Abstract
Coated microneedles have emerged as a promising drug delivery system for inflammatory pain treatment. We have previously shown that tramadol injection into the rat temporomandibular joint (TMJ) induces an antinociceptive and anti-inflammatory effect. In this study, microneedles coated with tramadol were investigated as a platform to treat TMJ pain. Male Wistar rats were administered tramadol using an intra-TMJ injection or with microneedles coated with tramadol, followed by 1.5% formalin nociceptive challenge administered 15 minutes later. The nociceptive behavior of rats was evaluated, and their periarticular tissues were removed after euthanasia for analysis. The duration of antinociceptive effect was determined by performing the formalin challenge at different time points extending up to 6 days post tramadol administration. Microneedles coated with tramadol produced an antinociceptive effect similar to injection of tramadol into the rat TMJ. Surprisingly, tramadol delivery using coated microneedles produced a more durable antinociceptive effect lasting as much as 2 days post tramadol delivery as compared with an antinociceptive effect lasting under 2 hours from intra-TMJ injection of tramadol. The proinflammatory cytokines tumor necrosis factor-α and interleukin-1β (IL-1β) were found to be reduced, whereas the anti-inflammatory cytokine IL-10 was found to be elevated in tramadol-treated groups. In conclusion, microneedles coated with tramadol can offer a therapeutic option for pain control of inflammatory disorders in the TMJ.
Introduction
Temporomandibular disorders (TMDs) include disorders of the masticatory muscles and the synovial temporomandibular joint (TMJ) (Scrivani et al., 2008). About 40%–75% of the US population has at least one symptom of TMD, and patients report facial pain as the most common symptom (Scrivani et al., 2008; Cairns, 2010; Gil-Martínez et al., 2018). Surveys from different parts of the world (Pow et al., 2001; Macfarlane et al., 2002; Johansson et al., 2003) put the estimated numbers of the world’s general population that has TMJ pain at 3.5%–33%. Around US$4 billion is spent annually in the United States alone to treat this disorder (National Institute of Dental and Cranofacial Research).
For management of TMJ pain, pharmacological treatment is commonly used, but it is largely empirical (Gil-Martínezet al., 2018). Nonsteroidal anti-inflammatory drugs, pain killers, and muscle relaxants are often indicated in cases of acute inflammation in the TMJ (Romero-Reyes and Uyanik, 2014; Gil-Martínezet al., 2018). However, prolonged use of these types of drugs is usually associated with adverse effects, such as increased risk of gastrointestinal and cardiovascular problems, which makes long-term use of these drugs problematic (Sostres et al., 2010). Moreover, there is no evidence to support that oral medications should be chosen as a standard treatment of chronic TMD (Mujakperuo et al., 2010). Intra-articular injections of nonsteroidal anti-inflammatory drugs, corticosteroids, or hyaluronate into the TMJ are available as other options for invasive treatments of inflammation or pain in the TMJ (Romero-Reyes and Uyanik, 2014). While one injection may not cause problems, multiple injections of steroids over time, especially to treat chronic pain, have the potential to negatively impact bone density and induce osteoclastogenic activity (Romero-Reyes and Uyanik, 2014; Knudsen et al., 2015).
Thus, there is need for both a pharmacological agent and a simple, painless, and possibly self-administrable method for administering the pharmacological agent. To facilitate delivery of drugs in a painless manner, we recently proposed microneedles (MNs) as a strategy for drug delivery to the periarticular tissues of the TMJ (Macedo et al., 2017). An MN patch consists of micron-scaled needles designed to rupture the stratum corneum layer of the skin. MNs are painless (Gill et al., 2008) and are preferred by patients over hypodermic injections (Birchall et al., 2011). We have previously demonstrated that by creating microscopic holes in the skin covering the TMJ by application of MNs and then topically applying 15-deoxy-Δ12,14-prostaglandin J2, development of inflammatory pain can be prevented in the rat TMJ (Macedo et al., 2017). Lidocaine is a common analgesic, and it has been delivered into the skin using microneedles for pain management (Gupta et al., 2012; Ma and Gill, 2014; Kathuria et al., 2016; Zhan et al., 2018). However, the efficacy of MNs in managing TMJ pain has not been demonstrated.
