation by dopamine or (+)-methamphetamine on DAT-mediated [3H]dopamine uptake. Dopamine and (+)-methamphetamine are not only TAAR1 agonists but also DAT substrates, and accordingly, it is difficult to distinguish between competitive and noncompetitive effects of dopamine or (+)-methamphetamine. Therefore, to determine the effect of TAAR1 activation on dopamine uptake, we used DAT-expressing cells that did not express TAAR1 as a control. Based on the CRE-Luc assay data, TAAR1 activation by 10 nM dopamine is very weak. Indeed, TAAR1 did not display any influence on [3H]dopamine uptake at 10 nM. However, at 100 nM or 1 μM[3H]dopamine, we observed an abrupt halt of [3H]dopamine uptake after 3 or 2 min, respectively, in the presence of TAAR1. Likewise, 90 nM (+)-methamphetamine added with 10 nM [3H]dopamine (total concentration of substrate 100 nM) induced uptake inhibition, but it was more prolonged than dopamine, perhaps due to its greater efficacy. This phenomenon may reflect time-dependent accumulation of dopamine or (+)-methamphetamine within the cells to reach a sufficient concentration to activate TAAR1 within a cellular compartment or agonist-induced translocation of TAAR1 to the membrane. With regard to DAT function, the effects of TAAR1 activation by dopamine or (+)-methamphetamine may result from a concurrent increase in substrate efflux, as suggested by the [3H]dopamine efflux experiments, rather than a pausing of the uptake process itself. Thereafter, an apparent increased rate (slope) of [3H]dopamine uptake was observed, particularly at 1 μM dopamine in TAAR1-DAT cells (Fig. 2C) and 100 nM dopamine in D1-DAT cells (Fig. 2E). Accordingly, there may be a potential of achieving greater uptake after 30 min, compared with DAT-only cells.
Elevated cAMP induced by TAAR1 activation may underlie TAAR1 effects on the DAT, because similar uptake inhibition was observed by D1 activation and could be mimicked by forskolin treatment. D1 dopamine receptor activation resulted in a similar [3H]dopamine uptake inhibition that was faster than the TAAR1 effect. Although D1 receptors are postsynaptic and are not expressed in dopamine neurons, we reasoned that D1 substitution for TAAR1 would be an important control experiment because, like TAAR1, D1 is a G protein-coupled receptor linked to adenylyl cyclase and responds to dopamine. D1, however, is largely expressed on the cell membrane (Trogadis et al., 1995), whereas TAAR1 is apparently largely intracellular (Bunzow et al., 2001; Miller et al., 2005). In addition, PKA or PKC inhibitors prevented TAAR1 effects on [3H]dopamine uptake, demonstrating that the dopamine uptake inhibition induced by TAAR1 activation was phosphorylation-dependent. It has been reported that protein kinase A activity mediates the kinetic up-regulation of DAT (Batchelor and Schenk, 1998). In our experiments, the inhibition of dopamine uptake was transient, and thereafter, an apparent increased rate (slope) of [3H]dopamine uptake was observed, which may reflect an increased [3H]dopamine uptake velocity at this time interval.
It has been known for decades that the DAT is a direct target of amphetamine-related psychostimulant drugs and that these drugs induce dopamine efflux via reversal of DAT transport direction (Azzaro and Rutledge, 1973; Fischer and Cho, 1979; Liang and Rutledge, 1982). However, it remains unclear whether the DAT is directly modulated by the drugs or the drugs affect the DAT through interaction with other cellular components. We compared [3H]dopamine efflux induced by methamphetamine or dopamine in DAT cells with or without TAAR1 coexpression. Spontaneous loss of [3H]dopamine was augmented by 10 μM dopamine or 10 μM(+)-methamphetamine, both of which promoted [3H]dopamine efflux in a dose-dependent manner in the TAAR1-DAT cells. Both the spontaneous and the substrate-induced [3H]dopamine efflux was blocked by either the dopamine transporter blocker methylphenidate (Wall et al., 1995; Johnson et al., 1998), or PKC inhibition, providing evidence that the [3H]dopamine efflux was via the DAT and dependent on PKC-driven phosphorylation. Substrate-induced efflux is a hall-mark of amphetamine-related psychostimulant action (for review, see Sulzer et al., 2005), but we did not observe any efflux in DAT cells in the absence of TAAR1 in our experiment that were performed in DMEM. We performed further experiments that substituted modified Krebs-HEPES assay buffer for DMEM, in an effort to replicate the reported spontaneous and methamphetamine-induced [3H]dopamine release observed in COS-7-hDAT cells (Eshleman et al., 1994). Indeed, we observed [3H]dopamine loss from the DAT-transfected HEK293 cells, but this loss was insensitive to either methylphenidate or PKC inhibition, indicating that the [3H]dopamine loss was not via DAT or dependent on phosphorylation. Limited studies using trypan blue exclusion indicated that the [3H]dopamine loss from the cells in assay buffer did not correlate with a greater incidence of cell death (data not shown). Apparently, DAT-mediated [3H]dopamine efflux, either spontaneous or induced by dopamine or (+)-methamphetamine, does not occur in DAT-transfected HEK293 cells, but it can be elicited via TAAR1 activation.
