In the above article [Maguire DR, Yang W, and France CP (2013) J Pharmacol Exp Ther 345:354–362], the authors have recently discovered inconsistencies for the self-administration data between the original data files and the data presented in the currently published article. These inconsistencies came to light after a recent personnel change in the laboratory. Below are the corrected results showing data that actually were obtained as determined from the original data files. In brief, for one monkey (NA), there is very little difference between the two sets of data; for the other three monkeys, the results are somewhat different, resulting largely from an increase in variance and a decrease in the dose-relatedness of the control heroin dose-effect curves. The major conclusion from this experiment originally was that the cannabinoid receptor agonists CP 55,940 and WIN 55,212 do not enhance the positive reinforcing effects of heroin. The corrected data are generally consistent with this conclusion. That is, CP 55,940 and WIN 55,212 decreased heroin self-administration, shifting the heroin dose-effect curves rightward or downward, and the effects of the cannabinoid receptor agonists were reversed by administration of the cannabinoid receptor antagonist rimonabant. All other data presented in the article have been confirmed to be free of errors. The authors deeply regret this error and apologize for any confusion and inconvenience it may have caused.
Corrected Results
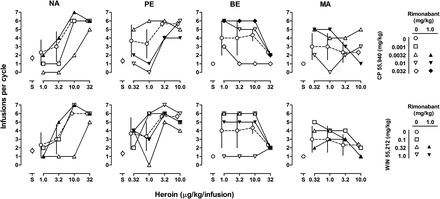
In all monkeys self-administering heroin, the number of infusions obtained per cycle increased, and in two monkeys (BE and MA), the number of infusions then decreased as the unit dose of heroin increased (Fig. 3, open circles). When saline was available (open circles above “S”), the mean (±S.E.M.) number of infusions obtained for all four cycles of the session was 1.9 ± 0.4 (NA), 1.4 ± 0.3 (PE), 0.8 ± 0.2 (BE), and 0.8 ± 0 (MA), and mean response rates were 0.06 ± 0.02 (NA), 0.05 ± 0.01 (PE), 0.03 ± 0.01 (BE), and 0.03 ± 0 (MA) responses per second (data not shown). The largest number of infusions was obtained at unit doses of 10 and 32 µg/kg/infusion heroin in NA, 3.2 and 10 µg/kg/infusion heroin in monkey PE, 10 µg/kg/infusion heroin in BE, and 0.32 and 1.0 µg/kg/infusion heroin in monkey MA; the largest average number of infusions varied from 3.0 ± 1.5 (MA) to 6.0 ± 0 (NA).
With the exception of 0.0032 mg/kg in monkey PE, CP 55,940 dose-dependently decreased heroin self-administration (Fig. 3, top panels), shifting heroin dose-effect curves rightward and downward. Monkey NA was most sensitive to the effects of CP 55,940; 0.0032 mg/kg (upright triangles) reduced the number of infusions obtained per cycle at the three largest unit doses of heroin (3.2, 10, and 32 µg/kg/infusion). For monkeys PE and MA, 0.01 mg/kg CP 55,940 (inverted triangles) reduced the number of infusions obtained per cycle at small and intermediate unit doses of heroin (0.32 and 1.0 µg/kg/infusion). For monkey BE, 0.01 mg/kg CP 55,940 had no significant effect on the number of infusions received; however, 0.032 mg/kg (diamonds) reduced the number of infusions obtained per cycle at each of the three largest unit doses of heroin (3.2, 10, and 32 µg/kg/infusion). With the exception of monkey PE, pretreatment with 1.0 mg/kg rimonabant (closed symbols) reversed the effects of CP 55,940 (i.e., 0.0032 mg/kg CP 55,940 in monkey NE, 0.01 mg/kg in monkey MA, and 0.032 mg/kg in monkey BE), resulting in dose-effect curves that were similar to those obtained with heroin alone (open circles).
WIN 55,212 also dose-dependently decreased heroin self-administration in three monkeys (NA, PE, and BE), without consistently affecting responding for heroin in a fourth monkey (MA), resulting in rightward and downward shifts in the heroin dose-effect curves (Fig. 3, bottom panels). Doses of 0.32 (monkeys NA and PE) and 1.0 mg/kg (monkey BE) WIN 55,212 generally reduced the number of infusions received. Pretreatment with a dose of 1.0 mg/kg rimonabant (filled symbols) attenuated the effects of WIN 55,212 on heroin self-administration when administered in combination with effective doses of WIN 55,212 (0.32 mg/kg for NE and 1.0 mg/kg for PE and BE).
- Copyright © 2014 by The American Society for Pharmacology and Experimental Therapeutics