Tramadol hydrochloride belongs to the opioid drug class; however, unlike classic opioids, it has fewer side effects and poses a lower risk of addiction and drug abuse (Bravo et al., 2017). It is widely prescribed as an oral medication to treat cancer-related pain, postoperative pain, and inflammatory pain (Bono and Cuffari, 1997; Wilder-Smith et al., 2001; Bravo et al., 2017). Although tramadol is considered a centrally acting drug (Miotto et al., 2017), when tramadol is administered locally into tissues, it also promotes analgesia. For example, in humans, tramadol has been evaluated as postoperative analgesia to manage pain for inguinal herniotomy (Demiraran et al., 2006; Prakash et al., 2006), extraction of molars (Al-Haideri, 2013), and pediatric tonsillectomy (Atef and Fawaz, 2008). Similarly, in animals, an analgesic effect was seen from local delivery of tramadol in the knee joint (Garlicki et al., 2006; Mert et al., 2007), in the hind paws (Sousa et al., 2008; Sawynok et al., 2013), and in the TMJ (Sipahi et al., 2015). We have also previously shown an antinociceptive effect of tramadol when injected into the rat TMJ (Lamana et al., 2017).
Considering the safety profile of tramadol over other opioids and its already-approved status as a pharmacological agent, we postulated that delivery of tramadol with MNs could offer a novel way to treat TMJ pain. Therefore, this study was the first to evaluate whether MNs coated with tramadol can inhibit nociceptive response in a formalin challenge rat model. We investigated the ability to coat MNs with tramadol, their ability to prevent development of nociception in a rat model, and the duration of effectiveness. A direct comparison was made to an intra-articular injection of tramadol into the TMJ.
Materials and Methods
Animals.
Animal experiments were performed under a protocol approved by the Institutional Animal Care and Use Committee at Texas Tech University. Male Wistar rats (6–8 weeks; Charles River Laboratories) were housed two per cage in a climate-controlled environment with a 12-hour dark and 12-hour light cycle, with food and water ad libitum. Animals were rested for 7 days to acclimatize them to the facility. Next, to acclimatize them to the testing procedure, animals were handled for 5 days by placing them in the experimental test chamber. The experimental test chamber was a 30 × 30 × 30–cm wooden chamber lined with mirrors on the inside on all sides and clear glass on the front side. These acclimatization steps are important so that the rats become accustomed to human handling and placement in the test chamber. This helps to reduce the influence of these variables on rat behavioral response. Nociceptive behavioral testing was performed during the light phase (between 8 AM and 5 PM) in a quiet room maintained at 23°C (Rosland, 1991). Animals were randomly assigned to the different groups.
MN Fabrication and Coating.
MN patches were fabricated from stainless steel (grade 316) sheets through a wet etch process. Each MN patch was 1 cm long × 1 cm wide and contained 57 MNs, each 700 μm long, 200 μm wide, and 50 μm thick. MNs on a patch were coated with microprecision dip coating equipment developed in house (Shakya and Gill, 2015). In brief, the coating solution was pipetted into an orifice that was attached to the z-axis computer-controlled stage. The MN array was attached on the x-y stage using alignment pins, and each microneedle of the array was sequentially dipped into the orifice to coat the entire array. The dipping process was fully automated. The coating solution was replenished after coating five MN arrays. The MN coating solution consisted of: 1) carboxymethyl cellulose [1% (w/v)] (low viscosity, USP grade; CarboMer, San Diego, CA) as a viscosity enhancer; 2) 0.5% (w/v) Lutrol F-68 NF (BASF, Mt. Olive, NJ) as a surfactant; and 3) tramadol hydrochloride (Sigma-Aldrich, St. Louis, MO) at different concentrations. A nomenclature of MNs-x% was used wherein x is 0, 5, 10, 20, 30, or 50, and it refers to patches prepared by dipping into a coating solution containing x% (w/v) tramadol. Use of tramadol was approved by a US Drug Enforcement Administration license.
Quantification of Tramadol Coated on an MN Patch.
The mass of tramadol in coatings was determined by using a UV-visible spectrophotometer (Cary 300; Varian Instruments). In brief, after coating the MN patches, each patch was placed in 1 ml of deionized water and vortexed for 10 minutes to dissolve the coatings. A standard curve obtained from known concentrations of tramadol was used to quantify tramadol in the sample by measuring absorbance through a quartz cuvette at a wavelength of 271 nm (Küçük and Kadioğlu, 2005).