In conclusion, the current data demonstrate that DAT-mediated uptake of dopamine can be modulated by G protein-coupled receptor activation. Furthermore, DAT-mediated efflux of dopamine may be dependent upon phosphorylation cascades, which can be triggered by G protein-coupled receptors on dopamine neurons. It is likely that, in brain, the regulation of DAT function is influenced by the integrative signaling from the repertoire of receptors expressed in the dopamine neurons, of which TAAR1 is a contributor. All of the data presented in this study were generated in HEK293 cells heterologously expressing the receptors and DAT, and accordingly, additional studies in native systems need to be done to confirm the present findings in separate studies. Nevertheless, this study provides the first evidence that TAAR1 activation by (+)-methamphetamine can result in inhibition of dopamine uptake and promotion of dopamine efflux by DAT. In this regard, TAAR1 is a potentially important target for therapeutics for the treatment of methamphetamine addiction.
Apparent [3H]dopamine efflux in Krebs-HEPES buffer is not via DAT. Stable DAT cells were exposed to 20 nM [3H]dopamine at 25°C in modified Krebs-HEPES buffer for 20 min. The uptake was terminated by aspiration of the buffer. A, cells were quickly washed and exposed to 10 μM(+)-methamphetamine or vehicle. B, cells were exposed to vehicle, 10 μM methylphenidate, or 10 μM Ro32-0432. C, cells were exposed to 10 μM(+)-methamphetamine with or without 10 μM methylphenidate or 10 μM Ro32-0432. There was a time-dependent loss of [3H]dopamine from the cells that is slightly enhanced by methamphetamine (A), but neither methylphenidate nor Ro32-0432 affected this spontaneous [3H]dopamine efflux (B) or the efflux in the presence of methamphetamine (C). Data shown are for three independent experiments performed in triplicate (means ± S.E.).
Acknowledgments
We thank Jennifer Carter for administrative support.
Footnotes
-
This work was supported by National Institutes of Health Grants DA016606, DA06303, DA021180, and RR00168. This work was presented as follows: Xie Z, Chen G, Yao W, Bahn M, and Miller GM (2006) Functional interactions of rhesus monkey trace amine receptor 1 (rhTA1) with monamine transporters and receptors, in 2006 Abstract Viewer/Itinerary Planner; 2006 Oct 17; Atlanta, GA. Program no. 451.14, Society for Neuroscience, Washington, DC.
-
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
-
doi:10.1124/jpet.106.117382.
-
ABBREVIATIONS: TAAR1, trace amine-associated receptor 1; DAT, dopamine transporter; HEK, human embryonic kidney; CRE, cAMP response element; Luc, luciferase; D1, dopamine D1 receptor; H89, N-[2-(4-bromocinnamylamino)ethyl]-5-isoquinoline; PLB, passive lysis buffer; RLU, relative light unit; PKA, protein kinase A; PKC, protein kinase C; Ro32-0432, 2-(8-[(dimethylamino)methyl]-6,7,8,9-tetrahydropyrido[1,2-a]indol-3-yl)-3-(1-methylindol-3-yl)maleimide.
- Received November 21, 2006.
- Accepted January 16, 2007.
- The American Society for Pharmacology and Experimental Therapeutics