Abstract
Antidepressant-like effects of metabotropic glutamate (mGlu)5 receptor antagonists have been reported previously. We now provide definitive identification of mGlu5 receptors as a target for these effects through the combined use of selective antagonists and mice with targeted deletion of the mGlu5 protein. In these experiments, the mGlu5 receptor antagonists 2-methyl-6-(phenylethynyl)-pyridine (MPEP) and the more selective and metabolically stable analog 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine (MTEP) decreased immobility in the mouse forced swim test, a test predictive of antidepressant efficacy in humans. mGlu5 receptor knockout mice had a phenotype in the forced swim test that was congruent with the effects of receptor blockade; mGlu5 receptor knockout mice were significantly less immobile than their wild-type counterparts. Consistent with mGlu5 receptor mediation of the antidepressant-like effects of MPEP, the effects of MPEP were not observed in mGlu5 receptor knockout mice, whereas comparable effects of the tricyclic antidepressant imiprimine remained active in the mutant mice. MPEP and imiprimine resulted in a synergistic antidepressant-like effect in the forced swim test. The drug interaction was not likely because of increased levels of drugs in the brain, suggesting a pharmacodynamic interaction of mGlu5 and monoaminergic systems in this effect. Thus, the present findings substantiate the hypothesis that mGlu5 receptor antagonism is associated with antidepressant-like effects. This mechanism may not only provide a novel approach to the therapeutic management of depressive disorders but also may be useful in the augmentation of effects of traditional antidepressant agents.
Monoamine-based antidepressants have been the primary pharmacological approach to the treatment of mood disorders, but these agents suffer from some limitations in both safety and efficacy that have prompted the continued search for novel therapeutic targets beyond the monoamine system (cf., Skolnick et al., 2001). Glutamate, the major excitatory neurotransmitter used by the mammalian brain, binds to both ion channel-associated (iontropic) and G protein-coupled (metabotropic) receptor subtypes. The glutamatergic system plays a critical role in the pathophysiology of depression and in the action of antidepressant drugs (Skolnick et al., 2001; Kugaya and Sanacora, 2005; Alt et al., 2006). Modulation of ionotropic (e.g., AMPA receptors and NMDA receptors) and metabotropic glutamate (mGlu) receptors (see below) produces antidepressant-like effects in preclinical models. The involvement of ionotropic glutamate receptors in the control of mood disorders and the mechanism of antidepressants has been studied much more extensively than the metabotropic glutamate receptors. However, recent attention has been directed to the potential role of the mGlu receptors.
The mGlu receptors are classified into three groups, with eight subtypes: group I (mGlu1 and -5), group II (mGlu2 and -3), and group III (mGlu4, -6, -7, and -8) (Schoepp and Conn, 1993) based on amino acid sequence homology, functional coupling, and pharmacology (Conn and Pin, 1997). Group I mGlu receptors are distributed primarily on postsynaptic excitatory synapses, which contain mGlu1 and mGlu5 receptor subtypes. Several studies indicate the mGlu1 and mGlu5 receptors are associated with a range of neurological and psychiatric disorders, such as anxiety, drug abuse, neurodegeneration, and depression (Spooren et al., 2003; Paul and Skolnick, 2003; Swanson et al., 2005).
The discovery of selective noncompetitive mGlu5 receptor antagonists, such as 2-methyl-6-(phenylethynyl)-pyridine (MPEP), together with the finding that antidepressant treatment changed the expression of mGlu5 receptors in rat hippocampus (Gasparini et al., 1999; Wieronska et al., 2001) prompted further investigations into the association of mGlu5 receptors and affective disorders (anxiety and depression). Data have shown that MPEP exerts antidepressant-like effects in behavioral tests (Wieronska et al., 2002). Antidepressant treatments and stress, known to affect neurobiological targets putatively relevant to depression, have also affected mGlu5 receptors (cf., Pilc and Legutko, 1995; Pilc et al., 1998; Wieronska et al., 2001; Smialowska et al., 2002).