In Vitro Evaluation of Delivery Efficiency.
Porcine skin was obtained from Innovative Research (Novi, MI) and stored at −80°C. Delivery efficiency of tramadol-coated MNs was determined as described previously (Serpe et al., 2016). In brief, the skin was thawed at room temperature, and hair on the skin was trimmed. Coated MN patches were inserted into the skin, held in place for 3 minutes, and removed. A prewetted cotton swab was gently rubbed on the application site to collect any tramadol left on the skin surface. The removed MN patch and swab were separately immersed in 1 ml of deionized water and vortexed for 10 minutes. The mass of tramadol in the solutions was determined as described in the previous section. The delivery efficiency was calculated using the following formula: where M1 is the initial mass of tramadol coated on the MN patch before insertion, and M2 and M3 are the residual mass of tramadol on the MN patch and skin, respectively.
where M1 is the initial mass of tramadol coated on the MN patch before insertion, and M2 and M3 are the residual mass of tramadol on the MN patch and skin, respectively.
Intra-articular TMJ Injection Procedure.
Animals were briefly anesthetized by inhalation of isoflurane (1.5%, 30-second period) through a nose cone. Subsequently, a 26-gauge needle attached to a Hamilton syringe (50 µl; 700 series) via a polyethylene tube was introduced into the TMJ. The needle was inserted into the lower portion of the posterior-inferior border of the zygomatic arch, being advanced in an anterior direction (Clemente et al., 2004). After performing the injection, anesthesia was stopped, and the animal regained consciousness about 30–60 seconds later.
MN Patch Application on Rat TMJ Skin.
Figure 1A provides a schematic describing how a coated MN patch enables tramadol delivery into the skin. MNs penetrate the skin carrying the coated payload. Once in the skin, contact of the coatings with the interstitial fluid initiates their dissolution process and detaches them from the MN surface. This occurs rapidly. Within 3–5 minutes, the MNs are removed and discarded. Figure 1B shows a digital photograph of an uncoated MN patch, and Fig. 1C shows a scanning electron micrograph of a portion of the uncoated MN patch. Twenty-four hours prior to MN patch application, hair was removed from the skin covering the rat TMJ by use of a clipper followed by application of a hair-removing lotion. Animals were briefly anesthetized by inhalation of isoflurane (1.5%, 30-second period) through a nose cone (Fig. 1E). A tramadol-coated MN patch was attached to the flat surface of a 15-ml Corning centrifuge tube cap (Fig. 1D) and applied manually on the hairless TMJ skin with a light pressure and held in place for 3 minutes (Fig. 1, F and G).
Coated microneedles for tramadol delivery to periarticular tissues. (A) Schematic describing the concept of coated microneedles for tramadol delivery into skin covering the TMJ. (B) Digital photograph of an uncoated microneedle patch held in fingers. (C) Scanning electron micrograph of an uncoated microneedle patch. (D) Coated microneedle patch attached to a test tube cap. (E) Animal under inhalational anesthesia. Hair was removed from its skin covering the temporomandibular area 24 hours prior to microneedle insertion. (F) Application of the microneedle patch in the temporomandibular area. (G) Pores observed on the skin after microneedle patch application.
MN Patch Application on TMJ Skin.
Twenty-four hours prior to MN patch application, hair was removed from the skin covering the TMJ with an electrical clipper followed by application of hair-removing lotion. A tramadol-coated MN patch was attached to the flat surface of a 15-ml Corning centrifuge tube cap, applied manually on the hairless TMJ skin with light pressure, and held in place for 3 minutes.
Assessment of Nociceptive Behavioral Response.
