Abstract
Neuronal hyperactivity in the spinal dorsal horn can amplify nociceptive input in diabetic neuropathic pain. The glutamate N‐methyl‐d‐aspartate and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (NMDA receptors and AMPA receptors, respectively) are involved in spinal nociceptive transmission. It is unclear, however, whether painful diabetic neuropathy is associated with changes in the activity of synaptic NMDA receptors and AMPA receptors in spinal dorsal horn neurons. AMPA receptors lacking GluA2 are Ca2+-permeable (CP-AMPA receptors), and their currents display characteristic inward rectification. In this study, we showed that evoked excitatory postsynaptic currents (EPSCs), induced by streptozotocin, exhibited inward rectification in spinal dorsal neurons in diabetic rats. Presynaptic and postsynaptic NMDA receptor activity in the spinal dorsal horn was similar in diabetic and control rats. In the dorsal spinal cord, the membrane GluA2 protein level was significantly lower in diabetic than in control rats, whereas the cytosolic GluA2 level was greater in diabetic than in control rats. In contrast, the GluA1 subunit levels in the plasma membrane and cytosol did not differ between the two groups. Blocking CP-AMPA receptors significantly reduced the amplitude of EPSCs of dorsal horn neurons in diabetic but not in control rats. Furthermore, blocking spinal CP-AMPA receptors reduced pain hypersensitivity in diabetic rats but had no effect on nociception in control rats. Our study suggests that diabetic neuropathy augments CP-AMPA receptor activity in the spinal dorsal horn by causing intracellular retention of GluA2 and impairing GluA2 membrane trafficking. Increased prevalence of spinal CP-AMPA receptors sustains diabetic neuropathic pain.
SIGNIFICANCE STATEMENT This study demonstrates that the prevalence of synaptic calcium-permeable AMPA receptors is increased in the spinal dorsal horn, which mediates pain hypersensitivity in diabetic neuropathy. Thus, calcium-permeable AMPA receptors play an important role in glutamatergic synaptic plasticity in the spinal cord in painful diabetic neuropathy. This new knowledge improves our understanding of the mechanisms involved in central sensitization associated with diabetic neuropathic pain and suggests that calcium-permeable AMPA receptors are an alternative therapeutic target for treating this chronic pain condition.
Introduction
Peripheral neuropathy is a prevalent complication that afflicts people with a long history of diabetes. Chronic pain caused by diabetic neuropathy is not adequately relieved by available treatments and represents a major clinical problem. A decreased availability of various neurotrophic factors, mitochondrial dysfunction, and abnormal protein kinase C activation in sensory nerves may contribute to the development of diabetic neuropathy (Ahlgren and Levine, 1994; Tomlinson et al., 1997; Russell et al., 2002). Insulin deficiency is considered a major cause of sensory neuropathy in type 1 diabetes because early insulin therapy can normalize or impede the development of diabetic neuropathic pain in streptozotocin (STZ)-induced diabetic animals and in patients with diabetes (Sasaki et al., 1998; Brussee et al., 2004; Romanovsky et al., 2006; Hoybergs and Meert, 2007); however, the pathophysiological mechanism leading to chronic pain development in diabetic patients is not fully understood.
Spinal dorsal horn neurons are critically involved in processing sensory input and play a major role in the development of chronic neuropathic pain. Although painful diabetic neuropathy is associated with hyperactivity of spinal dorsal horn neurons (Chen and Pan, 2002), the underlying mechanisms remain unclear. In the spinal dorsal horn, glutamate, via acting on synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and N‐methyl‐d‐aspartate (NMDA) receptors, is the most dominant excitatory neurotransmitter for the relay of nociceptive input. Previous work on targeting glutamate receptors for treating neuropathic pain has focused primarily on NMDA receptors because NMDA receptors contribute critically to the development of chronic pain caused by traumatic nerve injury and chemotherapy (Chen et al., 2014b, 2018; Xie et al., 2016, 2017); however, changes in the activity of presynaptic and postsynaptic NMDA receptors in the spinal cord in painful diabetic neuropathy remain unclear.
