Abstract
Myeloproliferative neoplasms (MPNs) are hematologic malignancies that result from acquired driver mutations in hematopoietic stem cells (HSCs), causing overproduction of blood cells and an increased risk of thrombohemorrhagic events. The most common MPN driver mutation affects the JAK2 gene (JAK2V617F). Interferon alpha (IFNα) is a promising treatment against MPNs by inducing a hematologic response and molecular remission for some patients. Mathematical models have been proposed to describe how IFNα targets mutated HSCs, indicating that a minimal dose is necessary for long-term remission. This study aims to determine a personalized treatment strategy. First, we show the capacity of an existing model to predict cell dynamics for new patients from data that can be easily obtained in clinic. Then, we study different treatment scenarios in silico for three patients, considering potential IFNα dose-toxicity relations. We assess when the treatment should be interrupted depending on the response, the patient’s age, and the inferred development of the malignant clone without IFNα. We find that an optimal strategy would be to treat patients with a constant dose so that treatment could be interrupted as quickly as possible. Higher doses result in earlier discontinuation but also higher toxicity. Without knowledge of the dose-toxicity relationship, trade-off strategies can be found for each patient. A compromise strategy is to treat patients with medium doses (60–120 μg/week) for 10–15 years. Altogether, this work demonstrates how a mathematical model calibrated from real data can help build a clinical decision-support tool to optimize long-term IFNα therapy for MPN patients.
SIGNIFICANCE STATEMENT Myeloproliferative neoplasms (MPNs) are chronic blood cancers. Interferon alpha (IFNα) is a promising treatment with the potential to induce a molecular response by targeting mutated hematopoietic stem cells. MPN patients are treated over several years, and there is a lack of knowledge concerning the posology strategy and the best timing for interrupting therapy. The study opens avenues for rationalizing how to treat MPN patients with IFNα over several years, promoting a more personalized approach to treatment.
Introduction
BCR-ABL–negative myeloproliferative neoplasms (MPNs) are hematologic malignancies that include essential thrombocythemia, polycythemia vera, and primary myelofibrosis leading to overproduction of myeloid cells (red cells, platelets, and granulocytes). These diseases are clonal due to the expansion over decades of a hematopoietic stem cell (HSC) that has undergone the acquisition of a genetic abnormality (Van Egeren et al., 2021; Hermange et al., 2022; Williams et al., 2022). After homologous recombination, homozygous malignant clones can develop in parallel to heterozygous clones. The mutations affect JAK2, calreticulin (CALR), and the thrombopoietin receptor (MPL) genes, with JAK2V617F being the most prevalent in more than 95% of polycythemia vera and around half of essential thrombocythemia and primary myelofibrosis. All these mutations are gain-of-function leading to constitutive activation of the JAK2/STAT pathway (Vainchenker et al., 2011).
Thrombohemorrhagic complications are the main comorbidities even if the transformation into secondary leukemia of dismal prognosis occurs in a significant fraction of cases (between 5% and 20%). Clinicians generally treat patients with MPN to normalize the blood parameters and/or to improve symptoms, mainly using aspirin, cytoreductive treatment (hydroxyurea), and JAK1/2 inhibitors [ruxolitinib and fedratinib (Inrebic)]. However, neither of these treatments impacts the malignant clone selectively, and the only curative option is bone marrow allogeneic transplantation in the most severe myelofibrotic patients. In this context, pegylated interferon alpha 2a (Peg-IFNα2a) is a promising treatment. When used, IFNα is mainly a second-line therapy. It harbors high rates of hematologic responses in JAK2V617F MPN patients and some molecular responses, including high molecular responses (HMRs), even after treatment arrest (Kiladjian et al., 2008; Gisslinger et al., 2020; Mascarenhas et al., 2022). In clinic, the molecular response refers to measuring the variant allele frequency (VAF) in mature cells and quantifying how the VAF decreases over the therapy. Despite these positive effects, some JAK2V617F MPN patients do not respond to IFNα, and HMR is only observed in ∼20% of JAK2V617F patients. Long-term treatment of 2–5 years is required to obtain HMR. Additionally, toxicity increases with the IFNα dose (Yamane et al., 2008), resulting in the choice of dose de-escalation in many patients after 1 year, potentially jeopardizing the success of long-term HMR (Mosca et al., 2021), all of which results in decision-making challenges for clinicians. Therefore, this therapy could be improved and optimized to increase the molecular response while limiting toxicity.
Previously, it has been shown that the IFNα targets the JAK2V617F HSCs in a preclinical mouse model (Hasan et al., 2013; Mullally et al., 2013; Austin et al., 2020). Due to the difficult access to human HSCs by biologic methods, the effect of IFNα on the mutated human HSC dynamics was investigated using a tailor-made compartmental mathematical model calibrated with data from a prospective, observational, and longitudinal cohort of treated patients. Our objective with this model was to quantify not only the molecular response among mature cells but also the response at the stem cell level. We inferred that the IFNα slowly targets human JAK2V617F HSCs and that the higher the dose, the better the response (Mosca et al., 2021). The latter mathematical model was then extended to account for the dose variations during the treatment when the mutated HSCs were targeted (Hermange et al., 2021). This model is used in this present work to develop a clinical decision-support tool for predicting the response of MPN patients to IFNα and optimizing their long-term therapy. First, we verify that we can get accurate predictions from the model, clinical observations from a new patient, and prior knowledge from a cohort of patients. Then, we explore several therapeutical strategies to find which one would minimize the IFNα toxicity until the end of the treatment. Finally, combining the previous mathematical model with another one describing how JAK2V617F-mutated HSCs—when not treated—expand over time (Hermange et al., 2022), we assess when the therapy could be interrupted depending on how the patient responds to IFNα, their age, and the proportion of homozygous and heterozygous JAK2V617F HSCs (i.e., the zygosity).
Materials and Methods
Experimental and Clinical Data
The experimental and clinical data used in this study are those of 19 JAK2V617F patients from Mosca et al. (2021). For each patient of this cohort, we have:
Their age at the beginning of the treatment.
Measurements of the VAF in mature cells (in granulocytes) at different time points during the treatment. We define them as clinical data since these observations are molecular biology data obtained during routine clinical exams.
Observations of the clonal architecture [i.e., measurement of the heterozygous and homozygous clonal fractions (CF)] at different time points during the treatment. We define them as experimental data since they are obtained in biology research laboratories.
The variations of the dose they received over time.
We display the experimental and clinical data of patient #32 and those of each patient (Fig. 1; Supplemental Fig. A, 1–19). We denote by D the dataset for all 19 patients (see Supplemental Methods A: Data and observation model).
Longitudinal observations of patient #32. The x-axis corresponds to the time from the start of the IFNα therapy. Clinical data consist of measurements of the VAF in mature cells (black squares); experimental data consist of measurements of the heterozygous (green circles) and homozygous (blue triangles) CF in progenitor cells. The shaded beige areas correspond to the weekly dose of IFNα received over time.
Predicting the Long-Term Dynamics
The model studied in Mosca et al. (2021) (see Fig. 2; Supplemental Methods B: Model) was calibrated from observations on 19 patients with MPN having the mutation JAK2V617F. A hierarchical Bayesian inference method was used to estimate the parameters of each individual in addition to a population effect, thus reducing the risk of overfitting. Then, the minimal IFNα dose 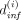 required for a patient i to reach long-term remission was estimated (Supplemental Methods C2.4). Our objective is now to demonstrate how the model could be used as a clinical decision-support tool. In clinical routines, trimestrial measurements of the clonal architecture of progenitor cells are not feasible. However, the clonal architecture of heterozygous and homozygous mutated immature cells (i.e., the measurement of CF) yields essential information that determines the response to the treatment as shown in Tong et al. (2021). In terms of cost, time, and human resources, it can be considered reasonable to measure the CF twice for each patient: one just before they start their therapy and one afterward (generally after about 300 days of therapy; we explore the impact of this choice in the Supplemental Methods F: Estimating the best timing for measuring the clonal architecture). The VAF in mature cells can easily be measured during routine clinical follow-up.
required for a patient i to reach long-term remission was estimated (Supplemental Methods C2.4). Our objective is now to demonstrate how the model could be used as a clinical decision-support tool. In clinical routines, trimestrial measurements of the clonal architecture of progenitor cells are not feasible. However, the clonal architecture of heterozygous and homozygous mutated immature cells (i.e., the measurement of CF) yields essential information that determines the response to the treatment as shown in Tong et al. (2021). In terms of cost, time, and human resources, it can be considered reasonable to measure the CF twice for each patient: one just before they start their therapy and one afterward (generally after about 300 days of therapy; we explore the impact of this choice in the Supplemental Methods F: Estimating the best timing for measuring the clonal architecture). The VAF in mature cells can easily be measured during routine clinical follow-up.
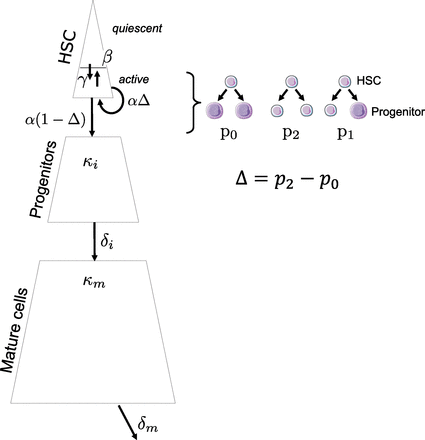
Schematic representation of the model for one cell population (e.g., mutated JAK2V617F heterozygous cells). Quiescent HSCs can exit quiescence at a rate γNq(t) with Nq(t) as the number of quiescent HSCs at time t. Active HSCs can become quiescent at a rate βNa(t) or be recruited to divide at a rate αNa(t) with Na(t) as the number of active HSCs at time t. In the latter case, an HSC could divide symmetrically (producing two HSCs) with probability p2, asymmetrically with probability p1, or produce two immature cells (progenitors) with probability p0. Δ = p2 − p0 corresponds to the balance between symmetrical and differentiated division. Progenitor cells expand with a rate constant κi and become mature cells with a rate constant δi. Mature cells expand with a rate constant κm and die at a death rate δm.
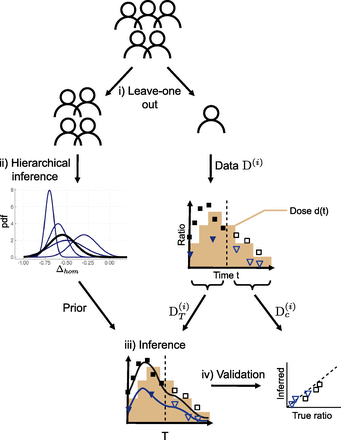
If the model were to be used by physicians, then we would have to evaluate its capacity to predict cell dynamics for new patients for whom such data can be easily obtained in clinical routines. Yet, we do not have access to patients outside the cohort of Mosca et al. (2021) to study the predictive capabilities of the model and then to study different dose strategies. Therefore, we choose to consider our 19 JAK2V617F-mutated patients with MPN, from which we will remove one individual (leave-one-out) and for whom we will perform data assimilation (Fig. 3i; Supplemental Methods C: Parameter estimation).
Illustration of the method for predicting the long-term cell dynamics over IFNα therapy. Removing one patient from the cohort (i), we run a hierarchical Bayesian inference procedure using the data of the remaining patients (ii). That is, for each model parameter (here, for example, Δhom), we estimate its probability density (pdf) for each patient (blue lines) as well as for the patients’ population (black line). The latter will be retained as prior knowledge. The data 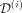 of the excluded patient from the initial cohort consists of VAF measurements in mature cells (black squares) and CF in progenitor cells, here homozygous CF (blue triangles), over time t. We also have the information on the dose of IFNα d(t) that the patient received (in brown). We split their dataset into two parts, the first one
of the excluded patient from the initial cohort consists of VAF measurements in mature cells (black squares) and CF in progenitor cells, here homozygous CF (blue triangles), over time t. We also have the information on the dose of IFNα d(t) that the patient received (in brown). We split their dataset into two parts, the first one 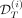 corresponding to observations before a time T (that we call assimilation time) and the second one
corresponding to observations before a time T (that we call assimilation time) and the second one 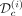 to the observations after that time. Based on the dataset
to the observations after that time. Based on the dataset 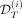 and the prior obtained from the remaining cohort of patients, we infer the dynamics of the mutated cells on treatment (iii). The black line corresponds to the dynamics of the VAF and the blue one to the dynamics of the homozygous CF. To evaluate the capacity of the model to predict the long-term dynamics correctly, we compare the model predictions and the control dataset
and the prior obtained from the remaining cohort of patients, we infer the dynamics of the mutated cells on treatment (iii). The black line corresponds to the dynamics of the VAF and the blue one to the dynamics of the homozygous CF. To evaluate the capacity of the model to predict the long-term dynamics correctly, we compare the model predictions and the control dataset 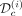 that was not used for estimating the model parameters.
that was not used for estimating the model parameters.
We denote 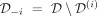 as the dataset for all patients except individual i (Table 1 summarizes the main notations). In this article, we will consider three individuals who were each in turn excluded from the cohort: patients #12, #18, and #32. These three patients are chosen because several observations of their response before t = 300 days of treatment are available, which is only the case for a few patients in the cohort (see Supplemental Methods A). Furthermore, each of the three patients exhibits a distinctive type of response to IFNα, making their study interesting: patient #12 has mostly homozygous cells, and they seem to be slowly but steadily targeted over the treatment; patient #18 presents both homozygous and heterozygous mutated cells initially, in the same proportions, and only the homozygous clone is targeted during the treatment; and patient #32 responds with a so-called “bell curve,” meaning that their VAF and CF first strongly increase at the beginning of the treatment and then decrease.
as the dataset for all patients except individual i (Table 1 summarizes the main notations). In this article, we will consider three individuals who were each in turn excluded from the cohort: patients #12, #18, and #32. These three patients are chosen because several observations of their response before t = 300 days of treatment are available, which is only the case for a few patients in the cohort (see Supplemental Methods A). Furthermore, each of the three patients exhibits a distinctive type of response to IFNα, making their study interesting: patient #12 has mostly homozygous cells, and they seem to be slowly but steadily targeted over the treatment; patient #18 presents both homozygous and heterozygous mutated cells initially, in the same proportions, and only the homozygous clone is targeted during the treatment; and patient #32 responds with a so-called “bell curve,” meaning that their VAF and CF first strongly increase at the beginning of the treatment and then decrease.
List of the main notations
We run three hierarchical estimation procedures (Supplemental Methods C1) corresponding respectively to the datasets 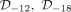 , and
, and  . Of these three calibrations, we will retain only the estimate of the population distribution (Fig. 3ii), which will be used as prior distribution to predict the response of the left-apart patient i. The progressive inclusion of observations for this patient (through what is called in Bayesian statistics the likelihood) will allow the updating of the previous distribution (i.e., the prior). We refer to this statistical process as “data assimilation” in this article (Fig. 3iii; Supplemental Methods C2). Let T ∈ {300,600,1000} [days] be the times (of assimilation) until which we consider the observations of patient i. The dataset of patient i used for the model calibration will be denoted by:
. Of these three calibrations, we will retain only the estimate of the population distribution (Fig. 3ii), which will be used as prior distribution to predict the response of the left-apart patient i. The progressive inclusion of observations for this patient (through what is called in Bayesian statistics the likelihood) will allow the updating of the previous distribution (i.e., the prior). We refer to this statistical process as “data assimilation” in this article (Fig. 3iii; Supplemental Methods C2). Let T ∈ {300,600,1000} [days] be the times (of assimilation) until which we consider the observations of patient i. The dataset of patient i used for the model calibration will be denoted by:  . Note that this dataset includes all VAF measurements before time T and only two measurements of the clonal architecture. The other observations of the CF among progenitor cells will not be used for estimating the model parameters. Indeed, in clinical routines, we cannot have repeated measurements of the progenitor clonal architecture. Thus, we choose to use only some of the observations available for progenitor cells before the assimilation time to treat the patient’s data as if they were obtained from realistic processes in clinical routine. We consider the observation of the progenitor CF at the start of the therapy and about 300 days afterward.
. Note that this dataset includes all VAF measurements before time T and only two measurements of the clonal architecture. The other observations of the CF among progenitor cells will not be used for estimating the model parameters. Indeed, in clinical routines, we cannot have repeated measurements of the progenitor clonal architecture. Thus, we choose to use only some of the observations available for progenitor cells before the assimilation time to treat the patient’s data as if they were obtained from realistic processes in clinical routine. We consider the observation of the progenitor CF at the start of the therapy and about 300 days afterward.
For patient i, for the assimilation time T ∈ {300,600,1000}, we will then estimate the posterior distribution of each parameter from the prior distribution and the observations 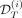 . The parameters will then be used to infer the on-treatment dynamics of the VAF in the mature cells as well as the heterozygous and homozygous CF in the progenitors and the HSCs (Fig. 3iii).
. The parameters will then be used to infer the on-treatment dynamics of the VAF in the mature cells as well as the heterozygous and homozygous CF in the progenitors and the HSCs (Fig. 3iii).
The confrontation of the predicted values with the observations not used for the model calibration will allow us to evaluate the quality of the predictions (Fig. 3iv). The control dataset for assessing the quality of the predictions will be denoted as:

This control dataset includes all observations made after time T as well as the observations of the CF among progenitors that were not used for estimating the model parameters.
To quantify how well the model fits the observations, either those used to estimate the model parameters (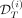 ) or those used as control (
) or those used as control (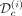 ), we will compute the mean square error (MSE) (see Supplemental Methods C2.5). In particular, we will check how MSE
), we will compute the mean square error (MSE) (see Supplemental Methods C2.5). In particular, we will check how MSE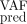 , which confronts the predicted VAF values to the ones truly observed after the assimilation time T (i.e., not used for the model calibration), evolves for higher T values.
, which confronts the predicted VAF values to the ones truly observed after the assimilation time T (i.e., not used for the model calibration), evolves for higher T values.
Optimizing the Therapy
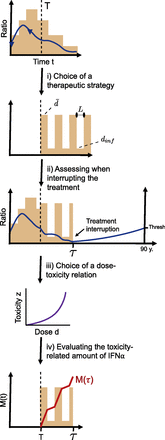
Once we have evaluated the ability of the model to predict the long-term response from data potentially available in clinical routines, we can explore different therapeutic strategies (see Therapeutic Strategies) and find the optimal one (Fig. 4). The optimality will be defined as the minimal dose of IFNα administered until treatment interruption (see Assessing When to Interrupt the Treatment) when penalizing higher doses associated with higher risks of toxicity (see Minimizing Drug-Related Toxicity during the Treatment). The complete method is presented in Supplemental Methods E: Optimizing the therapy, whereas the focus here is on the most important aspects for understanding the article.
Illustration of the method for optimizing the IFNα therapy. Considering the observations for a patient until time T and their model parameters estimated, we can obtain the dynamics of the mutated cells (here, for example, the CF of homozygous progenitors in blue) over time t for a given dose d(t). We can then consider different therapeutic strategies [i.e., different ways d(t) will evolve for t ≥ T (i)]. Here, for example, we illustrate a therapeutic strategy that is periodic. We can estimate the dynamics of the mutated cells for this new posology and assess the time τ when the CF will be low enough so that, after treatment interruption, the further clonal expansion will not lead to the onset of MPN symptoms before the patient is 90 years old (ii). Note that the illustration is done considering progenitor cells but that in our calculations, we estimate τ based on the inferred CF among HSCs. We then consider one scenario for the increase of IFNα toxicity z with the dose d (here, convex) (iii). Finally, we can compute M(t) for T ≤ t ≤ τ, which describes the amount of IFNα administered over the time interval [T,τ] when penalizing for high doses (iv). The quantity of interest is M(τ), which we want to minimize across different therapeutic strategies and for which we want to evaluate the impact of the dose-toxicity relation.
Assessing When to Interrupt the Treatment
IFNα therapy against MPN is a long-term therapy. It has been proven to induce, in some cases, an HMR, which is an undetectable level of JAK2V617F-mutated cells in peripheral blood (Kiladjian et al., 2008). However, we cannot clinically assess whether all mutated HSCs have been completely eradicated and whether a relapse might occur in the future. JAK2V617F-positive MPNs are hematologic malignancies that develop over decades before the onset of the symptoms, as shown recently in different studies (Van Egeren et al., 2021; Hermange et al., 2022; Williams et al., 2022). It was shown that heterozygous (het) JAK2V617F-mutated HSCs, when escaping stochastic extinction, would expand on average according to the following dynamics (Hermange et al., 2022):
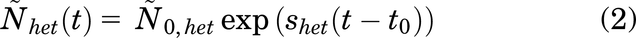 with a fitness estimated to be equal to shet = 20.4% per year and Ñhet(t) corresponding to the total number of mutated heterozygous HSCs over time t during the MPN development in the absence of IFNα therapy.
with a fitness estimated to be equal to shet = 20.4% per year and Ñhet(t) corresponding to the total number of mutated heterozygous HSCs over time t during the MPN development in the absence of IFNα therapy.
The dynamic of the homozygous clonal expansion was not studied in Hermange et al. (2022). Here, we generalize previous findings and assume that the homozygous clone would grow exponentially like the heterozygous one but with a higher fitness shom > shet, as shown by Williams et al. (2022). We estimate shom = 1.21 × shet (Supplemental Methods D3).
It is further assumed that the clonal expansion given by eq. 2, inferred from individuals before they received IFNα, will also be valid after they interrupt the treatment. Then, when inferring the CF of heterozygous and homozygous mutated HSCs from the model, at any time during the therapy, we could estimate using eq. 2 what would be the future development of the malignant clones after a treatment interruption at time τ. We can thus deduce the time t > τ at which the VAF among HSCs would exceed 7.5%. This value corresponds to the classic risk threshold above which it is considered that there could be a risk of thrombosis or cardiovascular events (i.e., a reappearance of the disease) (Dupont et al., 2007). The later this time, the better for the patient. Ideally, the therapy should be stopped at a time τ so that the relapse does not occur during the patient’s life. In practice, considering a life expectancy at 65 years old to be equal to 90 years old (Kontis et al., 2017), we will consider interrupting the treatment of a patient at an age τ such that the VAF among HSCs stays below 7.5% when the patient is under 90.
Thus, the criterion to interrupt the treatment depends on the age of the patients, the way they respond to the treatment, and their zygosity.
In this study, mutations associated with MPN such as DNMT3A, TET2, and TP53, which could potentially undergo a clonal expansion independent of the JAK2V617F clonal dynamics, are not considered.
In the Supplemental Methods D: Interrupting the therapy, we detail the choice of the criterion for the treatment interruption (Supplemental Methods D1), the model used for describing the clonal expansion (Supplemental Methods D2), and the estimation of the homozygous JAK2V617F fitness (Supplemental Methods D3).
Therapeutic Strategies
If we can correctly estimate the model parameters of a new patient (and thus correctly predict their mutated cell dynamics when they receive the dose d(t) for t ≥ T, with T as the assimilation time), we could explore alternative therapeutic strategies to those currently used by the clinicians. In practice, clinicians generally increase the dose until they observe a hematologic response and then de-escalate the dose, as was observed in the cohort of Mosca et al. (2021). However, this strategy might not be optimal since it can lead to early relapse.
In this article, different treatment strategies are studied, all of which will involve some parameters to optimize (see Minimizing Drug-Related Toxicity during the Treatment). Then, the strategies can be compared and the best one can be chosen (according to a criterion that we define later).
The list of strategies studied in this article is far from exhaustive, and we stick to simple ones that clinicians could easily employ. It is considered that the strategy will be set up from the assimilation time T to the interruption time τ. Before time T, the clinician might have treated the patient with a dose escalation, allowing an assessment of the patient’s tolerance to the treatment. During the period from the start of the therapy to T, measurements are made and will allow the estimation of the model parameters.
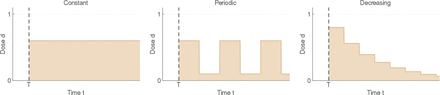
The three different treatment strategies studied are the following (Fig. 5):
Illustration of the three different therapeutic strategies that we consider. On the left, a constant dose over time (here,  ). In the middle, a periodic strategy (here,
). In the middle, a periodic strategy (here,  , L = 4 months, and dinf assumed to be equal to 0.1). On the right, a decreasing strategy (here,
, L = 4 months, and dinf assumed to be equal to 0.1). On the right, a decreasing strategy (here, 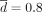 , L = 3 months, and λ = 0.7).
, L = 3 months, and λ = 0.7).
Constant:

Periodic:
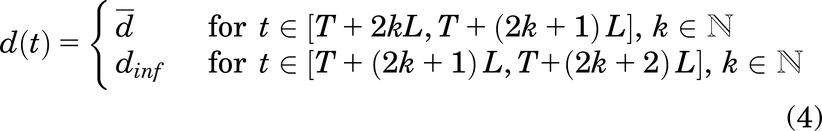
Decreasing:

The first strategy involves only one parameter  to optimize, the second strategy two parameters (
to optimize, the second strategy two parameters (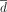 and the period L), and the last strategy three parameters, namely
and the period L), and the last strategy three parameters, namely 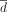 , λ, and the period L. This third strategy is the one often implemented in practice. Note that in the equation above, the expression of the decreasing function d is recursive (see Supplemental Methods E3 for another way to express the decreasing strategy).
, λ, and the period L. This third strategy is the one often implemented in practice. Note that in the equation above, the expression of the decreasing function d is recursive (see Supplemental Methods E3 for another way to express the decreasing strategy).
dinf corresponds to the minimal personalized dose that can be computed from the estimated parameter vector (Supplemental Methods C2.4). For all strategies, we consider 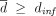 , λ ∈ [0,1]. We also want L ≥ 3 months to avoid too-frequent changes of posologies (regarding the treatment duration of generally many years). Besides, for too-high values of L, the decreasing and periodic strategies are equivalent to the constant one. To avoid finding ourselves in that case, the following condition is set: L ≤ 2 years. In addition, λ is constrained to be inferior to 0.95 to avoid the case where the decreasing strategy would approach the constant one when λ → 1. The optimal parameters will be estimated by doing a grid search (Supplemental Methods E1).
, λ ∈ [0,1]. We also want L ≥ 3 months to avoid too-frequent changes of posologies (regarding the treatment duration of generally many years). Besides, for too-high values of L, the decreasing and periodic strategies are equivalent to the constant one. To avoid finding ourselves in that case, the following condition is set: L ≤ 2 years. In addition, λ is constrained to be inferior to 0.95 to avoid the case where the decreasing strategy would approach the constant one when λ → 1. The optimal parameters will be estimated by doing a grid search (Supplemental Methods E1).
To note, we voluntarily choose not to consider stop-and-go strategies. Indeed, patients might need some time to tolerate the treatment; therefore, it should not be stopped to be resumed later to avoid this adaptation phase unless, of course, the interruption is permanent.
The periodic strategy, as defined by imposing the minimal dose to be equal to dinf, is then the strategy that is the closest to the stop-and-go strategy.
Minimizing Drug-Related Toxicity during the Treatment
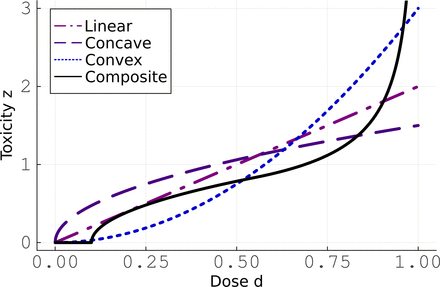
The main objective of the study is to optimize the IFNα therapy against MPN. For that purpose, several potential therapeutic strategies were considered in Therapeutic Strategies. We should define the clinically relevant quantity that has to be optimized. Intuitively, we want the treatment to be as short as possible. With the considered model, the corresponding optimization problem would be solved by choosing to give the maximal IFNα dose of 180 μg/week. However, the toxicity of IFNα is known to increase with the dose (Yamane et al., 2008), so optimizing only the duration of treatment would result in high-toxicity strategies. Thus, a penalty has to be applied for high doses. Toxicity includes everything harmful to the patient, either hematologic or not. In particular, IFNα is known to be associated with side effects such as depression (Trask et al., 2000; Lotrich et al., 2007), flu symptoms, or thrombocytopenia. Since no data exist to quantify the dose-toxicity relation, we will consider several hypothetical scenarios and evaluate their impact on the results. We define z(d) the drug toxicity as a function of the dose and consider four potential behaviors for the dose-toxicity relation (see Fig. 6):
The four different scenarios that we consider for modeling the way the IFNα toxicity may increase with the dose.
• Linear:
z(d) = 2d
Convex:
z(d) = 3d2
• Concave:
• Composite:
For normalization, the average toxicity is set to 1 for the four formulations:

For the composite relation, we assume that there is no toxicity below a low threshold of 0.1 (corresponding to 18 μg/week), then a concave behavior for d ∈ [0.1,0.55] followed by a convex one where the toxicity tends to infinity for d → 1. The value delimiting both behaviors (i.e., d = 0.55) is chosen so that the derivative of z is continuous over [0.1,1]. More details concerning the construction of the composite relation are provided in Supplemental Methods E4.
M(t) is defined as the toxicity-related amount of IFNα administered over [T, t]:
 where d (as a function of time) is assumed to be either constant, periodic, or decreasing and z (as a function of d) is either linear, convex, concave, or composite. Then, the quantity to minimize is M(τ). In the linear scenario, M(τ) would then correspond to the total amount of IFNα administered between T and τ. To note that, in eq. 8 we do not consider the interval [0, T] since we only want to optimize the treatment from t = T.
where d (as a function of time) is assumed to be either constant, periodic, or decreasing and z (as a function of d) is either linear, convex, concave, or composite. Then, the quantity to minimize is M(τ). In the linear scenario, M(τ) would then correspond to the total amount of IFNα administered between T and τ. To note that, in eq. 8 we do not consider the interval [0, T] since we only want to optimize the treatment from t = T.
We study for patients #12, #18, and #32 three therapeutic strategies and four scenarios of dose toxicity (i.e., twelve different conditions). For each of them, we will estimate the parameters related to the therapeutic strategy that minimizes the quantity M(τ) (Supplemental Methods E1). The strategies are then compared based on this value, for a given hypothetical scenario of toxicity, to find the optimal one. Moreover, in the absence of prior knowledge on how IFNα toxicity increases with the dose, a trade-off strategy can be proposed (Supplemental Methods E2).
Results
Minimal Observations Are Sufficient To Predict the Long-Term Mutated Cell Dynamics
First, we investigated whether some measurements of the VAF and only two measurements of the progenitor clonal architecture were sufficient to predict the long-term response to the treatment. To present the results in this section, we will focus on patient #18 (all of our results are detailed in Supplemental Material G: Detailed results; the results for patients #12 and #32 concerning the prediction part are detailed in Supplemental Material G1). The cell dynamics observed during IFNα therapy for patient #18 are interesting since they initially presented both heterozygous and homozygous clones (and in the same proportions), but the homozygous clone was targeted during the treatment when the heterozygous one continued to expand. Such dynamics might be challenging to predict when the parameter estimation is mainly based on VAF measurements.
For patient #18, we assess whether we could predict the evolution of their VAF correctly, but also both their heterozygous and homozygous CF in progenitors (Fig. 7), from:
only two measurements of the clonal architecture in immature cells (at the start of the treatment and 248 days afterward),
several measurements of the VAF before a time T ∈ {300,600,1000} [days], and
prior knowledge obtained from the remaining patients.
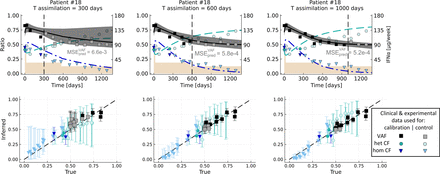
Data assimilation results for patient #18. From left to right, the assimilation times are T = 300, 600, and 1000 days (vertical dash line). At the top, we present the predicted dynamics. In black are shown the inferred VAF (median value and 95% credibility interval), and in green and blue are shown the inferred CF (median values) of heterozygous and homozygous mutated progenitors, respectively. The squares correspond to the clinical data and the circles and triangles to the experimental data, either used to calibrate the model (dark colors, 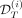 ) or to control the quality of the predictions (light colors, control dataset
) or to control the quality of the predictions (light colors, control dataset 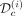 ). We represent the IFNα dose variations during treatment (in μg/week) in brown. The MSE displayed just below the VAF dynamics is computed on the VAF measurements not used for the calibration (i.e., the VAF measurements after the assimilation time T) such that MSE
). We represent the IFNα dose variations during treatment (in μg/week) in brown. The MSE displayed just below the VAF dynamics is computed on the VAF measurements not used for the calibration (i.e., the VAF measurements after the assimilation time T) such that MSE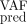 quantifies the predicted error on the VAF. At the bottom, we compare the inferred values (median values, on the y-axis) with those observed (on the x-axis). The error bars correspond to the 95% credibility intervals.
quantifies the predicted error on the VAF. At the bottom, we compare the inferred values (median values, on the y-axis) with those observed (on the x-axis). The error bars correspond to the 95% credibility intervals.
By progressively adding more information on the VAF dynamics (i.e., increasing the assimilation time), we increase the confidence in our predictions. It is particularly true for the VAF and the homozygous CF. As early as T = 300 days, when only four VAF measurements are used for the parameter estimation, we observe a good agreement between the predicted values, both for the VAF and the CF, and the experimental values not used for estimating the model parameters.
MSE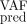 —quantifying the error between the observed and predicted VAF for different assimilation times T—is equal to:
—quantifying the error between the observed and predicted VAF for different assimilation times T—is equal to:
6.6 ⋅ 10−3 for T = 300 (10 VAF measurements used for computing MSE
 )
)5.8 ⋅ 10−4 for T = 600 (six measurements used for computing this MSE)
5.2 ⋅ 10−4 for T = 1000 (three measurements used for computing this MSE)
We show that adding more observations for the VAF improves the quality of our prediction. However, the (median) inferred dynamics for the mutated heterozygous progenitors are closer to the observations for T = 600 days than T = 1000, resulting for the latter in a higher MSE on the heterozygous CF measurements not used for the inference (Supplemental Table G2). For T = 1000, we overestimate the heterozygous CF, illustrating the difficulty in correctly predicting both the dynamics of heterozygous and homozygous cells when the inference is mainly based on the VAF measurements.
For patient #12, we also observe a good agreement between the predicted VAF and the observed ones, with an MSE of 1.1 ⋅ 10−3 and 1.5 ⋅ 10−3 for T equal to 300 and 600, respectively. However, we systematically underestimate the true homozygous CF (Supplemental Fig. G1; Supplemental Material G1.1; Supplemental Table G1). For patient #32, we obtained good predictions, both for the VAF and the homozygous CF (patient #32 has almost no heterozygous clones) for T = 300 or 1000 days (with MSE
of 1.1 ⋅ 10−3 and 1.5 ⋅ 10−3 for T equal to 300 and 600, respectively. However, we systematically underestimate the true homozygous CF (Supplemental Fig. G1; Supplemental Material G1.1; Supplemental Table G1). For patient #32, we obtained good predictions, both for the VAF and the homozygous CF (patient #32 has almost no heterozygous clones) for T = 300 or 1000 days (with MSE respectively equal to 2.17 ⋅ 10−2 and 2.35 ⋅ 10−2) but, surprisingly, poor predictions for T = 600 days (MSE
respectively equal to 2.17 ⋅ 10−2 and 2.35 ⋅ 10−2) but, surprisingly, poor predictions for T = 600 days (MSE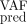 = 0.25) (Supplemental Fig. G5; Supplemental Material G1.3; Supplemental Table G3). But, if instead of considering the measurement of the clonal architecture at time t = 392, we chose to take the observation at t = 508, we observed that the predictions for T = 600 days were far better (MSE
= 0.25) (Supplemental Fig. G5; Supplemental Material G1.3; Supplemental Table G3). But, if instead of considering the measurement of the clonal architecture at time t = 392, we chose to take the observation at t = 508, we observed that the predictions for T = 600 days were far better (MSE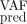 = 7.5 ⋅ 10−3) (Supplemental Fig. G6; Supplemental Material G1.4; Supplemental Table G4), illustrating the importance of the experimental design to make accurate predictions. We explore this question in Supplemental Material G3.
= 7.5 ⋅ 10−3) (Supplemental Fig. G6; Supplemental Material G1.4; Supplemental Table G4), illustrating the importance of the experimental design to make accurate predictions. We explore this question in Supplemental Material G3.
From the results on the three patients considered in this study, we conclude that it is possible to make predictions senseful of the mutated cell dynamics from VAF observations and two measurements of the CF among progenitor cells but that the choice of the time point for the clonal architecture matters, raising the concern of how the observation time should be chosen. After 300 days of treatment, it is possible to get good predictions of the cell dynamics and after 600 days to have good confidence in our predictions.
The Dose Should Be Kept Constant until the Treatment Is Stopped
We studied whether other therapeutic strategies could lead to better results (i.e., faster molecular response while limiting the potential IFNα toxicity). To evaluate these strategies, we focused on patient #32 (results for patients #12 and #18 are detailed in Supplemental Material G2). For this patient, the model predicts that a decrease in the dose after 2000 days of therapy might slow down the molecular response and even induce a risk of relapse (Supplemental Fig. G6). The way the patient was treated, with a dose escalation over the first 3 years of therapy up to the maximal dose of 180 μg/week, then a de-escalation, is also characteristic of the strategies applied in clinical routine and already observed in Mosca et al. (2021).
We consider T = 600 days. Using the observations before that time (five VAF observations, two clonal architectures: one at t = 0, the second one at t = 508) and the prior knowledge obtained from the 18 remaining patients, we estimated the posterior distribution of the model parameters and showed that we obtain good predictions (Supplemental Material G1.4).
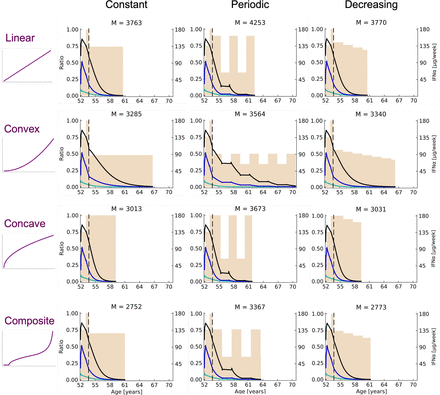
We now consider that the actual parameter vector is the estimated one (mean of the posterior distribution) and study how different dose strategies impact the response of the treatment after T = 600 days. With the estimated parameter vector, the minimal dose (under which the treatment would not sufficiently target the mutated HSCs, resulting in a relapse) is estimated to be dinf = 0.345 (i.e., 62 μg/week). We consider different scenarios for how the drug toxicity increases as a function of the dose, as presented in Fig. 6. For each scenario, we study the three therapeutic strategies presented in Therapeutic Strategies, for which we find the parameters (e.g., the choice of the dose  ) that minimize the value of M(τ). This latter value corresponds to the toxicity-related amount of IFNα administered from T to the time τ when the therapy could be interrupted (see Assessing When to Interrupt the Treatment). We can compare the three therapeutic strategies for a given drug-toxicity relation and select the one with the best (i.e., the lowest) value for M(τ). Results are presented in Fig. 8. It turns out that the constant strategy is the optimal one among the four studied scenarios, also for patients #12 (Supplemental Fig. G8) and #18 (Supplemental Fig. G9). With the two other strategies, namely the periodic one and the decreasing one, we obtain at the optimum lower values of M(τ)/(τ − T) (that we interpret as values of the yearly toxicity-related amount of IFNα) compared with the constant strategy. However, having periods with low doses or decreasing the dose over time also delays treatment interruption. Therefore, these two strategies are less effective in terms of treatment duration.
) that minimize the value of M(τ). This latter value corresponds to the toxicity-related amount of IFNα administered from T to the time τ when the therapy could be interrupted (see Assessing When to Interrupt the Treatment). We can compare the three therapeutic strategies for a given drug-toxicity relation and select the one with the best (i.e., the lowest) value for M(τ). Results are presented in Fig. 8. It turns out that the constant strategy is the optimal one among the four studied scenarios, also for patients #12 (Supplemental Fig. G8) and #18 (Supplemental Fig. G9). With the two other strategies, namely the periodic one and the decreasing one, we obtain at the optimum lower values of M(τ)/(τ − T) (that we interpret as values of the yearly toxicity-related amount of IFNα) compared with the constant strategy. However, having periods with low doses or decreasing the dose over time also delays treatment interruption. Therefore, these two strategies are less effective in terms of treatment duration.
Results of the treatment optimization for patient #32. Four hypothetical relationships between IFNα toxicity and the dose are studied: linear (at the top), convex (on the second line), concave (on the third line), and composite (at the bottom). For each of them, three therapeutic strategies—constant on the left, periodic on the middle, and decreasing on the right—are optimized to minimize M(τ) (denoted by M on the figures), which is the toxicity-related amount of IFNα administrated from T (vertical dashed line) to the treatment interruption time τ (when the weekly dose of interferon, represented in beige, drops to zero). The dynamics of the VAF (black line), the heterozygous CF (green line), and the homozygous CF (blue line) will differ for t ≥ T according to how the doses vary (depending on the considered therapeutic strategy and its parameter values). M values are only comparable for a given dose-toxicity relation—the lower, the better. If the actual dose-toxicity relation were linear, the constant strategy would be optimal for 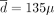 g/week (with the associated age of treatment interruption τ = 61 years and an optimal value of M(τ) = 3763); the periodic strategy would be optimal for
g/week (with the associated age of treatment interruption τ = 61 years and an optimal value of M(τ) = 3763); the periodic strategy would be optimal for  g/week and L = 22 months; and the decreasing strategy would be optimal for
g/week and L = 22 months; and the decreasing strategy would be optimal for  g/week, L = 2 years, and λ = 0.95. Among these three strategies, the constant one is the best. We can see how the optimal parameters of the decreasing strategy actually tend to make it approach a constant strategy, which would be the limiting case when λ → 1 and/or L → ∞. It is precisely to avoid finding oneself in this limiting case that constraints were imposed on the parameters (Supplemental Methods E1). If the actual dose-toxicity relation were convex, the constant strategy would be optimal for
g/week, L = 2 years, and λ = 0.95. Among these three strategies, the constant one is the best. We can see how the optimal parameters of the decreasing strategy actually tend to make it approach a constant strategy, which would be the limiting case when λ → 1 and/or L → ∞. It is precisely to avoid finding oneself in this limiting case that constraints were imposed on the parameters (Supplemental Methods E1). If the actual dose-toxicity relation were convex, the constant strategy would be optimal for  g/week; the periodic strategy would be optimal for
g/week; the periodic strategy would be optimal for 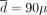 g/week and L = 23 months; and the decreasing strategy would be optimal for
g/week and L = 23 months; and the decreasing strategy would be optimal for  g/week, L = 2 years, and λ = 0.95. Among these three strategies, the constant one is the best. If the actual dose-toxicity relation were concave, the constant strategy would be optimal for
g/week, L = 2 years, and λ = 0.95. Among these three strategies, the constant one is the best. If the actual dose-toxicity relation were concave, the constant strategy would be optimal for 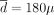 g/week; the periodic strategy would be optimal for
g/week; the periodic strategy would be optimal for 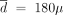 g/week and L = 20 months; and the decreasing strategy would be optimal for
g/week and L = 20 months; and the decreasing strategy would be optimal for 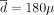 g/week, L = 2 years, and λ = 0.95. Among these three strategies, the constant one is the best. If the actual dose-toxicity relation were composite, the constant strategy would be optimal for
g/week, L = 2 years, and λ = 0.95. Among these three strategies, the constant one is the best. If the actual dose-toxicity relation were composite, the constant strategy would be optimal for  g/week; the periodic strategy would be optimal for
g/week; the periodic strategy would be optimal for 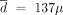 g/week and L = 2 years; and the decreasing strategy would be optimal for
g/week and L = 2 years; and the decreasing strategy would be optimal for  g/week, L = 2 years, and λ = 0.95. Among these three strategies, the constant one is the best.
g/week, L = 2 years, and λ = 0.95. Among these three strategies, the constant one is the best.
However, the choice of the optimal dose highly depends on how the IFNα toxicity increases with the dose. If there were a sharp increase in the toxicity for low doses followed by a slower one (concave scenario), we would recommend using high doses (about 180 μg/week for patient #32). Indeed, only slightly decreasing the dose would not strongly impact the toxicity, whereas decreasing the dose to a too-large extent would delay the treatment interruption or even induce a relapse if the dose is below a minimum value, as shown in Hermange et al., (2021). If, on the contrary, the toxicity would only sharply increase for high doses (convex scenario), then we would recommend using medium doses (about 90 μg/week for patient #32). Eventually, for the linear dose-toxicity relation (which is both concave and convex), or for the composite relation (which is first concave, then convex), we find that the recommended dose for patient #32 would be about 135 μg/week. Given the lack of existing data quantifying the relationship between the dose and the toxicity of IFNα, we cannot conclude which dose level would be optimal. Our results highlight the need to estimate such relations since we demonstrate their importance in deciphering how patients with MPN should be treated.
In the absence of prior knowledge on whether the dose toxicity would better be described by a linear, concave, convex, or composite relationship, we can determine the strategy that, even if not optimal, would be the best compromise. Finding a trade-off strategy is important since the best strategy under the hypothesis that the dose-toxicity relation would be concave would be detrimental under another hypothesis. We illustrate that point with patient #32. In the concave scenario, the best strategy is to treat patient #32 at a constant dose of 180 μg/week over about 5 years. This value would actually correspond to the highest dose considered in the study (i.e., a very high dose). If the dose-toxicity relation were actually not concave but linear, such a strategy would still be acceptable, with a value for M(τ) equal to 4020, placing it in the top 3.4% and better than the best periodic strategy. But, in the case where the dose-toxicity relationship was convex, treating patient #32 with such a high dose would be harmful. Indeed, it turns out that the optimal strategy in the concave scenario is also the worst one in the convex scenario.
Therefore, without having assessed in the first 2 years of the therapy how the patient responds to the treatment or in the absence of data that would quantify the dose-toxicity relation for IFNα, the best choice would be to select the trade-off strategy.
This strategy is the one that gives good results under the four hypothetical dose-toxicity relationships. For patient #32, the trade-off would be to treat him with a constant dose of 115 μg/week over 8 years until the treatment could be interrupted, here at age 62 (i.e., after about 10 years of IFNα therapy since patient #32 began the treatment at age 52). This trade-off strategy would be:
in the top 1.7% if the dose-toxicity relation were linear,
in the top 2.3% if the dose-toxicity relation were convex,
in the top 2.9% if the dose-toxicity relation were concave, and
in the top 0.2% if the dose-toxicity relation were composite.
For patient #18, the trade-off strategy would also be the constant one, with  g/week, until treatment discontinuation at the age of 72 (i.e., after about 15 years of IFNα therapy). For patient #12, the trade-off strategy would be the decreasing one, with
g/week, until treatment discontinuation at the age of 72 (i.e., after about 15 years of IFNα therapy). For patient #12, the trade-off strategy would be the decreasing one, with 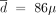 g/week, λ = 0.45, and L = 16 months, until treatment discontinuation at the age of 77 (i.e., after about 14 years of IFNα therapy) (Supplemental Material G2).
g/week, λ = 0.45, and L = 16 months, until treatment discontinuation at the age of 77 (i.e., after about 14 years of IFNα therapy) (Supplemental Material G2).
Discussion
We proposed a mathematical approach combining modeling, statistical inference, and optimization to rationalize the manner in which we treat JAK2V617F patients with MPN in the long term using IFNα. Clinicians often proceed to a dose de-escalation when they observe a hematologic response. The rationale behind this strategy is that IFNα is associated with general or hematologic side effects such as depression (Trask et al., 2000; Lotrich et al., 2007), flu symptoms, or thrombocytopenia and that the toxicity increases with the dose (Yamane et al., 2008). In the absence of both data quantifying the dose-toxicity relation and information on the response of mutated HSCs to the treatment, their therapeutic strategy, albeit empirical, is relevant. However, as observed from the cohort of Mosca et al. (2021), clinicians sometimes have to increase the dose again after a treatment interruption or a strong decrease when a relapse is observed. To avoid such behaviors that could harm the patient, we explored which therapeutic strategy would be optimal given hypothetical dose-toxicity relations. In particular, we estimated which dose level should be administered. We found that treating the patients with a constant dose until the therapy is interrupted rather than decreasing or alternating a low and a higher dose periodically should be more relevant. Even if not considered initially as a selection criterion, the simplicity of the constant strategy involving only one value to choose (i.e., the IFNα dose to be kept constant until the treatment interruption) makes it easy to deploy in clinics. Such a simple strategy appears more optimal than more sophisticated ones. However, the number of strategies that we explored was far from exhaustive; thus, we could not exclude that patients with MPN could be treated more successfully with different strategies. In our mathematical approach, we do not guarantee that, after interrupting the treatment, there will not be a relapse. On the contrary, we quantified this risk and chose to interrupt the treatment so that the reappearance of the MPN disease would not occur before the age of 90. This age threshold is based on the estimated life expectancy of 65-year-old individuals in developed countries (Kontis et al., 2017). Increasing this age, thus reducing the risk, would result in treating the patient for a longer time. It might also seem relevant to adjust this criterion regarding the sex of the patient, given that women have a higher life expectancy than men. Concerning the reappearance of the MPN disease, we considered that the relapse might occur when the VAF in HSCs exceeds 7.5% (Dupont et al., 2007). However, some patients with essential thrombocythemia might exhibit lower VAF, whereas a higher VAF is necessary for others to get the MPN symptoms. The choice of this value particularly matters when considering the problem of early screening (Hermange et al., 2022). In our case, however, the patients who have interrupted their treatment would still be followed so that clinicians might detect quickly if and when a relapse occurs.
Given hypothetical dose-toxicity relations, we found that treating a patient with a constant dose is optimal. However, the value of the dose depends on how IFNα toxicity increases with the dose. High doses should be recommended if the patient tolerates the treatment well; medium or low doses should be recommended otherwise. No data quantify, in the general case, what the dose-toxicity relation could be. Such a relation could actually be patient dependent. In our approach, we looked for the best therapeutic strategy once the patient had already been treated for 600 days. Therefore, we could already get prior insight into how the patient responded to a dose escalation. In other words, the clinician might provide information on the potential dose-toxicity relation, so we could recommend the constant dose value to administer to the patient. Involving the clinicians in getting such prior information would benefit our method, which could, in turn, provide more relevant guidelines on how it might be optimal to treat the patient. Without such dose-toxicity relation, we can find a trade-off strategy. This strategy is such that the dose-toxicity-related quantity of IFNα administered to the patient would be as little as possible for all four scenarios of dose-toxicity that we considered. It is worth noting that, for optimizing the therapy, we only considered minimizing a quantity related to the drug toxicity and did not account for economic criteria, for example. The issue of the economic cost of the therapy was studied by Pedersen et al. (2020). We also limit ourselves to four potential dose-toxicity relations, where the toxicity would strictly increase with the dose. It should not be excluded that more complex relations might exist and that the toxicity could, for example, reach a plateau. With the four dose-toxicity relations that we considered, we found that the trade-off strategy would also be when the dose is maintained at a constant value for two of the three patients studied. For the third patient, the decreasing strategy was found to be the best compromise; our results are patient dependent, and more patients should be studied to identify general patterns. In particular, the dose level is specific to each patient and, more precisely, to how they respond to the treatment. For the two patients whose trade-off strategy was the constant one, we found different optimal values of 61 and 115 μg/week. Our study considered any potential dose between 0 and 180 μg/week. In practice, clinicians can inject 135 or 180 μg of IFNα. By choosing the period between two medication intakes, we can have a range of potential doses, but some dose values would still not be clinically relevant. Furthermore, when the frequency of IFNα injection is low, below one every 10 days (Xu et al., 1998), the modeling assumption that the dose input is a piecewise constant function might not be justified. It would then be worth studying pharmacokinetic/pharmacodynamic (PK/PD) models [for a review see, for example, Gabrielsson and Green (2009)].
Our mathematical approach focuses on the mutated JAK2V617F HSCs: how IFNα targets them and then how they expand after the treatment interruption. The fact that HSCs cannot be observed in vivo justifies using mathematical models to infer their dynamics. Here, we combined two models: one that describes the dynamics of mutated cells during the therapy (Mosca et al., 2021) and one that describes the clonal expansion of the mutated cells in the absence of IFNα (Hermange et al., 2022). We assumed that the stochastic effect could be neglected. However, when the treatment lasts for a long time, the number of mutated cells might reach a low value, so deterministic models might not be appropriate anymore. It would then be relevant to propose a stochastic model for how IFNα would selectively target a few mutated HSCs within a large pool of wild-type (normal) HSCs. Concerning the wild-type HSCs, our model is based on the hypothesis that their number stays constant, even during disease development. Alternative models could also be proposed, either for the effect of the treatment (Ottesen et al., 2020; Pedersen et al., 2021) or the clonal expansion without IFNα (Van Egeren et al., 2021; Williams et al., 2022). In particular, the model from Pedersen et al. (2021) and Ottesen et al. (2020) presents the advantage of having been initially designed for calibration from VAF measurements; thus, it might be more relevant for use in clinical routine. The counterpart is that they do not account for the zygosity, which is a determinant of the response to IFNα (Mosca et al., 2021; Tong et al., 2021). A roundabout way exists to access information on zygosity in mature cells from a VAF measurement: using the 46/1 haplotype (Hasan et al., 2014) for patients heterozygous for this polymorphism (it would also be possible to determine other polymorphisms as informative). Indeed, it has been shown that generally, in the case of mitotic recombination by which a heterozygous JAK2V617F-mutated cell gives a homozygous mutated cell, the cell will also become homozygous for the 46/1 haplotype. Thus, measuring the VAF for this haplotype will tell us the proportion of homozygous mutated cells. Even if the model from Mosca et al. (2021) was not originally calibrated from data that could be easily obtained in clinical routine, we showed that having only two observations of the progenitor clonal architecture would be sufficient to get accurate predictions when having some prior information on the parameter distribution. This prior knowledge came from a hierarchical Bayesian inference when using all available information about the heterozygous and homozygous CF dynamics in progenitor cells (and not only two measurements of the clonal architecture) from other patients. Still, the prediction quality is uneven depending on the timing for measuring the clonal architecture. Poor predictions also mean that our recommendations for treating the patient could not be adapted. This is why we explored the issue of optimal experimental design: how to choose the best timing for measuring the clonal architecture (i.e., the one leading to the most accurate inferences). Intensive in silico investigations have shown that the time for the second CF measurement is important, but a search for any systematic was inconclusive (Supplemental Materials F and G3). It turned out that the best timing for a given patient might not be the best for another, preventing us from finding a general pattern of when it would be optimal to measure the CF among progenitor cells for a new patient. Thus, it might be worth adding more patients to the study. In addition, the experimental design question could be extended to the dates at which the VAF should be measured. We only superficially addressed the issue of experimental design, which deserves to be studied in more detail. However, if clinicians can easily use our model-based predictions and treatment recommendations, it would be more complicated to make recommendations on when they should collect patient blood samples. Indeed, the timing for collecting patient observations is subject to scheduling constraints over which we have no influence. It would be difficult to impose the dates when the patient should have an appointment with their clinicians. For that reason, we limited ourselves to the experimental design study and only considered the question of the best timing for the second measurement of the progenitor clonal architecture. Even if we could not conclude when it would be optimal for a new patient to get that measurement, we showed that not all choices were equal.
Finally, we demonstrated how a mathematical model calibrated from real data could predict MPN patients’ response to IFNα and optimize their long-term therapy. The proposed methods were designed to be easily applied as a decision-support tool in clinical routine. Our mathematical approach can be extended to study other drugs against MPN such as ropeg-IFNα2b (Barbui et al., 2021; Gisslinger et al., 2020; Mascarenhas et al., 2022), which is a different pegylated IFNα from the one studied in this article, or even its combination with ruxolitinib (Kiladjian et al., 2022). Furthermore, our methods could be applied more broadly to studying other chronic hematologic malignancies.
Acknowledgments
The authors thank D. Madhavan for her help in editing the manuscript.
Data Availability
The authors declare that all of the data supporting the findings of this study are available within the paper and its Supplemental Material.
Authorship Contributions
Participated in research design: Hermange, Cournède, Plo.
Performed data analysis: Hermange, Cournède, Plo.
Wrote or contributed to the writing of the manuscript: Hermange, Cournède, Plo.
Footnotes
- Received December 15, 2022.
- Accepted June 21, 2023.
This work is supported in part by a grant from the Prism project and by the Agence Nationale de la Recherche [Grant ANR-18-IBHU-0002] (to P.-H.C. and I.P.). This work was also supported by grants from INCA Plbio2018, 2021 (to I.P.) and Ligue Nationale Contre le Cancer (équipe labellisée 2019) and by the Appel à Projets Pré-néoplasies 2021 (C21021LS) with financial support from Institut Thématique Multi-Organismes (ITMO) Cancer of Aviesan within the framework of the 2021–2030 Cancer Control Strategy, with funds administered by INSERM.
The authors declare no competing financial interests.
↵
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
Abbreviations
- CF
- clonal fraction
- HMR
- high molecular response
- HSC
- hematopoietic stem cell
- IFN
- interferon
- MPN
- myeloproliferative neoplasm
- MSE
- mean square error
- VAF
- variant allele frequency
- Copyright © 2023 by The American Society for Pharmacology and Experimental Therapeutics