The test procedure is summarized in Fig. 2. Prior to any procedure, animals were removed from their cages and kept in the test chamber for 15 minutes for acclimatization. Animals (n = 5/group) were then treated with MN patches coated with tramadol or intra-articular TMJ (intra TMJ) injection of tramadol (0 or 90 µg/TMJ). After this treatment, animals were kept in the test chamber for 15 minutes. Subsequently, they were moved to the anesthesia station, and 1.5% formalin (Sigma-Aldrich) prepared in saline was injected as a noxious challenge into the same TMJ via intra-articular injection, as described in the previous section (under Intra-articular TMJ Injection Procedure). Each animal regained consciousness within approximately 30–60 seconds after discontinuing the anesthesia. The animal was immediately placed in the test chamber to measure the behavioral response over a 45-minute observation period (Roveroni et al., 2001; Clemente et al., 2004). This response was defined as the cumulative total number of seconds that the animal spent rubbing the orofacial region asymmetrically with the ipsilateral fore or hind paw, plus the number of head flinches counted during the observation period. Since each head flinch lasted 1 second in duration, each flinch was expressed as 1 second. At the end of each behavior test, the animals were immediately euthanized, and their periarticular tissue was removed for further analyses.
Experimental design. Schematic outlining the experimental protocol for tramadol delivery and challenge with formalin at different time periods.
To study longevity of treatment, animals were treated with tramadol-coated MN patches, and instead of being challenged after 15 minutes, they were challenged with formalin after 2 hours, 8 hours, 1 day, 2 days, 4 days, or 6 days. Each animal was used only once.
Cytokine Measurement in Periarticular Tissue.
Periarticular tissue was isolated as described before (Lamana et al., 2017). In brief, skin covering the TMJ was removed and discarded. Temporalis and posterior deep masseter muscles were carefully dissected with careful attention to anatomic landmarks (zygomatic arch and tympanic bulla) until exposure of the condylar process. The samples included all of the tissues surrounding the condylar process, including the masticatory muscles (temporalis, posterior deep masseter, and pterygoideus externus), articular cartilage, fibrocartilage of the disc, and lateral ligaments. A tissue sample measuring 1 × 1 × 0.5 cm was homogenized in 500 μl of radioimmunoprecipitation assay buffer containing protease inhibitors (RIPA Lysis Buffer; Santa Cruz Biotechnology, Dallas, TX) and then centrifuged at 10,000 rpm for 10 minutes at 4°C. The supernatants were collected and stored at −20°C until analysis. Tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-10 in periarticular tissue homogenates were evaluated with ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocol. Results were expressed as picograms per milliliter.
Statistical Analysis.
To determine if there were significant differences (P < 0.05) among groups, the data were analyzed using one-way analysis of variance (ANOVA) with post hoc contrasts using Tukey’s test. Data are presented in figures as the mean ± S.D.
Results
Effect of Dip Coating Parameters on Tramadol Coating and Delivery Efficiency.
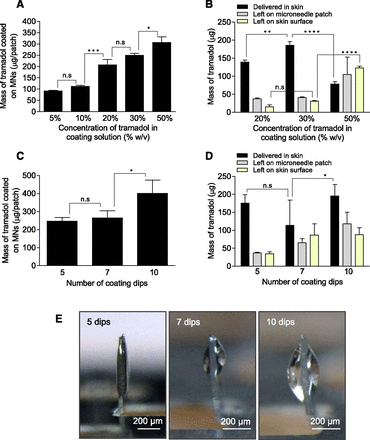
First, we fixed the number of coating dips to five and evaluated how an increase in tramadol concentration in the coating solution affects coatings. As tramadol concentration in the coating solution was increased from 5% to 50%, the amount of tramadol coated on an MN patch increased (Fig. 3A). Since at tramadol concentrations of 5% and 10% the tramadol coated on MNs was low, we decided not to pursue these two coating concentrations further. We next determined the delivery efficiency of coated patches. The efficiency of tramadol delivery into skin for the MN-20%, MN-30%, and MN-50% groups was about 73% (139.4 ± 5.4 μg), 72% (185.5 ± 10.2 μg), and 26% (78.3 ± 6.5 μg), respectively (Fig. 3B). In the MN-50% group, a large fraction of residual tramadol remained attached to MNs (34% equivalent to 104.8 ± 48.4 μg), and an even greater fraction was lost on the skin surface (40% equivalent to 123.3 ± 4.5 μg).
Optimization of tramadol coating on microneedle patches and its delivery efficiency in pig skin in vitro. (A and B) Effect of tramadol concentration in coating solution. Microneedles were dip coated with five dips into coating solutions with tramadol at different concentrations. Amount of tramadol coated on microneedles (A) and in vitro delivery efficiency into porcine skin (B). (C and D) Effect of number of coating dips. Microneedles were dip coated into 30% tramadol coating solution by performing 5, 7, or 10 dips. Amount of tramadol coated on microneedles (C) and in vitro delivery efficiency into porcine skin (D). (E) Stereomicrographs of a single MN of the MN patch coated by dipping into a coating solution with 30% tramadol and 5, 7, or 10 dips. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. n.s, not significant.
Since tramadol delivery was highest in the MN-30% group, we selected it to evaluate the impact of the number of coating dips by changing them from 5 to 7–10 dips (Fig. 3C). The mass of tramadol coated on MNs did not change significantly when the dips were increased from five to seven; however, it increased significantly for 10 dips (Fig. 3C). Efficiency of tramadol delivery into skin was highest at 71% (175.4 ± 23.5 μg) for five dips and decreased to 43% (113.7 ± 70.2 μg) for seven dips and 49% (195.5 ± 32.1 μg) for 10 dips (Fig. 3D). The cumulative amount of tramadol that was lost (either by remaining stuck to MNs or via loss on skin surface) was 29% (70.8 μg) for five dips, 57% (151.7 μg) for seven dips, and 51% (205.6 μg) for 10 dips.
When we visualized the MN coatings under a stereomicroscope (Fig. 3E), the coatings obtained with 7 and 10 dips in the coating solution were rather bulky. This bulkiness hindered penetration of the MNs into the skin, and some of the coating was sloughed off on the skin during insertion, which led to loss of tramadol and thus lower delivery efficiency.
MNs Coated with Tramadol Prevent Formalin-Induced Nociceptive Effect.
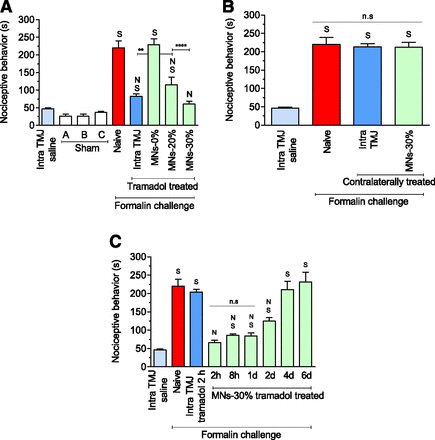
To test the efficacy of tramadol-coated MNs, we inserted them into the skin covering the TMJ of rats and injected formalin as a noxious challenge 15 minutes later. High nociceptive response was seen in the naïve group; however, intra-articular TMJ injection of tramadol significantly blunted it (Fig. 4A). As expected, MNs coated with just the coating excipients (MN-0%) were unable to inhibit formalin’s nociceptive response. However, MN-20% and MN-30% both significantly reduced formalin’s nociception, with MN-30% showing a significantly better blunting effect than MN-20%. Intra TMJ also had a significantly better effect than MN-20%. Treatment with MN-30% (but not with MN-20%, nor with intra TMJ tramadol injection) was able to inhibit formalin nociception to an extent that it was no longer different from the low nociception that was produced just by saline injection control. Other sham treatments of: 1) anesthesia procedure and 2) anesthesia followed by empty hypodermic needle insertion into TMJ without liquid injection also showed low nociceptive responses (Fig. 4A), indicating that the respective procedures by themselves do not contribute significantly to the nociceptive response. Because of the superior antinociceptive effect of MN-30%, we selected this treatment for further evaluation.