Similar to NMDA receptors, AMPA receptors (AMPARs) are crucially involved in various physiologic functions, such as learning and memory, and in the pathophysiology of many neurologic and psychologic conditions (Franciosi, 2001; Shepherd and Huganir, 2007; Traynelis et al., 2010). AMPARs are ion channels composed of a combination of GluA1, GluA2, GluA3, and GluA4 (Traynelis et al., 2010). At excitatory synapses, AMPARs contain predominantly heteromeric GluA1/GluA2 subunits and, in some instances, of GluA3/GluA2 subunits (Kauer and Malenka, 2006; Derkach et al., 2007). GluA2 is highly significant for the biophysical properties of AMPARs because GluA2-containing AMPARs are impermeable to Ca2+. On the other hand, GluA2-lacking AMPARs show inward-rectifying currents and have a high Ca2+ permeability and are thus referred to as Ca2+-permeable AMPAs (CP-AMPARs) (Sommer et al., 1991; Chen et al., 2013). The prevalence of synaptic CP-AMPARs is markedly increased in the dorsal spinal cord after nerve ligation (Chen et al., 2013); however, it is unknown whether diabetic neuropathic pain is associated with increased activity of CP-AMPARs in the spinal cord. Therefore, in this study, we determined whether spinal NMDA receptors and AMPARs are differentially regulated in streptozotocin (STZ)-induced diabetic neuropathy.
Materials and Methods
Animal Model of Diabetic Neuropathic Pain.
All experimental procedures were approved by the Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center (approval no. 1186-RN01). Male Sprague-Dawley rats (8 weeks old; Harlan Sprague-Dawley, Indianapolis, IN) were used. Experimental diabetes was induced by intraperitoneal administration of 60 mg/kg of STZ, a pancreatic β-cell toxin (Chen and Pan, 2002; Chen et al., 2011). Age-matched saline-injected rats were used as the control group. This rat diabetic model mimics the clinical symptoms of diabetic patients and shows pain hypersensitivity and diminished opioid analgesic effects (Chen and Pan, 2002; Wang et al., 2007; Chen et al., 2011). Diabetes was confirmed by measuring the glucose concentration in the tail-vein blood samples using ACCU-CHEK test strips (Roche Diagnostics, Basel, Switzerland) 3 weeks after STZ injection. All animals were housed (two to three rats per cage) in a specific-pathogen-free facility and received standard chow and water ad libitum.
Intrathecal catheters were inserted in some rats anesthetized with inhalation of 2% to 3% isoflurane 2 weeks after diabetic induction. In brief, a small opening in the atlanto-occipital membrane was made, and a PE-10 catheter was introduced such that the caudal tip was situated at the L5-L6 spinal cord level (Chen et al., 2000). The animals were allowed to recover for at least 4 days before being used for intrathecal injections.
Assessment of Neuropathic Pain.
To measure changes in the tactile sensitivity of the hind paw, rats were placed individually in chambers placed on a mesh floor. Calibrated von Frey filaments were applied vertically to the plantar surface of the hind paw to bend the filament for 6 seconds. A swift withdrawal was considered a positive response. The tactile stimulus producing a 50% likelihood of withdrawal response was calculated by using the method described previously (Chen et al., 2000; Chen and Pan, 2002). To measure the mechanical nociceptive threshold, we applied a pressure stimulus on the hind paw using an analgesiometer (Ugo Basil, Varese, Italy). The device was activated to apply a constantly increasing force. When the animal displayed a paw withdrawal response, the device was instantly stopped to register the animal’s withdrawal threshold (Chen and Pan, 2002; Chen et al., 2014a). The investigators testing the nociceptive behaviors were blinded to the treatments.
Spinal Cord Slice Preparation and Whole-Cell Recordings.
All terminal electrophysiological experiments were performed on rats 3 weeks after STZ or vehicle treatment. Spinal cord slices at the L5-L6 levels were obtained from diabetic and control rats as we described previously (Li et al., 2002; Wang et al., 2007). We removed the lumbar spinal cord through laminectomy during isoflurane-induced anesthesia. We sliced the spinal cord to 400 μm in thickness using a vibratome and continuously superfused the slices with artificial cerebrospinal fluid containing (in millimolars) 117.0 NaCl, 2.5 CaCl2, 1.2 NaH2PO4, 3.6 KCl, 1.2 MgCl2, 11.0 glucose, and 25.0 NaHCO3. Neurons in the lamina II outer zone were chosen for recording because they mainly receive nociceptive input from primary afferent nerves (Pan and Pan, 2004; Wang et al., 2018). Whole-cell voltage clamp recordings were performed at 34°C by using pipettes filled with a solution containing (in millimolars) 110 Cs2SO4, 0.5 CaCl2, 5.0 HEPES, 5.0 EGTA, 5 TEA, 2.0 MgCl2, 5.0 ATP-Mg, 0.5 Na-GTP, 0.1 spermine, and 10 lidocaine N-ethyl bromide. We included 0.1 mM spermine in the intracellular recording solution to compensate for a possible loss of endogenous polyamines during whole-cell recording in spinal cord slices (Li et al., 2012; Chen et al., 2013).