Although these biochemical findings cannot yet bring clarity to the precise role that mGlu5 receptors may play in depression or antidepressant activity, these data along with an antidepressant-like profile for an mGlu5 receptor antagonist suggest that a role for mGlu5 in the effects of antidepressants is likely. The mGlu5 antagonist hypothesis would be strengthened by data on additional selective ligands. This is especially important because MPEP exhibits weak interactions with other receptors such mGlu4 (Mathiesen et al., 2003), NMDA receptors, and noradrenaline transporters (Heidbreder et al., 2003). An MPEP analog, MTEP, was discovered with increased potency, in vivo selectivity (Cosford et al., 2003), and improved bioavailability (Roppe et al., 2004). We evaluated MTEP in the forced swim test, a model with high predictive validity for the detection of antidepressants (cf., Porsolt et al., 1977). Additional assessments of the mGlu5 receptor target were made through the use of mice with targeted deletions of the mGlu5 receptor protein. To this end, we evaluated whether MPEP evokes an antidepressant-like effect in mutant mice lacking mGlu5 receptors. We also hypothesized that there might be a potential synergistic effect of imipramine and mGlu5 antagonism that has been observed previously with another potential glutamatergic antidepressant (Li et al., 2003). The results of the present experiments provide additional pharmacological validation and transgenic mouse data consistent with the idea that mGlu5 receptor blockade might be a valuable target for novel antidepressants. Moreover, the drug interaction data suggest that mGlu5 receptor blockade may interact in a positive manner with other antidepressant mechanisms to provide additional efficacy in treatment-resistant patients.
Materials and Methods
Animals. Male NIH Swiss mice (purchased from Harlan, Indianapolis, IN) weighing 20 to 25 g were housed in plastic cages (40.6 × 20.3 × 15.2 cm) with 10 to 12 mice/cage in a vivarium at least 7 days before the experiments. Male mGlu5 knockout (KO) and wild-type (WT) control mice were generated as described by Jia et al. (1998) and bred at Taconic Farms (Transgenic Models and Services Division, Germantown, NY) by heterozygous × heterozygous breeding. In this way, mGlu5 receptor knockout mice and their age-matched littermate wild-type controls were used in these experiments. The receptor KO and WT mice were housed in plastic cages (31.5 × 17.5 × 15 cm) with two to five mice per cage. KO and WT mice were housed in separate cages in our laboratory vivarium for ease of identification. It should be noted that the background strain for the KO and WT mice is C57/BL6. Water and rodent chow were available freely during this acclimation period, with lights on at 6:00 AM and off at 6:00 PM. Experiments were conducted between 12:00 PM and 4:00 PM. Animals were removed from the vivarium to the testing area in their home cages and allowed to adapt to the new environment for at least 1 h before testing. All experiments were conducted according to the Guidelines for Care and Use of Laboratory Animals under protocols approved by a local animal care and use committee. All mice were experimentally and drug naive at the time of testing and were used for only one experiment.
Forced Swim Test. This test was performed using the original method described by Porsolt et al. (1977). In brief, mice were placed individually in clear plastic cylinders (10 cm in diameter by 25 cm in height) filled to 6 cm with 22-25°C water for 6 min. The duration of immobility was recorded during the last 4 min of a 6-min trial. A mouse was regarded as immobile when floating motionless or making only those movements necessary to keep its head above the water.
Acute Dosing Experiment. Animals were weighed and pretreated with either vehicle, 10 or 30 mg/kg MPEP, 3, 10 or 30 mg/kg MTEP, or 15 mg/kg imipramine 15 min before the test. In drug combination experiments, mice were dosed with vehicle or 5 or 15 mg/kg imipramine approximately 1 min before a second dosing of either vehicle or 3 and 10 mg/kg MPEP. The FST was conducted 15 min after the second injection.
Subchronic Dosing Experiment. Vehicle or 10 or 30 mg/kg MPEP was injected once a day at the same time daily for five consecutive days. Mice were then tested in the FST on day 5, 15 min after the final drug administration.
Locomotor Activity. Locomotor activity was assessed in Plexiglas locomotor activity chambers (40.6 × 20.3 × 15.2 cm) in a 20 station photobeam activity system (San Diego Instruments, San Diego, CA) with seven photocells per station in lighted room. Mice not previously exposed to the locomotor chambers were dosed with 30 mg/kg MPEP or MTEP and placed in the activity chambers 15 min later. Data are expressed as total ambulation (where ambulation was defined as the sequential breaking of adjacent photobeams) in 3-min (MPEP) or 5-min (MTEP) intervals for the entire observation period.
Brain Sample Preparation and Drug Concentration Determination. Seven groups (four mice/group) of naive NIH Swiss male mice were evaluated for brain concentrations of MPEP or imipramine after systemic treatment with combinations of MPEP and imipramine (5 mg/kg). Mice received either saline or imipramine approximately 1 min before a second injection with either vehicle or MPEP. Animals were decapitated 15 min after the second injection, and whole brains were rapidly removed and placed on dry ice. Each brain was bisected mid-sagittally, and half of the brain was homogenized with an ultrasonic dismembrator probe (model 60; Fisher Scientific, Pittsburgh, PA) in 4 volumes (w/v) of acetonitrile (ACN) containing 0.1% formic acid. The homogenates were centrifuged (model 5417R; Eppendorf, Westbury, NY) at 14,000 rpm (20,000g) for 16 min. Then, 100 μl of the supernatant was added to 500 μl of water in an autosampler vial for analysis. Separation was achieved by injecting 10 μl onto a high-performance liquid chromatography (Shimadzu, Columbia, MD) using a C18 column (2.1 × 50 mm, 3.5-μm particle size, SB Zorbax; Agilent Technologies, Wilmington, DE), with a gradient elution of water/ACN, both with 0.1% formic acid, from 20 to 90% ACN. Compounds eluting from the column were detected using an API3000 triple quadrapole mass spectrometer (Applied Biosystems, Foster City, CA) using positive electrospray ionization and multiple reaction monitoring mode. The mass transitions monitored were m/z 194 and 152 for MPEP and 281 and 193 for imipramine. Chromatographic assays were calibrated using standard curves generated by extracting a series of mouse brain tissue samples to which known quantities of analyte had been added.
Compounds. Imipramine was purchased from Sigma-Aldrich (St. Louis, MO). MPEP and MTEP were synthesized at Lilly Research Laboratories (Indianapolis, IN). Imipramine was dissolved in water, whereas MPEP and MTEP were suspended in 1% carboxymethylcelluose/0.25% Tween 80/0.05% antifoam (Dow Corning, Midland, MI) by homogenization. All compounds and vehicle were administered intraperitoneally in a volume of 10 ml/kg.
Statistical Analyses. All data were presented as the mean ± S.E.M. and analyzed with appropriate analysis of variance followed post hoc Dunnett's test for comparison of treated groups with vehicle controls. Individual contrast hypotheses were evaluated with Student's t test. Comparisons with P < 0.05 were considered statistically significant.
Results
Effects of MPEP and MTEP in the FST. Both MPEP (10-30 mg/kg) and MTEP (3-30 mg/kg) produced a dose-dependent reduction in immobility in the FST after acute administration. Statistically significant reductions in the duration of immobility were detected at a dose of 30 mg/kg of either compound (Fig. 1). The maximal reduction of immobility produced by MPEP or MTEP was comparable with that produced by 15 mg/kg imipramine (Fig. 1). When 5 mg/kg MPEP was administered daily for five consecutive days and tested for activity in the FST after dosing on day 5, MPEP significantly reduced immobility as with acute dosing (Fig. 1).
Effects of MPEP and MTEP on Locomotor Activity. Because increases in motor activation by a compound could register a false positive in the FST, MPEP and MTEP were tested for their ability to increase locomotor activity at antidepressant-like doses. MPEP (30 mg/kg) did not affect exploratory locomotor activity in mice when locomotor activity was accessed for 30 min after a 15-min pretreatment (Fig. 2A). Although MTEP tended to increase locomotor activity, these effects were not statistically different from that of vehicle control animals (Fig. 2B).
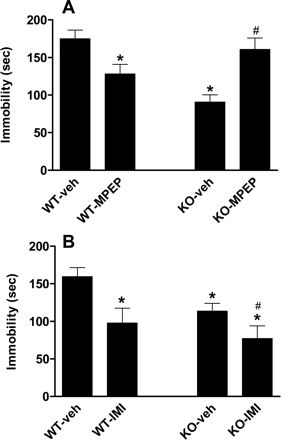
Antidepressant-Like Phenotype of mGlu5 Receptor Knockout Mice. The mGlu5 receptor KO mice had a comparable body weight compared with the WT mice. The KO mice treated with vehicle displayed a significant reduction of immobility compared with WT mice treated with vehicle in the forced-swim test (Fig. 3A).
Effect of MPEP and Imipramine in mGlu5 Receptor Knockout Mice. Treatment of WT mice with 30 mg/kg MPEP significantly reduced the duration of immobility compared with the vehicle control mice, whereas it did not decrease immobility when administered to mGlu5 receptor KO mice (Fig. 3A). MPEP did however increase immobility in the KO mice compared with vehicle administration in the mGlu5 receptor KO. In contrast, when 15 mg/kg imipramine was administered to either WT or KO mice, imipramine significantly reduced the duration of immobility compared with their respective vehicle control mice in the same experiment (Fig. 3B).
Effects of MPEP and MTEP on immobility in the forced swim test. MPEP, MTEP, or imipramine (15 mg/kg) was given i.p., 15 min before the test. In another experiment, MPEP was administered i.p. once a day for 5 days. On day 5, the mice were tested for the first time, 15 min after dosing. The data are expressed as the means ± S.E.M. of eight animals. Asterisks indicate significant differences from vehicle control (P < 0.05).
Effects of MPEP and MTEP on locomotor activity. MPEP or MTEP was given as described under Materials and Methods. Locomotor activity was measured immediately after placing the animals pretreated with 30 mg/kg MPEP into the activity chambers (A) or in mice dosed with 30 mg/kg MTEP (B). Values represent the means ± S.E.M. of six animals.
Effects of Coadministration of MPEP and Imipramine in the FST. MPEP at 3 and 10 mg/kg or imipramine at 5 mg/kg was ineffective in the FST when given alone. However, joint administration of these subactive doses of MPEP and imipramine resulted in a significant reduction in immobility time in this test with the combination of 10 mg/kg MPEP and 5 mg/kg imipramine (Fig. 4).
Effect of MPEP on Brain Concentrations of Imipramine in Mice. Under identical conditions as used in the drug combination experiment in the FST, mouse brains were removed 15 min after MPEP administration, and the concentrations of MPEP and imipramine were determined. Brain levels of MPEP (at doses of 3 and 10 mg/kg) were not significantly altered by the coadministration of imipramine (Fig. 5A). MPEP (at doses of 3 and 10 mg/kg) also did not significantly affect the concentrations of 5 mg/kg imipramine in the mouse brains (Fig. 5B). The brain imipramine concentration was approximately three times higher when mice were dosed with 15 mg/kg instead of 5 mg/kg imipramine.
Discussion
A key role for glutamate in depressive disorders has been suggested, and behavioral, pharmacological, and biochemical data have implicated mGlu5 receptors as a critical feature of the glutamate transduction pathways relevant to these disorders (see Introduction). The principle pharmacological support for the involvement of mGlu5 receptors in depression has come from a limited number of studies using MPEP. Because MPEP exhibits weak interactions with other receptors, such as mGlu4 (Mathiesen et al., 2003), NMDA receptors, and noradrenaline transporters (Heidbreder et al., 2003), MTEP was synthesized. MTEP is an MPEP analog with increased potency, in vivo selectivity (Cosford et al., 2003), and improved bioavailability (Roppe et al., 2004). We now demonstrate that both MPEP and MTEP produce antidepressant-like effects in the mouse forced swim test. Comparable antidepressant-like effects have also just been reported by Belozertseva et al. (2006) in the mouse tailsuspension test. However, it still remains to be shown that a nonalkyne mGlu5 receptor antagonist can produce antidepressant-like effects.
Effects of MPEP and imipramine on immobility in mGlu5-receptor knockout mice. A, the knockout and wild-type mice were given 30 mg/kg MPEP or vehicle 15 min before testing. B, the knockout and wild-type mice were dosed with 15 mg/kg imipramine or vehicle 15 min before testing. The data are expressed as the means ± S.E.M. of seven to 17 animals. *, significant differences from wild-type vehicle control (P < 0.05). #, significant difference from knockout vehicle control.
Effects of a combination of MPEP and imipramine on immobility in the forced swim test. Vehicle or MPEP (3 and 10 mg/kg) was administered with 5 mg/kg imipramine or vehicle jointly 15 min before the test. The data are expressed as the means ± S.E.M. of eight animals. *, P < 0.05 compared with vehicle (v) + H2O (Dunnett's test); #, P < 0.05 compared with 10 mg/kg MPEP + H2O(t test). $, P < 0.05 compared with v + 5 mg/kg imipramine (t test).
Effect of joint administration of MPEP and imipramine on brain concentrations of MPEP (A) and imipramine (B) in NIH Swiss mice. Drug concentrations were determined as described under Materials and Methods. Brains were removed 15 min after MPEP (3 and 10 mg/kg) given jointly with 5 mg/kg imipramine. The data are expressed as the means ± S.E.M. of four animals.
Additional support for the hypothesis that mGlu5 receptors are the transducer of the antidepressant-like efficacy of these antagonists comes from the data on mGlu5 receptor knockout mice. Here, we demonstrate for the first time that 1) the mGlu5 receptor KO mouse displays an antidepressant-like behavioral phenotype in that this mouse has less immobility when placed in water that is not escapable than that of wild-type littermate control mice, 2) the antidepressant-like effects of MPEP are deleted in the mGlu5 receptor KO mice, and 3) imipramine retains its antidepressant-like effect even in the absence of mGluR5 receptors. In addition, like the AMPA receptor potentiators that display antidepressant-like effects in the forced swim test (Li et al., 2003), mGlu5 receptor antagonists also interact synergistically with the tricyclic antidepressant imipramine.
To date, MPEP has been the most extensively investigated mGlu5 antagonist in studies of psychiatric disorders, especially in rodent models of anxiolytic activity (Spooren and Gasparini, 2004; Swanson et al., 2005). MTEP like MPEP is also effective in rodent models of anxiety, such as fear-potentiated startle and Geller-Seifter conflict in rats (Cosford et al., 2003; Pietraszek et al., 2005), Vogel conflict and elevated plus-maze in rats as well as the four-plate test in mice (Klodzinska et al., 2004). A recent report has also confirmed activity of MPEP and also of MTEP in the mouse tail-suspension test, olfactory bulbectomy test in rats, but not in rats in the forced swim test (Palucha et al., 2005). The in vivo potency of MTEP has sometimes been reported to be greater than that of MPEP in rats (cf., Varty et al., 2005) but not always (cf., Pietraszek et al., 2005) and has also been reported to be similar in mice (Varty et al., 2005; Belozertseva et al., 2006; present study). This apparent differential potency difference between MPEP and MTEP in mice and rats could be complicated by the more prolonged occupation of rat than mouse receptors by these compounds when administered systemically (Anderson et al., 2003). However, the true potency differences between MTEP and MPEP in vivo remain to be conclusively identified.
In the current study, we observed the antidepressant-like effect in the mouse behavioral despair model (forced swim test) after acute administration of MPEP and MTEP as well as after subchronic administration of MPEP (5 days), indicating that the antidepressant-like effects of MPEP do not undergo tolerance under these conditions. The results are consistent with findings in the rat olfactory bulbectomy model of depression after multiple administration of MPEP (Pilc et al., 2002). Although not pressed to prolonged treatment regimens, the lack of tolerance is an important piece of data in light of the report that MTEP may have less efficacy in increasing punished responding of rats (an anxiolytic-sensitive assay) after several treatments (Busse et al., 2004).
Given that mGlu5 receptors have been implicated in a range of psychiatric and neurological disorders, such as pain, anxiety, cognition, Parkinson's disease, and schizophrenia, mGlu5 mutant mice have been the subject of broad behavioral scrutiny. For example, mGlu5(-/-) mice exhibited impairment in prepulse inhibition of the startle reflex, a measure of preattentional sensorimotor gating to model the sensorimotor gating deficits observed in schizophrenia (Kinney et al., 2003; Brody et al., 2004b). However, none of the antipsychotic drugs, including raclopride, clozapine, and serotonin 2A receptor antagonist MDL 100,907, could reverse the deficit in prepulse inhibition found in mGlu5 KO mice (Brody et al., 2004a). Despite showing similar dopamine increases induced by cocaine in nucleus accumbens in wild-type animals, mice lacking the mGlu5 receptor gene did not exhibit increases in locomotor activity after cocaine administration, and they failed to self-administer cocaine (Chiamulera et al., 2001). In addition, the mGlu5 receptor knockout mice displayed an anxiolytic-like phenotype, which manifested by a significant reduction of stress-induced hyperthermia compared with wild-type controls. These mice also failed to exhibit MPEP-induced anxiolytic-like effects, which were observed in mGlu5(+/+) mice (Brodkin et al., 2002). Although MPEP has demonstrated antidepressant-like effects in animal models, genetic confirmation of an mGlu5 receptor target for these effects has not been reported previously. Here, we showed that mGlu5 receptor KO mice exhibited a behavioral phenotype in the forced swim test that was congruent with the effects of pharmacological receptor blockade. In addition, we demonstrated that the antidepressant-like effects of MPEP, robustly detected in wild-type mice, were absent in KO mice, whereas the antidepressant-like effects of imipramine were retained. However, although MPEP did not decrease immobility in the KO mice, increases in immobility with MPEP were observed. The reasons for this effect are not clear from the data at hand but could suggest compensatory changes in other receptors that could be responsible. Nonetheless, these data combined with pharmacological data strongly support the blockade of mGlu5 receptors as a novel potential target for the discovery of antidepressant agents.
The findings that 1) chronic imipramine alters the sensitivity of hippocampal neurons to group I mGlu receptor agonists (Palucha et al., 1997), 2) mGlu5 receptor antagonists produce antidepressant-like effects through mGlu5 receptor antagonism, and 3) other potential glutamate antidepressants (e.g., AMPA receptor potentiators) synergize with imipramine in animal models of antidepressant activity (Li et al., 2003) led us to investigate the interaction of imipramine and MPEP in the forced swim test in the present study. The synergistic interaction between inactive doses of MPEP and imipramine observed here was shown to likely be due to pharmacodynamic rather than pharmacokinetic dynamics because neither the concentration of brain imipramine nor MPEP was significantly altered when the compounds were coadministered. Although the combination of compounds showed a trend toward an increase in MPEP brain levels that may have contributed to the synergy observed, a decreased trend in imipramine brain levels was observed with the drug combination. The mechanisms underlying the synergistic interplay between monoamine and glutamate receptor blockade via mGlu5 receptors need to be defined. A recent study reported that MPEP and MTEP decreased basal and stress-induced norepinephrine in rat frontal cortex (Page et al., 2005), whereas a facilitation of monoamine neurotransmission in cortex might be one predicted mechanism for the synergy observed in the present study. The effect of imipramine and mGlu5 receptor antagonists may also share a common cellular pathway that may be centered upon the induction of BDNF and its trophic effects such as neurogenesis (for discussion, see Alt et al., 2006).
The findings of the present study in conjunction with data existing in the literature strongly support the hypothesis that mGlu5 receptor blockade could serve the dual function of producing anxiolytic and antidepressant effects. Novel compounds with these dual activities could be welcome new entities for the treatment of these comorbid disorders with the potential promise of increased efficacy and reduced side effect potential.
Acknowledgments
We thank Drs. David L. McKinzie and Jim Monn for encouragement and support of this research and for helpful comments on the manuscript.
Footnotes
-
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
-
doi:10.1124/jpet.106.103143.
-
ABBREVIATIONS: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; NMDA, N-methyl-d-aspartate; mGlu, metabotropic glutamate; MPEP, 2-methyl-6-(phenylethynyl)-pyridine; MTEP, 3-[2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine; KO, knockout; WT, wild-type; FST, forced-swim test; MDL 100,907, R-(+)-a-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidinem ethanol.
- Received February 22, 2006.
- Accepted June 23, 2006.
- The American Society for Pharmacology and Experimental Therapeutics