Antinociceptive effect of tramadol-coated microneedles on formalin-induced nociceptive response in the TMJ. (A) Effect of tramadol-coated microneedles on formalin-induced in the TMJ. (Sham A) Animals were anesthetized, allowed to recover, and nociceptive effect was observed. This control allowed assessment of the effect of just the anesthesia procedure. (Sham B) Animals were anesthetized, a 26-gauge hypodermic needle was inserted into the TMJ but no fluid was injected, and nociceptive effect was observed. This control allowed assessment of the effect of needle insertion into the TMJ. (Sham C) Animals were anesthetized, microneedles coated with just the excipients but without tramadol were applied, and nociceptive effect was observed. This control allowed assessment of the effect of MN insertion. (Naïve) Animals did not receive tramadol. (Intra TMJ saline) Control group that received just saline injection into TMJ but no formalin injection. (Intra TMJ) Tramadol was given through intra-articular injection into the TMJ. (MN-0%) Microneedles coated with coating excipients but not tramadol were used. (MN-20%) Tramadol was given through microneedles coated by dipping into 20% tramadol coating solution with five dips. (MN-30%) Tramadol was given through microneedles coated by dipping into 30% tramadol coating solution with five dips. (B) Antinociceptive effect of tramadol is local. Tramadol was given in one TMJ and formalin challenge was given in the other (contralateral) TMJ. (Naïve) Animals did not receive tramadol. (Intra TMJ) Tramadol was given through intra-articular injection into the TMJ. (MN-30%) Tramadol was given through microneedles coated by dipping into 30% tramadol coating solution with five dips. (Intra TMJ saline) Control group that received just saline injection into the TMJ but no formalin injection. (C) Longevity of the antinociceptive effect of tramadol. Nociceptive response of animals from intra-articular formalin challenge with a wait of 2 hours, 8 hours, 1 day, 2 days, 4 days, or 6 days between tramadol delivery and formalin challenge. (Naïve) Animals did not receive tramadol. (Intra TMJ tramadol 2 hours) Tramadol was given through intra-articular injection into the TMJ and formalin challenge was given 2 hours later. (MN-30%) Tramadol was given through microneedles coated by dipping into 30% tramadol coating solution with five dips. (Intra TMJ saline) Control group that received just saline injection into the TMJ but no formalin injection. The data are expressed as the mean ± S.D. of five animals per group. N, nociceptive behavior significantly lower than naïve group (P < 0.05: ANOVA, Tukey’s test); n.s, not significant; S, nociceptive behavior significantly higher than saline control group (intra TMJ saline) (P < 0.05: ANOVA, Tukey’s test). **p<0.01, ****p<0.0001.
To investigate whether the antinociceptive effect induced by MNs coated with tramadol was local or if tramadol was acting through the systemic route, we administered tramadol into one TMJ and then challenged the contralateral TMJ with formalin (Fig. 4B). MN-30% and intra TMJ injection were both unable to blunt nociception from contralateral formalin injection, demonstrating that the antinociceptive effect from tramadol-coated MNs and intra TMJ injection was local and not systemic (P < 0.0001).
To assess the longevity of tramadol’s effect, animals were treated with intra TMJ injection of tramadol (90 µg/TMJ) or MN-30% and challenged with formalin after 2 hours, 8 hours, 1 day, 2 days, 4 days, or 6 days. For the intra TMJ injection group, tramadol was not able to inhibit formalin-induced nociception even for 2 hours. Surprisingly, MN-30% sustained the antinociceptive effect against formalin challenge for 2 days (Fig. 4C). Of note, the antinociceptive effect of MNs-30% was quite strong until 2 hours, so much so that the nociception from formalin challenge after treatment with MNs-30% was no different than the nociception by just saline injection in naïve mice. This indicates that treatment with MNs-30% treatment was able to counter the noxious formalin challenge. This benefit was, however, abrogated on days 4 and 6 (Fig. 4C). These results show that MNs enhance the longevity of tramadol effectiveness.
MN Coated with Tramadol Reduces Levels of Proinflammatory Cytokines TNF-α and IL-1β but Enhances the Anti-inflammatory Cytokine IL-10.
To better understand the mechanism of the antinociceptive effect of MNs coated with tramadol, we quantified the proinflammatory cytokines TNF-α and IL-1β and the anti-inflammatory cytokine IL-10 in the periarticular tissues. For the 15-minute-wait protocol, intra TMJ injection of tramadol significantly reduced the levels of TNF-α (Fig. 5A) and IL-1β (Fig. 5C) and significantly increased IL-10 expression (Fig. 5E) over that of the naïve group. However, at the 2-hour point in the longevity assessment study, intra TMJ tramadol injection showed no significant change in any of the three cytokines relative to the naïve group (Fig. 5, B, D, and F). This suggests that intra TMJ injection produces a rather short-term effect. In contrast, upon use of tramadol-coated MNs, a much longer-lasting effect was observed. TNF-α and IL-1β expression was downregulated for up to 6 days, while IL-10 expression was upregulated for up to 6 days (Fig. 5). MN-30% and MN-20% produced similar TNF-α and IL-1β expression levels; however, in terms of IL-10 expression, MN-30% was significantly higher than MN-20% (Fig. 5E).