The attached dorsal root was electrically stimulated (0.6 mA, 0.2 millisecond, and 0.1 Hz) to evoke EPSCs. Monosynaptic EPSCs were identified when the evoked EPSCs showed a constant latency and no conduction failure in response to a 20-Hz electrical stimulation, as we described previously (Li et al., 2002; Pan et al., 2002). Neurons were voltage-clamped at −60 mV, and AMPAR-mediated excitatory postsynaptic currents (AMPARs-EPSCs) were recorded in the presence of 2 μM strychnine, 10 μM bicuculline, and 50 μM DL-2-amino-5-phosphonovaleric acid (AP5), a specific NMDA receptor antagonist (Chen et al., 2014a, 2018). GluA2-lacking CP-AMPARs exhibit inward rectification at positive holding potentials (Sommer et al., 1991; Bowie and Mayer, 1995; Chen et al., 2013). AMPAR-EPSCs were recorded at various membrane potentials ranging from −70 to +70 mV in 20-mV steps to determine the current-voltage relationship. The evoked EPSCs are rapidly blocked by bath application of the AMPAR antagonist cyanquixaline (6-cyano-7-nitroquinoxaline-2,3-dione) (Li et al., 2002; Pan et al., 2002).
Miniature EPSCs (mEPSCs) were recorded at a holding potential of −60 mV in the presence of 1 μM strychnine, 10 μM bicuculline, and 1 μM tetrodotoxin. Presynaptic NMDA receptor-mediated glutamate release was tested by bath application of 50 μM AP5 (Chen et al., 2014a, 2018), whereas postsynaptic NMDA receptor currents were elicited by puff application of 100 μM NMDA to the recorded neuron using a positive-pressure system (4 p.s.i., 15 milliseconds; Toohey Company, Fairfield, NJ). To minimize the Mg2+ block of NMDA receptors, the puff NMDA currents were recorded in an extracellular solution containing no Mg2+, 10 μM glycine, and 1 μM tetrodotoxin at a holding potential of −60 mV (Chen et al., 2014a, 2018).
AP5, cyanquixaline (6-cyano-7-nitroquinoxaline-2,3-dione), and bicuculline were obtained from Ascent Scientific (Princeton, NJ). IEM-1460 (N,N,H,-Trimethyl-5-[(tricyclo[3.3.1.13,7]dec-1-ylmethyl)amino]-1-pentanaminiumbromide hydrobromide) was purchased from Tocris Bioscience (Ellisville, MO). All drugs were prepared immediately before the experiments and delivered at the indicated final concentrations.
Immunoblotting.
Plasma membrane and cytosolic proteins were isolated from the dorsal spinal cord at L5 and L6 levels using Mem-PER Plus Membrane Protein Extraction Kit (#89842; Thermo Fisher Scientific, Waltham, MA). Tissues were added with 400 µl permeabilization buffer (no. 78440; Thermo Fisher Scientific) and homogenized with tissue grinder. After incubating for 10 minutes at 4°C with constant mixing, the homogenate was centrifuged at 16,000g for 15 minutes at 4°C. The supernatant containing cytosolic proteins was carefully transferred to a new tube. The pellet was resuspended in 400 µl of solubilization buffer in the presence of a protease and phosphatase inhibitor cocktail and incubated for 30 minutes at 4°C with constant mixing. After centrifugation at 16,000g for 15 minutes at 4°C, the supernatant containing solubilized membrane and membrane-associated proteins was transferred to another new tube. A total of 40 µg of protein was loaded and separated via electrophoresis using NuPAGE 4%–12% Bis-Tris Protein gels (cat. no. NP0321BOX; Invitrogen, Carlsbad, CA) and then transferred to a polyvinylidene difluoride membrane (cat. no. IPVH00010; Millipore, Burlington, MA) for immunoblotting. As we described previously (Chen et al., 2013), primary antibodies used were as follows: rabbit anti-GluA2 (1:1000 dilution, cat. no. AB10529; Millipore), rabbit anti-GluA1 (1:1000 dilution, cat. no. AB1504; Millipore), mouse anti-β-actin (1:2000 dilution, cat. no. ab8226; Abcam, Cambridge, MA), and mouse anti-Na+-K+-ATPase (1:2000 dilution, cat. no. ab7671; Abcam). Horseradish peroxidase-conjugated anti-rabbit IgG (cat. no. 111-036-003, 1:10,000; Jackson ImmunoResearch, West Grove, PA) and anti-mouse IgG (cat. no. 115-036-062, 1:10,000; Jackson ImmunoResearch) were used as secondary antibodies. The protein bands were detected using a chemiluminescence kit (cat. no. 34580; Thermo Fisher Scientific).
Data Analysis.
Data are presented as mean ± S.E.M. Only one neuron was recorded from each spinal cord slice, and at least five animals were used for each recording protocol. The mEPSCs were analyzed using a peak detection program (MiniAnalysis; Synaptosoft, Leonia, NJ). The amplitudes of evoked EPSCs were analyzed with Clampfit 9.2 (Molecular Devices, San Jose, CA). The rectification index was obtained by dividing the amplitude of AMPAR-EPSCs recorded at +50 mV by that at −50 mV (Li et al., 2012; Chen et al., 2013). Two-tailed Student’s t tests were used to compare two groups, and one-way analysis of variance (with Dunnett’s post-hoc test) was used to determine the differences between more than two groups. All statistical analyses were performed using Prism software (version 7; GraphPad Software Inc., La Jolla, CA). P value of less than 0.05 was considered statistically significant.
Results
Diabetic Neuropathy Does Not Change Spinal NMDA Receptor Activity at Presynaptic and Postsynaptic Sites.
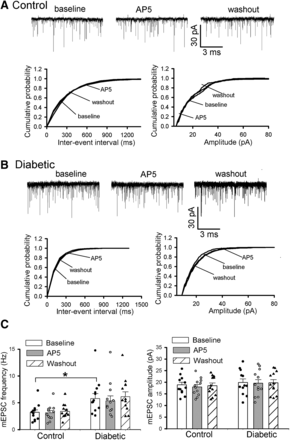
NMDA receptors expressed presynaptically in the spinal cord are not activated under normal conditions but are spontaneously activated in neuropathic pain after peripheral nerve injury and treatment with calcineurin inhibitors and certain chemotherapeutics (Chen et al., 2014a,b, 2018; Xie et al., 2016, 2017; Deng et al., 2019). To determine whether painful diabetic neuropathy is associated with increased presynaptic NMDA receptor activity in the spinal dorsal horn, we first recorded mEPSCs of lamina II neurons, which represent glutamate release from presynaptic terminals (Chen et al., 2014a, 2018), and examined the effect of blocking NMDA receptors with AP5. As reported previously (Li et al., 2010), the baseline frequency of mEPSCs was significantly greater in diabetic than in vehicle-treated control rats (P = 0.03, F(5,66) = 4.46; n = 12 neurons in each group; Fig. 1); however, bath application of 50 µM of AP5 for 6 minutes had no significant effect on the frequency or amplitude of mEPSCs of lamina II neurons recorded from diabetic or control rats (Fig. 1).
Painful diabetic neuropathy is not associated with changes in presynaptic NMDA receptor activity in the spinal dorsal horn. (A and B) Original recording traces and cumulative plots show the effect of 50 μM AP5 on the frequency and amplitude of mEPSCs of lamina II neuron from control (A) and diabetic (B) rats. (C) Mean data show the effect of AP5 on the frequency and amplitude of mEPSCs in control and diabetic rats (n = 12 neurons in each group). Data are presented as means ± S.E.M. *P < 0.05, compared with the baseline in control rats (one‐way ANOVA followed by Dunnett’s post-hoc test).
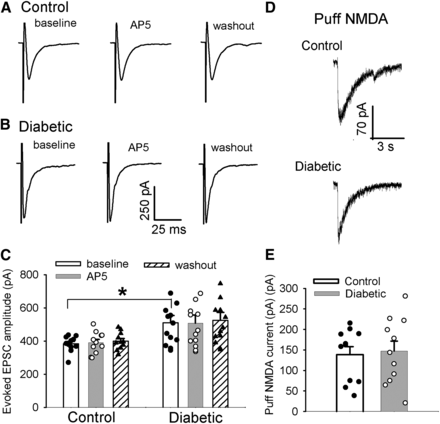
Diabetic neuropathic pain is maintained by augmented sensory input from primary afferent nerves (Chen and Pan, 2002; Khan et al., 2002; Cao et al., 2011). We thus determined specifically whether diabetic neuropathic pain alters the activity of NMDA receptors expressed specifically at central terminals of primary sensory nerves in the spinal dorsal horn. We recorded EPSCs of lamina II neurons evoked monosynaptically from the dorsal root to assess NMDA receptor-mediated glutamate release (Zhou et al., 2010; Chen et al., 2014a, 2018). The amplitude of evoked EPSCs at baseline was significantly greater in the diabetic (n = 13 neurons) than in the control group (n = 11 neurons; P = 0.04, F(5,66) = 3.99; Fig. 2, A–C), which is in agreement with our previous reports (Wang et al., 2007; Li et al., 2010); however, bath application of 50 µM of AP5 did not have any effect on evoked EPSCs in lamina II neurons tested from diabetic or control rats (Fig. 2, A–C).
Synaptic NMDA receptor activity in the spinal dorsal horn is not altered in painful diabetic neuropathy. (A–C) Original traces (A and B) and summary data (C) show the effect of bath application of 50 μM AP5 on evoked monosynaptic excitatory postsynaptic currents (EPSCs) of lamina II neurons from control (n = 11 neurons) and diabetic rats (n = 13 neurons). (D and E) Representative traces (D) and mean data (E) show NMDA receptor currents elicited by puff application of 100 μM NMDA onto lamina II neurons from control and diabetic rats (n = 11 neurons in each group). Data are presented as means ± S.E.M. *P < 0.05 compared with baseline in control rats (one‐way ANOVA followed by Dunnett’s post-hoc test).
We next studied whether diabetic neuropathic pain alters the activity of postsynaptic NMDA receptors in spinal dorsal horn neurons. We recorded NMDA receptor currents in lamina II neurons elicited by puff application of 100 µM of NMDA (Chen et al., 2014a; Xie et al., 2017). The amplitude of puff NMDA-elicited currents in lamina II neurons between diabetic and control rats did not show any significant difference (n = 11 neurons in each group, Fig. 2, D and E). Collectively, these results suggest that painful diabetic neuropathy is not associated with altered activity of presynaptic or postsynaptic NMDA receptors in the spinal dorsal horn.
Activity of CP-AMPA Receptors in the Spinal Dorsal Horn Is Increased in Diabetic Neuropathy.
Spinal AMPARs are critically involved in the relay of nociceptive information in traumatic nerve injury-induced neuropathic pain (Chen et al., 2000, 2013). To determine whether diabetic neuropathic pain alters the activity of GluA2-lacking, CP-AMPAR$s in the spinal cord, we examined the current-voltage relationship of AMPARs-EPSCs of dorsal horn neurons in the presence of AP5. GluA2-lacking AMPARs are permeable to Ca2+ and display inward rectification at positive holding potentials owing to the voltage-dependent block by intracellular polyamines. We therefore measured the current-voltage relationship of AMPARs-EPSCs in lamina II neurons electrically evoked from the dorsal root at various holding potentials. The EPSCs recorded showed a linear current-voltage relationship in all lamina II neurons from control rats (n = 13 neurons; Fig. 3, A and B), indicating that EPSCs are mediated mainly by GluA2-containing AMPARs. Remarkably, the amplitude of EPSCs in diabetic rats was reduced at positive holding potentials (n = 14 neurons; Fig. 3, A and B). The rectification index (I+50 mV/I−50 mV) of AMPAR-EPSCs was significantly smaller in diabetic rats (0.71 ± 0.05, n = 14 neurons) than in control rats (1.09 ± 0.07, n = 13 neurons; P = 0.003, t(12) = 4.19; Fig. 3C).
Painful diabetic neuropathy increases CP-AMPA receptor activity of spinal dorsal horn neurons. (A) Representative recording traces of evoked EPSC of lamina II neurons (A) and their current-voltage curves (B) recorded at holding potentials from −70 to +70 mV in control (n = 13 neurons) and diabetic (n = 14 neurons) rats. (C) Mean data of the rectification index (I+50 mV/I−50 mV) of evoked EPSCs of lamina II neurons in control (n = 13 neurons) and diabetic (n = 14 neurons) rats. Data are presented as means ± S.E.M. **P < 0.01, compared with control rats (two-tailed Student’s t test).
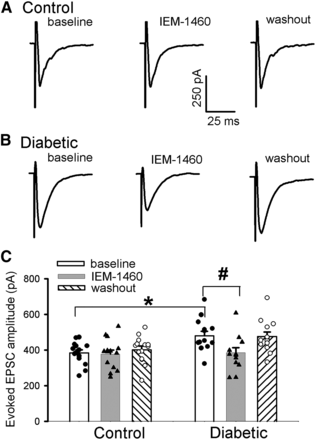
To determine to what extent GluA2-lacking AMPARs contribute to the overall glutamatergic synaptic input to dorsal horn neurons, N,N,H,-trimethyl-5-[(tricyclo[3.3.1.13,7]dec-1-ylmethyl)amino]-1-pentanaminiumbromide hydrobromide (IEM-1460), a highly selective blocker of GluA2-lacking AMPARs (Samoilova et al., 1999; Rossi et al., 2008; Chen et al., 2013), was used. We tested the effect of IEM-1460 on monosynaptic AMPARs-EPSCs electrically evoked from the dorsal root at a holding potential at −60 mV. IEM-1460 (100 μM), via bath perfusion, did not affect the amplitude of EPSCs in dorsal horn neurons in control rats (n = 14 neurons, Fig. 4). In contrast, IEM-1460 significantly attenuated the amplitude of evoked AMPARs-EPSCs in diabetic rats (n = 13 neurons, F(5,75) = 4.375, P = 0.041, Fig. 4). Together, these results suggest that painful diabetic neuropathy potentiates the activity of GluA2-lacking, CP-AMPARs in the spinal dorsal horn.
CP-AMPA receptors contribute to increased glutamatergic input in the spinal dorsal horn in diabetic neuropathic pain. (A–C) Representative recording traces (A and B) and summary data (C) show the differential effect of bath application of 100 μM IEM-1460 on evoked EPSCs of lamina II neurons in control (n = 14 neurons) and diabetic rats (n = 13 neurons). The mean value in the control group was set at 1. Data are presented as means ± S.E.M. *P < 0.05 compared with baseline in control rats. #P < 0.05 compared with the baseline in diabetic rats (one‐way ANOVA followed by Dunnett’s post-hoc test).
Diabetic Neuropathy Reduces Plasma Membrane Trafficking of GluA2 in the Spinal Cord.
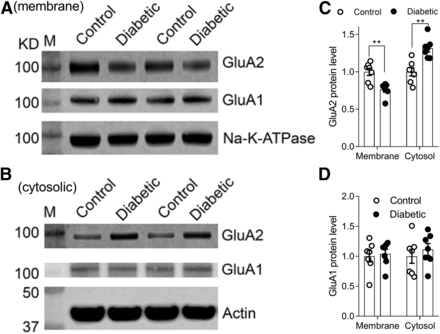
GluA1 and GluA2 subunits are the main AMPARs on the postsynaptic sites in the superficial spinal dorsal horn (Kerr et al., 1998). We therefore determined whether the protein amounts of spinal GluA1 and GluA2 subunits in the plasma membrane are altered in diabetic rats. Immunoblotting was used to measure the protein amount of GluA1 and GluA2 in the plasma membrane and cytosolic fractions. The protein amount of GluA2 in the plasma membrane in the diabetic group was significantly lower than that in the control group (P = 0.0017, t(12) = 4.016, n = 7 rats per group; Fig. 5). Also, the GluA2 protein level in the cytosol in the diabetic rats was much greater than that in the control rats (P = 0.0011, t(12) = 4.296, n = 7 rats per group; Fig. 5); however, the level of GluA1 in the plasma membrane and cytosol was similar in control and diabetic groups (n = 7 rats per group, Fig. 5). These results indicate that diabetic neuropathy causes intracellular GluA2 retention and attenuates GluA2 plasma membrane trafficking in the spinal dorsal horn.
The plasma membrane trafficking of GluA2 is reduced in the dorsal spinal cord of diabetic rats. (A and B) Representative blotting images show the protein level of GluA1 and GluA2 in the plasma membrane (A) and cytosol (B) in the dorsal spinal cords between diabetic rats and control groups. Na+-K+-ATPase and β-actin were used as loading controls for plasma membrane and cytosolic proteins, respectively. M, molecular weight marker. (C and D) Group data show the protein level of GluA2 (C) and GluA1 (D) in the plasma membrane and cytosolic fractions of the dorsal spinal cord tissues in control and diabetic rats. Data are shown as means ± S.E.M., n = 7 rats in each group. **P < 0.01 compared with the control group (two-tailed Student’s t test).
Spinal CP-AMPA Receptors Mediate Pain Hypersensitivity Associated with Diabetic Neuropathy.
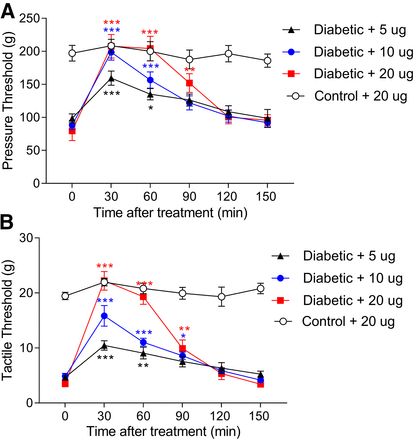
We next determined whether CP-AMPARs at the spinal level are involved in maintaining chronic pain in STZ-induced diabetic rats. We tested the effect of IEM-1460 injected intrathecally on tactile allodynia and pressure hyperalgesia in diabetic rats. We did not test thermal sensitivity because the heat withdrawal threshold was similar in control and STZ-induced diabetic rats (Chen and Pan, 2002). Intrathecal administration of saline has no effect on the withdrawal thresholds tested with tactile and pressure stimuli in diabetic rats (Li et al., 2010; Chen et al., 2011). In diabetic rats, intrathecal administration of IEM-1460 at 5–20 μg significantly elevated the tactile and pressure withdrawal thresholds in a dose-dependent manner (n = 7 rats; Fig. 6). In contrast, intrathecal administration of 20 μg of IEM-1460 did not significantly alter the tactile and pressure withdrawal thresholds in control rats (n = 6 rats; Fig. 6). These findings suggest that spinal CP-AMPARs contribute to sustaining pain hypersensitivity associated with diabetic neuropathy.
CP-AMPA receptors at the spinal cord level contribute to diabetic neuropathic pain. (A and B) Time course of the inhibitory effect of of IEM-1460 injected intrathecally on the withdrawal threshold in response to a noxious pressure stimulus (A) and von Frey filaments (B) in seven diabetic rats and six control rats. Data are shown as means ± S.E.M. *P < 0.05; **P < 0.01; ***P < 0.001 compared with respective baseline values (time 0; repeated measures ANOVA with Dunnett’s post-hoc test).
Discussion
Spinal cord NMDA receptors are generally thought to be central for neuropathic pain development (Zhou et al., 2011; Deng et al., 2019). For example, nerve ligation in rats potentiates the activity of both presynaptic and postsynaptic NMDA receptors in the spinal dorsal horn (Chen et al., 2014b, 2018). Furthermore, chemotherapy-induced neuropathic pain selectively potentiates presynaptic NMDA receptor activity of spinal dorsal horn neurons (Xie et al., 2016, 2017). In this study, however, we found that painful diabetic neuropathy induced by STZ is not associated with significant changes in the activity of presynaptic and postsynaptic NMDA receptors in the spinal dorsal horn. Thus, it is less likely that pain hypersensitivity in diabetic neuropathy results from altered NMDA receptor activity in the spinal dorsal horn. Although systemic treatment with an NMDA receptor blocker, meantime or MK801, for 1 to 2 weeks can reduce pain hypersensitivity in STZ-induced diabetic rats (Malcangio and Tomlinson, 1998; Daulhac et al., 2006; Chen et al., 2009), its site of action may be in the supraspinal region. Our findings reinforce the concept that the molecular mechanisms involved in neuropathic pain development are disease-specific and that mechanism-based treatments should be considered for different types of neuropathic pain conditions.
The most important finding of our study is the increased CP-AMPA receptor prevalence in the spinal dorsal horn synapses in diabetic neuropathic pain. Because AMPARs in the spinal dorsal horn are essential for synaptic and nociceptive transmission, determining the AMPAR-associated synaptic plasticity in neuropathic pain is fundamentally important. GluA2 acts in a dominant fashion to control several receptor properties including Ca2+ permeability and block by intracellular polyamines. Most AMPARs in the brain and spinal cord contain GluA2, which makes AMPARs Ca2+-impermeable (Dingledine et al., 1999; Isaac et al., 2007). This is crucial for maintaining a low level of cytoplasmic Ca2+ under physiologic conditions. Thus, alterations in GluA2 composition could have a dramatic impact on the quality and strength of synaptic transmission (Plant et al., 2006; Derkach et al., 2007; Li et al., 2012). GluA2-deficient mice display facilitated nociceptive plasticity and long-lasting pain hypersensitivity after tissue inflammation (Hartmann et al., 2004). We found that diabetic neuropathic pain remarkably changed the current-voltage relationship of AMPARs from linear to inward-rectifying, suggesting a change from GluA2-containing AMPARs to GluA2-lacking CP-AMPARs at synaptic sites in the spinal dorsal horn. A persistent incorporation of CP-AMPARs in neurons could cause excessive Ca2+ influx (Kwak and Weiss, 2006), leading to central sensitization in diabetic neuropathy (Chen and Pan, 2002).
The exact molecular mechanisms responsible for the augmented activity of CP-AMPARs in the spinal cord in painful diabetic neuropathy remain unknown. In neuropathic pain caused by traumatic nerve injury, increased NMDA receptor activity seems to play a main role in the switch to CP-AMPARs in spinal dorsal horn neurons (Chen et al., 2013); however, we found no evidence of increased NMDAR activity in the spinal cord in STZ-induced diabetic rats. CP-AMPARs are present in spinal dorsal horn neurons (Engelman et al., 1999; Youn et al., 2008). It is likely that some CP-AMPARs are present in the extrasynaptic site and can substitute postsynaptic GluA2-containing AMPARs in diabetic neuropathy. We showed that in the spinal cord of diabetic rats, the GluA2 protein level was reduced in the plasma membranes but was increased in the cytosol. Thus, augmented GluA2 internalization and reduced GluA2 membrane trafficking in the spinal dorsal horn can reduce the amount of GluA2-containing (Ca2+-impermeable) AMPARs and increases the prevalence and activity of GluA2-lacking, CP-AMPARs at postsynaptic sites in painful diabetic neuropathy.
We showed that the activity of CP-AMPARs is increased and mediates the augmented nociceptive input to spinal dorsal horn neurons in STZ-induced diabetic neuropathy. Reducing the activity of AMPARs, particularly CP-AMPARs, at the spinal cord level effectively relieves neuropathic pain induced by traumatic nerve injury (Chen et al., 2000, 2013). In this study, we found that IEM-1460, a selective CP-AMPAR blocker (Samoilova et al., 1999; Kobylecki et al., 2010), preferentially reduced the glutamatergic EPSCs of dorsal horn neurons in diabetic but not in control rats. Furthermore, we found that blocking CP-AMPARs at the spinal cord level markedly attenuated pain hypersensitivity in STZ-induced diabetic rats but produced no effect on the nociceptive threshold in control rats. These data suggest that in the spinal dorsal horn, increased activity of CP-AMPARs may serve to strengthen nociceptive transmission in painful diabetic neuropathy. We showed that the amplitude of glutamatergic EPSCs evoked from the dorsal root is significantly larger in diabetic than in control rats (Wang et al., 2007; Li et al., 2010). The increased nociceptive glutamatergic input from primary sensory neurons to the spinal dorsal horn in painful diabetic neuropathy is caused by reduced K+ channel activity and/or increased activity of voltage-activated Ca2+ channels and mGluR5 in dorsal root ganglion neurons (Hall et al., 2001; Cao et al., 2010, 2011; Li et al., 2010).
In conclusion, by using electrophysiological, biochemical, and behavioral approaches, we provide new evidence that the prevalence of synaptic CP-AMPARs is increased in the spinal dorsal horn, which mediates pain hypersensitivity in diabetic neuropathy. Thus, CP-AMPARs play a key role in glutamatergic synaptic plasticity in the spinal cord in painful diabetic neuropathy. This new information advances our knowledge of the underlying mechanisms responsible for synaptic plasticity in diabetic neuropathic pain and suggests that CP-AMPARs are an alternative therapeutic target for treating this chronic pain condition.
Authorship Contributions
Participated in research design: S.-R. Chen, Pan.
Conducted experiments: S.-R. Chen, Zhang, H. Chen.
Performed data analysis: S.-R. Chen, Zhang, H. Chen.
Wrote or contributed to the writing of the manuscript: S.-R.Chen, Pan.
Footnotes
- Received July 8, 2019.
- Accepted August 20, 2019.
This work was supported by a grant from the National Institutes of Health [R01 NS101880] and by the N.G. and Helen T. Hawkins Endowment.
Abbreviations
- AMPA
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AP5
- 2‐amino‐5‐phosphonopentanoic acid
- AMPAR
- AMPA receptor
- CP
- Ca2+-permeable
- EPSC
- excitatory postsynaptic current
- mEPSC
- miniature excitatory postsynaptic current
- NMDA
- N‐methyl‐d‐aspartate
- STZ
- streptozotocin
- Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics