Abstract
The uptake of serotonin (5HT) into mouse uterine horns, the localization of sites at which this amine could be stored and the effect of oxytocin on 5HT uptake were studied. To analyze the characteristics of the 5HT uptake process, the tissue was incubated with [3H]serotonin. The uptake of [3H]5HT was Na+ dependent and saturable (Kmapp: 166 ± 15 nM, Vmax: 404 ± 25 fmol/mg tissue, 30 min (diestrous); and Km: 165 ± 39 nM, Vmax: 276 ± 43 fmol/mg tissue, 30 min (estrous), n = 6), and was inhibited by imipramine, fluoxetine and 6-nitroquipazine (IC50: 2; 0.09 and 0.5 nM, respectively). In the myometrium the main 5HT uptake process was localized in uterine mast cells. This was determined by treating the uterine horns with 6-hydroxydopamine, by using an immunocytochemical approach and by studying the outflow of3H under the action of stimuli directed to either mast cells (compound 48/80: 10 μg/ml) or sympathetic nerves (high K+: 100 mM and veratridine: 20 μM) in uterine preparations. Oxytocin inhibited [3H]5HT uptake into uterine mast cells during estrus, but not in ovarectomized mice treated with progesterone. Maximal inhibition was attained at 0.03 nM, with a significant reduction in both Kmapp and Vmax (87 ± 15 nM and 184 ± 36 fmol/mg tissue/30 min,n = 3, respectively). This effect was reversed by the addition of OVT16, an oxytocin antagonist, at a concentration of 4 nM (Kmapp 158 ± 35 nM, Vmax: 278 ± 24 fmol/mg tissue, 30 min, n = 3). These findings support a new potential role of oxytocin and mast cells as a local regulators of serotonin bioavailability in myometrium. Because serotonin is recognized as an important endogenous uterotonic compound, this effect could be considered as an indirect action of oxytocin that may contribute to its potency as a labor inducer after genomic effects of estrogens are expressed in uterine tissue.
Parturition is the result of complex processes governed by endocrine and paracrine events and characterized by an increase in myometrial contractility. Uterine mast cells mediators released under specific stimuli seem to contribute to that response, suggesting that these cells may be important for labor induction (Rudolph et al., 1993, 1997).
The preparation of the myometrium for parturition is dependent on the action of estrogens, whose pleiotropic effects are mainly exerted by the modulation of the transcription of target genes in response to binding of the hormone. An increase in the synthesis of compounds that contract uterine smooth muscle and in the number of the receptors for those ligands are observed by the action of estrogens. One of the most characteristic examples is oxytocin, that is recognized as a potent stimulant of myometrium contractility, thought to be essential for the contractile events of parturition (Zingg et al., 1995).
Oxytocin receptors from the uterine tissue have been widely studied. They have been found in the plasma membranes of smooth muscle myometrium mediating a direct contractile effect (Soloff and Sweet, 1982; Anwer and Sanborn, 1989; Magocsi and Penniston, 1991) and in epithelial cells of endometrium being responsible to evoke the activation of arachidonate metabolism and production of prostaglandins, specifically PGF2α (Chan et al., 1996;Edgerton et al., 1996; Fuchs et al., 1996).
Interestingly, uterine mast cells are more sensitive to degranulate and release its mediators under the action of estrogens (Padilla et al., 1990; Cocchiara et al., 1992; Jeziorska et al., 1995). Nevertheless, the possible endogenous mediators that may trigger the activation of mast cells in this tissue are unknown. Taking into account that oxytocin is an important labor inducer, we decided to investigate whether oxytocin could also interact with uterine mast cells. This piece of information is important to analyze if any participation of these cells on muscle contractility regulation could be expected.
We present some evidences that oxytocin inhibits the uptake of 5HT into uterine mast cells. This effect depends on the predominance of estrogens in the tissue. 5HT is a mast cell mediator and a potent uterotonic compound being actively transported into uterine mast cells when myometrial tissue is incubated with 5HT at low concentrations. The inhibition of 5HT uptake may increase uterine contractility. An evaluation of the possible physiological relevance of this effect of oxytocin was made by using 6-nitroquipazine, a specific inhibitor of the 5HT transporter (Hashimoto and Goromaru, 1990). The results showed that 6-nitroquipazine potentiates contractions evoked by low concentrations of 5HT which allow us to postulate that oxytocin may have a dual action on uterine contractility: 1) a well-known direct one, by acting on smooth muscle myometrium and 2) an indirect one, by inhibiting the uptake of 5HT.
Methods
Materials.
5-[1,2-3H(N)]-Hydroxytryptamine, creatinine sulfate (sp. act. 27.6 Ci/mmol) was purchased from New England Nuclear Corp. (Boston, MA). Oxytocin was a commercial oxytocin distributed by Sandoz Laboratories in 5 UI/ml solution. 6-Nitroquipazine was purchased from RBI (Natick, MA). The other compounds were purchased from Sigma Chemical Co. (St. Louis, MO). DesGly9,d(CH2)5[Tyr(Me)2Thr4] (OVT16) was a gift from Dr. M. Manning, Medical School of Toledo, OH). Rabbit polyclonal serum anti-5HT was purchased from Incstar Corp. (Stillwater, MN). The second antibody and the PAP complexes were obtained from Dako Corp. (Carpinterı́a, CA).
The RBS used in these experiments had the following composition (in mM): NaCl, 153; KCl, 5.3; MgCl2, 1.8; NaHCO324.9; CaCl2 2; ascorbic acid 1.2 and glucose 2.7. It was bubbled with 95% O2 and 5% CO2. Na-free buffer was made by iso-osmotic substitution with LiCl. High K+ (100 mM) was made by iso-osmotic substitution of NaCl with KCl.
Animals.
Female albino mice, weighing 30 to 40 g, maintained under a dark/light cycle (12 hr dark/12 hr light) in a controlled temperature room (24–25°C) with free access to drinking water and laboratory food were used. The stage of the estrous cycle of the animal was determined through a cytochemical analysis of a vaginal smear and confirmed by both physical and anatomical aspects of the uterine horns once dissected. In addition ovariectomized mice were used, they were treated with progesterone as described (Montesinoet al., 1995).
Sympathectomy of mouse uterine horns with 6-OHDA.
A total of 50 μl of an l-ascorbic acid (10 mM) solution containing 5 mg of 6-OHDA was injected into the uterus through a dorsal incision made under anesthesia with pentobarbital (100 mg/kg). Control mice were injected with an equal volume of 10 mM of l-ascorbic acid. Animals were sacrificed 48 hr after 6-OHDA (Donoso et al., 1992).
Serotonin uptake measurements.
Uterine horns from mice in estrus, diestrus and ovariectomized-treated with progesterone were dissected, fragmented into two pieces, weighed and mounted on a support. The time course of the 5HT uptake was determined by incubating each uterine horn fragment (20–25 mg) at different periods in beakers containing 2 ml of RBS without Ca++ at 30°C in which [3H]5HT (38 nM) was added. For estimating the kinetic parameters of 5HT uptake, seven concentrations of [3H]5HT were used, ranging from 25 to 240 nM, using a 30-min incubation period. Corresponding incubations were conducted in a Na+-free medium to correct for nonspecific uptake. Radioactivity not incorporated by the tissue was washed off by further incubation for 10 min in 100 ml of RBS. The tissue was homogenized in 2 ml of 10% perchloric acid at the end of the incubation. The resulting suspension was centrifuged at 600 × g for 5 min and 0.1 ml of the supernatant was analyzed for radioactivity.
Samples of tissue suspensions were lyophilized, redissolved in acetic acid and analyzed by paper chromatography to determine the proportion of radioactivity that remained as intact 5HT after incubations (Verbeuren et al., 1987).
Effect of oxytocin on 5HT uptake into uterine horns.
Horn fragments (30–40 mg) were incubated as indicated above to evaluate the inhibitory effect of oxytocin, in uterine horns from mice in estrus and in diestrus. To evaluate the magnitude of this inhibitory effect, different concentrations of oxytocin were used ranging from 0.1 fM to 30 nM, although [3H]5HT concentration was kept constant at 38 nM. Results were expressed as the ratio of the values of the [3H]5HT taken up in uterine fragments treated with and without oxytocin. A similar protocol was followed to evaluate the action of vasopressin. Concentration-response curves for inhibiting 5HT uptake into uterine horns were developed for imipramine, fluoxetine and 6-nitroquipazine to evaluate IC50. A single concentration was tested for some other endogenous compounds, as indicated in the results. These experiments were done in triplicate by incubating tissue fragments in 2 ml of RBS without Ca++ at 30°C for 40 min with [3H]5HT (38 nM) and a high concentration of the inhibitor as indicated. Results were corrected for nonspecific uptake as described.
Immunocytochemistry of 5HT.
Both native and 5HT (38 nM) incubated tissues were used for immunocytochemistry. The tissue was fixed in a 4% paraformaldehyde-phosphate buffer (pH 7.4) at 4°C. The PAP method was used with rabbit polyclonal serum anti-5HT, 1:500 dilution. The second antibody (goat anti-rabbit immunoglobulin, whole serum) was used at a 1:25 dilution in a Tris buffer, pH 7.8, containing 0.7% λ-carrageenan and 0.25% Triton X-100. The negative controls were performed by omitting the first antibody in the incubation and replacing it with normal serum at a dilution of 1:100. They did not show any positive reaction. Mast cells were stained with 0.1% toluidine blue in 0.7 mol/liter HCl for 15 min and counterstained with 0.01% fast green and picrosyrius 0.1%.
Serotonin release studies.
To study the basal and evoked3H outflow from [3H]5HT previously taken up by the tissue, the horns were incubated in a beaker containing [3H]5HT (38 nM) in 4 ml of RBS without Ca++at 30°C for 40 min. Then the tissue was washed with 100 ml of the RBS and placed into beakers containing 2 ml of RBS and sequentially transferred after 1 min of incubation into a series of nine beakers. The stimulus to evoke 3H outflow (10 μg/ml Compound 48/80, 100 mM K+ or 20 μM veratridine) was applied when the tissue was in beaker no. 6. At the end of perfusion, uterine horns were weighed and homogenized in 2 ml of 10% perchloric acid. The resulting suspension was centrifuged at 600 × g for 5 min. Aliquots from each beaker and from the supernatant were analyzed for 3H. 3H outflow was calculated for every beaker as a percentage of fractional release, i.e., the percentage of the total amount of 3H remaining in the uterine horn, to reduce the error due to metabolization of [3H]5HT (Hughes and Roth, 1971). Basal outflow was monitored in beakers 4 and 5. The stimulus-evoked release was calculated as the net fractional3H outflow above basal levels from the application of the stimulus.
Contraction studies in mouse uterine horns.
The objective of this experiment was to evaluate the indirect effect of oxytocin on uterine contractility that could be masked by the direct one, due to the presence of oxytocin receptors in smooth muscle myometrial cells. Therefore, 6-nitroquipazine, a specific inhibitor of the 5HT transporter (Hashimoto and Goromaru, 1990) was used instead of oxytocin. Longitudinal pieces of uterine horns of mice in estrus were mounted vertically into an isolated organ bath containing oxygenated RBS at 35°C, as described (Rudolph et al., 1992). After a stabilization period, increasing concentrations of 5HT were added in a nonaccumulative protocol. The evoked contractions were recorded on a Grass model 7 polygraph. The effect of 6-nitroquipazine on the contractile action evoked by 5-HT, was studied by pretreating the uterine horns with the drug for 15 min before 5-HT was added.
Data analysis.
The Kmapp value, which represents the concentration of the 5HT giving half-maximal uptake, and Vmax, which is the maximal entry rate, were determined on the basis of different experiments in which specific uptake was measured in function of different concentrations of [3H]5HT. The results were expressed by using a Lineweaver and Burk plot and linear regression analysis. Data are expressed as means ± S.E.M. of a number of different experiments (n). The difference was assessed by using a t test or analysis of variance. The level of significance was 0.05 in each comparison.
Results
[3H]5HT uptake.
The time course of [3H]5HT uptake showed that this process was linear for a minimum of 30 min (data not shown). Therefore, uptake measurements to determine the kinetic parameters of the transport process were done for 30 min at 30°C. The uptake, which occurred in the Na-free medium, was subtracted from the total uptake, occurring in normal incubation medium, and the difference was considered to be specific uptake.
In mouse uterine horns, the uptake was concentration dependent. The double reciprocal plot of these data was linear (r > 0.99). TheKmapp values for the transport system at estrus and diestrus were similar (Kmapp: 165 ± 39 and 166 ± 15 nM, respectively, n = 6). Nevertheless, there were significant differences in Vmax (estrus: 276 ± 43 fmol/mg tissue, 30 min, n = 6; diestrus: 404 ± 25 fmol/mg tissue, 30 min, n = 6) (table1). Treatment of mice with 6-OHDA did not influence [3H]5HT uptake parameters.
Kmapp and Vmax values for [3H]5HT uptake into mouse uterine horns
Paper chromatography analysis of the radioactivity extracted from tissue previously incubated with [3H]5HT showed that between 60 and 65% of the radioactivity recovered from the tissues consisted of intact amine.
Imipramine, fluoxetine and 6-nitroquipazine inhibited 5HT uptake with IC50 values of 2; 0.09 and 0.5 nM, respectively. Noradrenaline (10 μmol/liter), bradykinin (1 μmol/liter), histamine (10 μmol/liter), PGF2α (10 μmol/liter) and PGE2 (10 μmol/liter) did not have any significant effect on 5HT uptake.
Immunohistochemistry of 5HT.
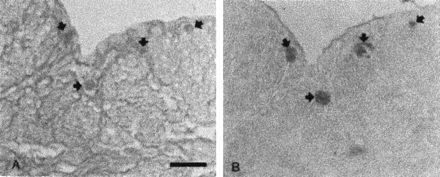
The distribution of cells containing 5HT was determined in mice uterine tissue. Mast cells were stained with acidic toluidine blue as already described in “Methods” and shown in figure 1(left). As shown in figure 1 (right), the pattern of the cells that reacted with the polyclonal antibody against 5HT corresponded to those of the mast cells marked with acidic toluidine blue. The pattern of labeling of the tissues preincubated with 5HT was similar to that of the native tissue (data not shown).
A, 5-μm frozen transverse section of a mouse uterine horn (left) stained with toluidine blue and counterstained with fast green and picrosyrius. (right): serial sections were immunostained with 5HT polyclonal antibody and recognized by the PAP method in mouse (B). (Bar: 25 μm). Arrows indicate mast cells.
Effect of oxytocin on [3H]5-HT uptake.
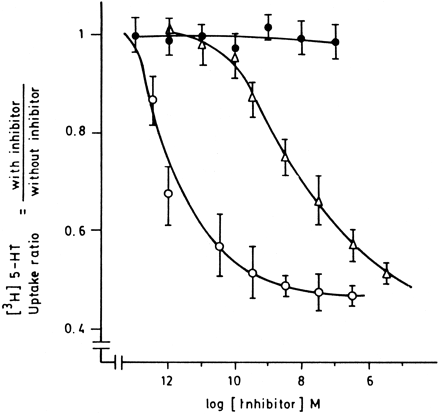
The study of the inhibitory effect of oxytocin on 5HT uptake into mouse uterine horns gave the following results. Oxytocin at a range of concentrations from 0.1 fM to 30 nM inhibited the uptake of 5HT. This effect was likely dependent on the predominance of estrogens in the tissues examined. Up to 50% inhibition was observed in uterine horns from mice in estrus, although no inhibitory effect of oxytocin was observed in uterine horns from diestrus or from ovariectomized and progesterone-treated mice (table 1; fig.2). In uterine horns from 15- and 21-day pregnant mice, maximal inhibition was increased, reaching 70 and 80%, respectively. The effect of oxytocin on 5HT uptake from mice in estrus was manifested as a reduction of both Kmapp and Vmax. The oxytocin antagonist DesGly9,d(CH2)5[Tyr(Me)2Thr4] (OVT16) blocked the action of oxytocin at a concentration of 4 nM (table 1). Vasopressin also inhibited serotonin uptake but at higher concentrations, concentration response curve was parallel and had the same maximal response (fig. 2).
Effect of oxytocin on [3H]5HT uptake into mouse uterine horns. Tissues were incubated in RBS containing 38 nM [3H]5HT and increasing concentrations of oxytocin (○) at the following two experimental conditions: estrus (empty symbols) and ovariectomized and treated with progesterone (full symbols) mice. Concentration-response curve for vasopressin (▵) in uterine horns from mice in estrus. Ordinate values are expressed as the ratio of [3H]5HT specific uptake between horns incubated with and without oxytocin or vasopressin at the corresponding concentration.
5HT release.
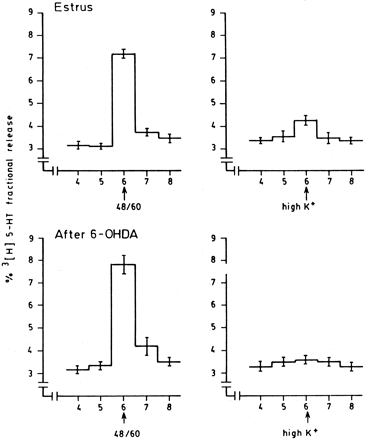
These experiments were developed to determine the sites that could uptake and store 5HT in uterine tissue. An example of the experiments developed to evaluate 5HT release is shown in figure3. Compound 48/80 (specific activator of mast cells) (Morrison et al., 1974) at a concentration of 10 μg/ml evoked the release of [3H]5HT previously taken up by uterine tissue (fractional release: 4.23 ± 0.12%,n = 3). High K+ (100 mM) and veratridine (20 nmol/ml) (which depolarizes nerves only) (Celuch and Sloley, 1989;Mohr and Fewtrell, 1987) also increased the outflow of 3H over resting levels. The magnitude of this release was near 20% of the [3H]5HT release evoked by compound 48/80 suggesting that adrenergic nerves can also take up some 5HT. Nevertheless, uterine horns treated with 6OHDA did not release [3H]5HT when incubated with high K+ solution (table2).
Example of the experiments developed to study the types of cells (nerves or mast cells) that could uptake and release 5HT in uterine tissue. The horns were preincubated with [3H]5HT and then superfused with RBS. When basal3H release became stabilized uterine horns were treated with compound 48/80 or high K+. The same procedure was developed with uterine horns denervated with 6-OHDA. Arrows indicate the beakers where uterine horns were stimulated. The stimulus-evoked release (arrows) was calculated as the net fractional 3H outflow above basal levels. Results of these experiments are given in table 2.
Characterization of [3H]5HT evoked release from mouse uterine horns
Consequences on contractility after inhibiting 5HT uptake in mouse uterine horns in vitro.
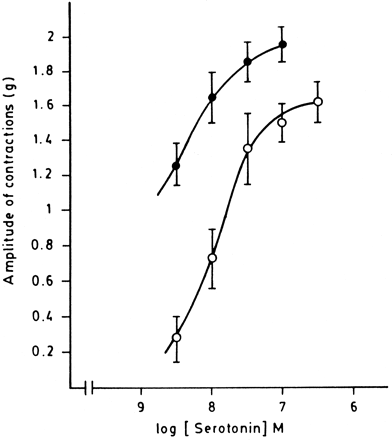
Figure4 depicts a concentration-response curve for the contractile action of 5-HT, and the potentiation evoked in the presence of 10 nM of 6-nitroquipazine. The inhibitor of 5-HT uptake did not evoke contractility but displaced up and to the left, in one order of magnitude, the concentration-response curve evoked by 5-HT.
Concentration-contractile response curves developed to study the influence of the inhibition of the 5HT uptake on contractions evoked by low doses of 5HT. Control (○), with 6-nitroquipazine 10 nM (•). Concentration-response curve for 5-HT in the presence of 6-nitroquipazine (a 5HT uptake inhibitor) was developed by previous incubating the tissue for 15 min with the drug. Statistical analysis showed that the displacement was significant.
Discussion
Similar to what has been described in other tissues, 5HT uptake into the mouse uterus was Na+ dependent and saturable, being inhibited by imipramine, fluoxetine and 6-nitroquipazine. To evaluate whether adrenergic nerves or mast cells were responsible for that process both neurochemical and immunocytochemical studies were designed. The results suggest that most 5HT was taken up by mast cells since: 1) in tissues previously charged with 3H-5HT the most significant 3H outflow over basal levels was observed after the addition of compound 48/80, a mast cell degranulator (Morrison et al., 1974), contrasting with the reduced effect of high K+ solution or veratridine, which stimulate nerve terminals (Celuch and Sloley, 1989; Mohr and Fewtrell, 1987), 2) 5HT uptake and release were not modified after lesioning adrenergic uterine horns with 6OHDA and 3) the polyclonal antibody against 5HT was only detected in mast cells, even after uterine tissue was incubated with 5HT (38 nM) before fixation.
This uptake process exhibited kinetic parameters that were dependent on sexual hormone predominance. The efficiency was reduced when estrogens effects predominated over progesterone (Vmax: 276 ± 43 fmol/mg tissue, 30 min, vs. diestrus: 404 ± 25 fmol/mg tissue, 30 min, n = 6 in estrus and diestrus, respectively). In uterine tissue of mice in estrus oxytocin evoked a further reduction of the efficiency of this uptake (Vmax: 184 fmol/mg tissue, 30 min, at 0.03 nM oxytocin, n = 3). This effect was dependent on the concentration of the hormone. Vasopressin also inhibited serotonin uptake, but at concentrations 1000 times superior than that of oxytocin, suggesting that the effect is mediated by an oxytocin receptor (Chan et al., 1996). According to these results, both estrogens and oxytocin appear to act as negative modulators of the 5HT transporter in the mast cell of the uterine tissue. The fact that the action of oxytocin was not observed under progesterone predominance could be explained on the basis that oxytocin receptors need to be expressed in mast cells. In this aspect estrogens and progesterone act in different directions, estrogens induce an increase in oxytocin binding while progesterone antagonizes it (Fuchs et al., 1983).
The mechanisms controlling the concentration of 5HT in the myometrium have not been elucidated. Peripheral 5HT is synthesized mainly in enterochromafin cells of the gastrointestinal tract, from where it is released to the portal circulation. In the blood, 5HT is found in platelets and basophils (Da Prada and Pletcher, 1968; Tamir et al., 1982). In addition, a variable pool of free 5HT has been reported in plasma which could be metabolized by monoamine oxidase (Thorpe et al., 1987) or diffused through capillary interendothelial clefts into intersticial space of visceral tissues where it may interact with the target cells (Fukuda et al., 1986; Moffet et al., 1988; Sarrias et al., 1990), unless it is taken up by some tissue components such as mast cells.
Our results suggest that uterine mast cells may mediate an estrogen-oxytocin endocrine regulation of free 5HT concentration in the myometrium. An inhibition in the rate of uptake could lengthen the time during which 5HT could interact with its receptors and may expand the range of diffusion of the compound. Whether this regulation may have a role in the regulation of uterine contractions, and/or any other physiological processes in vivo, remains to be determined. Our results suggest that the inhibitory action of oxytocin on 5HT uptake may have some relevance on uterine contractility as it was shown by using 6-nitroquipazine, a specific 5HT uptake inhibitor (Classenet al., 1984; Hashimoto and Goromaru, 1990). The drug inhibited [3H]5HT uptake and also potentiated 5HT evoked contractions at low concentrations (fig.4). Threshold of 5HT required to evoke contractions in uterine tissues is near 30 nM. Therefore, if 5HT reaches the myometrium at a higher concentration, myometrium smooth muscle cells should contract due to the potentiation that results when 5HT interacts with endogenous compounds such as histamine or PGF2α (Rudolph et al., 1992, 1993). The fact that the uptake of 5HT into mast cells has aKmapp value near 100 nM means that the 5HT transporter expressed in mast cells may have the capacity to maintain the extracellular concentration of the amine near that threshold. Therefore, the efficacy of the process should depend on Vmax,i.e., the number and efficiency of the sites available for 5HT uptake in the mast cells. This mechanism may keep the serotonergic contractile response of the myometrium to a minimum under progesterone predominance and thus lessen the likelihood of contractions when “quietness” is necessary. Nevertheless, at the end of pregnancy, when oxytocin receptors are expressed, the efficacy of the 5HT uptake becomes reduced, increasing 5HT bioavailability in the tissue, which may contribute to labor.
Contractile properties of 5HT on myometrium have had applications in obstetrics with the use of ergometrine (a partial agonist of 5HT) to contract the uterus postpartum to reduce hemorrhaging (Hollingsworthet al., 1988). Furthermore, 5HT stimulates myometrial smooth muscle cells to synthesize collagenase at the end of gestation that indicates that this amine has a possible contributory role in the ripening of the cervix and the timing of human parturition (Wilcoxet al., 1992). The above results constitute important information on the possible mechanisms that could regulate 5HT bioavailability in the myometrium.
Acknowledgment
The authors gratefully thanks Dr. M. Manning for providing the oxytocin antagonist DesGly9,d(CH2)5[Tyr(Me)2Thr4] (OVT16).
Footnotes
-
Send reprint requests to: Dr. M. Isolde Rudolph, Departamento de Farmacologı́a, Universidad de Concepción, casilla 160-C, Concepción, Chile.
-
↵1 This work was supported by Research Grants from FONDECYT (197-0842) and Dirección de Investigacı́on, Universidad de Concepción (97.032.005-1.0).
- Abbreviations:
- 5HT
- serotonin
- 6-OHDA
- 6-hydroxydopamine
- RBS
- Ringer’s bicarbonate solution
- PAP
- peroxidase-antiperoxidase
- Received October 7, 1997.
- Accepted May 22, 1998.
- The American Society for Pharmacology and Experimental Therapeutics