Protein expression levels of TNF-α, IL-1β, and IL-10 in periarticular TMJ tissues. Protein expression levels of TNF-α (A), IL-1β (C), and IL-10 (E) when formalin challenge was performed 15 minutes after tramadol delivery. Protein expression levels of TNF-α (B), IL-1β (D), and IL-10 (F) when formalin challenge was performed with a wait of 2 hours, 8 hours, 1 day, 2 days, 4 days, or 6 days between tramadol delivery and formalin challenge. (Naïve) Animals did not receive tramadol. (Intra TMJ and intra TMJ tramadol 2 hours) Tramadol was given through intra-articular injection into the TMJ but formalin was given 15 minutes or 2 hours later, respectively. (MN-0%) Microneedles coated with coating excipients but not tramadol were used. (MN-20%) Tramadol was given through microneedles coated by dipping into 20% tramadol coating solution with five dips. (MN-30%) Tramadol was given through microneedles coated by dipping into 30% tramadol coating solution with five dips. (Intra TMJ saline) Control group that received just saline injection into the TMJ but no formalin injection. The data are expressed as the mean ± S.D. of five animals per group. N, nociceptive behavior significantly lower than naïve group (P < 0.05: ANOVA, Tukey’s test). n.s, not significant; S, protein level significantly higher than saline control group (intra TMJ saline) (P < 0.05: ANOVA, Tukey’s test). ****, p<0.0001.
Discussion
We were motivated to perform this study with the long-term goal of developing a safe, self-applied, painless, and effective method of treating TMJ pain using MNs. Coated MNs offer a distinctive delivery system wherein the drug is integrated into the device in the form of coatings. This integrated drug delivery system could simplify the drug application procedure and perhaps even make the process self-applicable in a home setting. Encouraged by our previous result where we showed potent analgesic and anti-inflammatory properties of tramadol in TMJ through local delivery (Lamana et al., 2017), in this study, we decided to coat tramadol onto MNs to evaluate its antinociceptive effect.
Treatment of rats with MN-20% and MN-30% or with an intra TMJ injection of tramadol was able to prevent nociception induced by a formalin challenge done 15 minutes post tramadol delivery. Notably, MN-30% blunted the formalin nociception the most and brought it down to the nociception level of the control group that received just saline injection (no subsequent formalin challenge). In the longevity assessment study, when the gap of time between tramadol delivery and formalin challenge was increased, we surprisingly saw that MN-30% provided a longer-lasting effect. MN-30% successfully inhibited formalin’s nociceptive stimuli even 2 days post application. In contrast, intra TMJ injection of tramadol was unable to sustain its effect for even 2 hours post tramadol delivery.
We have previously observed a similar prolongation of antinociception (up to 6 hours) when we used MNs to topically deliver an anti-inflammatory compound, 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) (Macedo et al., 2017). One explanation could be that when liquid is injected, the liquid can leave the injection site quickly due to convective flow. Similarly, it is possible that after intra TMJ injection of tramadol, it can be lost from the local tissue in a short duration. In contrast, when coated MNs are used to deliver the payload in the skin, the payload is in a solid phase and it may take longer to diffuse away, which could create a high local concentration of tramadol for a longer duration.
To better understand the possible mechanism of tramadol’s peripheral action, we chose to analyze levels of TNF-α and IL-1β, the two major proinflammatory cytokines, and IL-10, an anti-inflammatory cytokine, in periarticular tissues. We selected these cytokines because tramadol has been shown to decrease TNF-α levels in in vitro studies (Liu et al., 2008; Bastami et al., 2013) and in patients with herniated disc and carpal tunnel syndrome (Kraychete et al., 2009). More recently, our group has also shown that intra TMJ injection of tramadol can significantly reduce the inflammatory chemotaxis through inhibition of neutrophil infiltration and reduction of inflammatory cytokines TNF-α and IL-1β (Lamana et al., 2017). Inflammatory pain is also modulated by simultaneous release of anti-inflammatory cytokines, such as IL-10 (Cunha et al., 1999; Verri et al., 2006; Zhang et al., 2017). In the MN-30% group, changes in TNF-α, IL-1β, and IL-10 lasted until 6 days post tramadol delivery, whereas for the intra TMJ injection group, the cytokines leveled to their basal value within 2 hours. This pattern of temporal cytokine change produced by coated MNs and intra TMJ injection matches quite well with their respective time intervals of effectiveness and could provide a possible explanation of why, despite a half-life of about 6 hours (Miotto et al., 2017), the effect of tramadol lasts longer when delivered using microneedles. It is possible to speculate that since the local delivery of tramadol using microneedles releases the entire dose locally, it can create a local high tissue concentration of tramadol. At this concentration, tramadol might initiate a secondary anti-inflammatory cascade that acts through cytokine secretion and whose half-life is more than the actual half-life of tramadol. One possible mechanism could be through the activation of macrophage cells, which are known to secrete cytokines such as IL-10 (da Silva et al., 2015). IL-10 not only provides anti-inflammatory effects but also causes polarization of macrophages to the M2 phenotype, which further provide anti-inflammatory and healing effects (Murray and Wynn, 2011). Additional studies are needed to test this hypothesis.
It should be noted that MN-30% delivered about 185 μg of tramadol versus the 90 μg that was delivered with intra TMJ injection. One could thus argue that MN-30% was more effective due to the larger dose. However, it should be noted that MN-20% also delivered a larger dose (about 139 μg) than the intra TMJ injection, yet intra TMJ produced a significantly better antinociceptive effect than MN-20%. This suggests that changing the route of delivery has a more complex effect that cannot be explained just on the basis of drug dose. Additional studies are needed to investigate this further.
The mechanism of how tramadol promotes an antinociceptive effect in the peripheral nervous system is not yet completely understood. However, based on emerging evidence, it is known that delivery of tramadol directly into the TMJ promotes antinociception mediated by the activation of the intracellular nitric oxide/cyclic guanosine monophosphate pathway, at least in part, independent of opioid receptors (Lamana et al., 2017). Tramadol and its metabolites can also activate peripheral adenosine A1 receptors (Sawynok et al., 2013). It has been shown that agonists of adenosine A1 receptors can produce pain-alleviating effects (Sawynok, 2016), and additionally, the antinociceptive effect of activation of adenosine A1 receptors is associated with intracellular nitric oxide/cyclic guanosine monophosphate pathway, resulting in membrane hyperpolarization (Lima et al., 2010).
In summary, we provide evidence that reinforces the suggestion that tramadol delivery directly into the periarticular tissues induces a potential anti-inflammatory effect. MNs coated with tramadol proved to be more effective than intra TMJ injection. Altogether, these data support a direct clinical approach to treat TMJ pain using MNs coated with tramadol.
Acknowledgments
We thank Md Jasim Uddin for help taking the scanning electron microscopy image of microneedle patch and Dr. Akhilesh Shakya for taking the digital photograph of the microneedle patch.
Authorship Contributions
Participated in research design: Abdalla, Jain, Napimoga, Clemente-Napimoga, Gill.
Conducted experiments: Abdalla, Jain.
Contributed new reagents or analytic tools: Abdalla, Jain, Napimoga, Clemente-Napimoga, Gill.
Performed data analysis: Abdalla, Jain, Napimoga, Clemente-Napimoga, Gill.
Wrote or contributed to the writing of the manuscript: Abdalla, Napimoga, Clemente-Napimoga, Gill.
Footnotes
- Received January 25, 2019.
- Accepted February 22, 2019.
This work was supported by grants from Brazilian governmental financial support by Coordination for the Improvement of Higher Education Personnel (CAPES/PDSE #88881.133786/2016-01) as a Doctoral Fellowship to H.B.A., CNPq for researcher productivity fellowship to J.T.C.-N. and M.H.N., and by FAPESP (#2017/22334-9). This work was also supported by Texas Tech University internal funds, and by endowment funds of Whitacre Endowed Chair in Science and Engineering (H.S.G.).
Abbreviations
- 15d-PGJ2
- 15-deoxy-Δ12,14-prostaglandin J2
- IL
- interleukin
- intra TMJ
- intra-articular TMJ
- MN
- microneedle
- TMD
- temporomandibular disorder
- TMJ
- temporomandibular joint
- TNF-α
- tumor necrosis factor-α
- Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics