Abstract
Apraglutide (FE 203799) is a glucagon-like peptide-2 (GLP-2) analog under development for the treatment of intestinal failure associated with short bowel syndrome (SBS-IF) and graft-versus-host disease (GvHD). Compared with native GLP-2, apraglutide has slower absorption, reduced clearance, and higher protein binding, enabling once-weekly dosing. This study evaluated the pharmacokinetic (PK) and pharmacodynamic (PD) profile of apraglutide in healthy adults. Healthy volunteers were randomized to receive 6 weekly subcutaneous administrations of 1, 5, or 10 mg apraglutide or placebo. PK and citrulline (an enterocyte mass PD marker) samples were collected at multiple time points. Kinetic parameters of apraglutide and citrulline were calculated using noncompartmental analysis; repeated PD measures were analyzed with a mixed model of covariance. A population PK/PD model was developed that also included data from a previous phase 1 study in healthy volunteers. Twenty-four subjects were randomized; 23 received all study drug administrations. Mean estimated apraglutide clearance was 16.5–20.7 l/day, and mean volume of distribution was 55.4–105.0 liters. A dose-dependent increase in citrulline plasma concentration was observed, with 5-mg and 10-mg doses inducing higher citrulline levels than 1-mg doses and placebo. PK/PD analysis showed that weekly 5-mg apraglutide induced the maximal citrulline response. Increased plasma citrulline levels were sustained for 10–17 days after the final apraglutide administration. Apraglutide displays predictable dose-dependent PK and PD profiles, with a 5-mg dose showing significant PD effects. Results suggest that apraglutide has early and enduring effects on enterocyte mass and supports the continued development of weekly subcutaneous apraglutide for SBS-IF and GvHD patient populations.
SIGNIFICANCE STATEMENT Once-weekly subcutaneous apraglutide results in dose-dependent elevations of plasma citrulline (an enterocyte mass pharmacodynamic marker) with parameters suggesting that apraglutide has lasting effects on enterocyte mass and the potential to provide therapeutic benefits. This is the first report of a model relating glucagon-like peptide-2 (GLP-2) agonism and its effects in intestinal mucosa, affording not only the ability to predict pharmacologic effects of GLP-2 analogs but also the exploration of optimal dosing regimens for this drug class across populations with different body weights.
Introduction
Glucagon-like peptide-2 (GLP-2) is a 33–amino-acid peptide secreted by enteroendocrine L cells in the distal ileum and colon in response to ingested nutrients (Drucker, 2002). In healthy individuals, GLP-2 is mediated via the GLP-2 receptor expressed in the stomach, small intestine, and large intestine (Drucker, 2002). Major biologic effects of GLP-2 include stimulation of enterocyte proliferation and intestinal perfusion, inhibition of enterocyte apoptosis, maintenance of intestinal barrier function, and regulation of gastrointestinal (GI) motility (Wojdemann et al., 1999; Benjamin et al., 2000; Brubaker, 2018; Baccari et al., 2022).
In both short bowel syndrome (SBS) and graft-versus-host disease (GvHD), there is a substantial loss of intestinal mucosa. SBS is a malabsorptive condition caused by physical or functional loss of significant portions of the small intestine (Jeppesen, 2014). In GvHD, intestinal mucosa is a major target of conditioning regimens administered before hematopoietic stem cell transplantation as well as donor T-cell–mediated immune response, with preclinical studies showing that L-cell counts were targeted in acute GvHD after chemotherapy conditioning (Ghimire et al., 2017; Norona et al., 2020). Both conditions may benefit from treatment with exogenous GLP-2; however, clinical use of GLP-2 has been hampered by the short half-life of the native peptide due to rapid catabolism by dipeptidylpeptidase-IV (DPP-IV) (Amato et al., 2016; Hargrove et al., 2020).
Several GLP-2 analogs (e.g., teduglutide, apraglutide, and glepaglutide) have been designed that are resistant to DPP-IV cleavage. Of these, only teduglutide (Gattex [Shire-NPS Pharmaceuticals, Lexington, MA] or Revestive [Boehringer Ingelheim, Vienna, Austria]) is approved in patients with SBS associated with intestinal failure (SBS-IF). People with SBS-IF require parenteral support (PS) to maintain macronutrients, fluids, and/or electrolytes at levels required to support health and/or growth (Pironi et al., 2015). Once-daily, subcutaneous administration of teduglutide effectively reduces PS volume after 24 weeks in SBS-IF patients receiving parenteral nutrition (Jeppesen et al., 2012); 11%–24% of patients achieved complete parenteral nutrition independence in real-world data from several clinical cohorts using teduglutide (Iyer et al., 2017; Joly et al., 2020; Pape et al., 2020). Although not currently indicated for GvHD, mouse studies suggest that teduglutide reduced de novo acute GvHD and steroid-refractory GvHD (Norona et al., 2020). However, long-term efficacy of teduglutide may be limited by its relatively short half-life, which necessitates daily subcutaneous injections.
Apraglutide (FE 203799) is a synthetic selective full agonist for the human GLP-2 receptor under development for patients with SBS-IF and GvHD (Hargrove et al., 2020; Eliasson et al., 2022a,b). Although its potency and selectivity are comparable to native GLP-2, apraglutide has a longer elimination half-life than both native GLP-2 and teduglutide (Marier et al., 2008; Marier et al., 2010) because of low clearance (Wisniewski et al., 2016) resulting from DPP-IV resistance and high plasma protein binding. Therefore, apraglutide can be administered less frequently than teduglutide, using a once-weekly dosing regimen in humans.
Preclinical studies in normal animals and animal models for SBS-IF showed that apraglutide promotes adaptation of intestinal structure and function through enhanced intestinal growth, resulting in increased mucosal mass and villus height (Slim et al., 2019; Hargrove et al., 2020; Martchenko et al., 2020; Pauline et al., 2021). Moreover, studies in patients with SBS-IF (Eliasson et al., 2022a,b) and in animals receiving conditioning therapy before hematopoietic stem cell transplantation (Dimitriadou and Minden, 2022) indicate that subcutaneous apraglutide preserves or increases levels of plasma citrulline, which is produced by enterocytes in the small bowel (Fragkos and Forbes, 2018), suggesting an increased enterocyte mass and intestinotrophic effects on the intestinal epithelium. Additionally, several studies demonstrated correlations between plasma citrulline levels, small bowel length, and PS independence (Crenn et al., 2000; Jianfeng et al., 2005; Luo et al., 2007; Santarpia et al., 2008). Thus, plasma citrulline concentration appears to be a useful biomarker for small bowel absorptive enterocyte mass, making it an excellent pharmacodynamic (PD) biomarker for the intestinotrophic effect of GLP-2 analogs (Crenn et al., 2000, 2008; Fragkos and Forbes, 2018).
Apraglutide doses investigated in the first-in-human study (3 weekly doses of 11.4, 28.4, and 56.9 mg) resulted in comparable elevations in citrulline, suggesting a plateauing of the PD effect at the lowest dose investigated (Bolognani et al., 2019). Furthermore, elevated levels of citrulline were observed in the last sample collected 64 hours after the final apraglutide administration. Consequently, it was not possible to estimate duration of the PD effect. The present study was designed to better characterize the pharmacokinetic (PK) and PD properties of multiple apraglutide doses in healthy volunteers and to construct a population PK/PD model.
Methods
This was a phase 1, randomized, multiple-dose, parallel-arm, double-blind, placebo-controlled trial in healthy adult volunteers. The study was conducted between May and September 2019 at the Centre for Human Drug Research, Leiden, Netherlands. The study protocol was approved by the independent medical ethics committee “Medisch Ethische Toetsingscommissie van de Stichting Beoordeling Ethiek Biomedisch Onderzoek” (Assen, Netherlands), and the study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants, and the study was registered in the Dutch CCMO trial registry (NL69596.056.19).
Subjects
Study participants were required to be healthy, 18–40 years of age, and have a body mass index between 18 and 28 kg/m2. Health status was verified by a detailed medical history, a complete physical examination, vital signs, 12-lead ECG, and laboratory testing (including hepatic and renal panels, complete blood count, virology, and urinalysis). Subjects were required to discontinue all medication (except paracetamol up to 4 g/day, ibuprofen up to 1 g/day, and hormonal contraceptives) at least 14 days before the first study drug administration and throughout the trial.
Study Treatment
Healthy adult volunteers were randomized to one of four treatment arms: apraglutide 1, 5, or 10 mg or placebo. Each treatment arm included at least two subjects of each sex. Subcutaneous apraglutide or placebo was administered once weekly, with a total of six administrations.
Study Assessments
Subjects were assessed on days –2, –1, 1, 2, 3, 5, 8, 15, 22, 29, 36, 37, 38, 40, 43, 46, 50, 53, 57, 60, 64, 71, 78, and 81, with the first administration of apraglutide on day 1. Fasting blood samples for PK and citrulline analyses were collected on days 1, 2, 3, 5, 8, 15, 22, 29, 36, 37, 38, 40, 43, 46, and 50. Additional blood samples for plasma citrulline were collected on days –2, –1, 53, 57, 60, 64, 71, and 78. Samples for serum anti-apraglutide antibody analyses were collected on days 1, 36, and 78. All study visits were scheduled between 8 and 10 am with subjects having fasted for at least 8 hours. Subjects were instructed to avoid citrulline-containing food products (e.g., watermelon and nuts) throughout the study, and strenuous exercise was excluded in the 24 hours preceding each visit. Safety was assessed by the report of treatment-emergent adverse events (TEAEs) until 45 days after the last study drug administration, concomitant medication, vital signs (pulse rate, systolic blood pressure, diastolic blood pressure), and ECG (heart rate and PR, QRS, QT, QTcF intervals). Chemistry panels, hematology and coagulation panels, body weight, and stool consistency (assessed using the Bristol Stool Scale) (Lewis and Heaton, 1997) were analyzed at various time points.
Bioanalysis
Plasma levels of apraglutide and L-citrulline were quantified using a fully validated liquid chromatography-mass spectroscopy–based method. For apraglutide, the lower limit of quantification (LLOQ) was 1 ng/ml and the upper limit was 200 ng/ml. Respective values for L-citrulline were 0.5 and 50 μg/ml. Anti-apraglutide antibodies were quantified using a validated enzyme-linked immunosorbent assay. Apraglutide, L-citrulline, and anti-apraglutide antibodies were measured by Altasciences (Laval, Canada).
Statistical Analysis
Statistical analysis was performed using SAS for Windows V9.4 (SAS Institute, Inc., Cary, NC), and noncompartmental analysis was performed using R for Windows version 3.6.1 (R Core Team, Vienna, Austria) using PKNCA 0.9.1. Demographic and baseline variables were summarized by treatment. For safety and tolerability endpoints, summary statistics for observed values were calculated for all continuous parameters. For each apraglutide dose, noncompartmental analyses of PK data were performed. Data below the LLOQ were set to zero if the LLOQ was reached before time to maximum concentration (tmax), and values were treated as missing if the LLOQ was reached after tmax. The elimination rate constant and its derived parameters were excluded when they could not be accurately estimated (negative value for elimination rate constant less than three data points after tmax, adjusted R-square <0.85, or span-ratio <1.5). PD endpoints measured at multiple time points after baseline were analyzed with a mixed model analysis of covariance (ANCOVA) with treatment, time, and treatment by time as fixed factors, subject as random factor, and the average prevalue as covariate. The average prevalue was calculated from all prevalues after screening and before dosing. The Kenward-Roger approximation was used to estimate denominator degrees of freedom, and model parameters were estimated using the restricted maximum likelihood method. The general treatment effect and specific contrasts were reported with the estimated difference and 95% confidence interval (CI), least squares means estimates, and P values.
A statistical steady-state analysis per dose level was performed using the Helmert approach. The first difference tested compared the mean response immediately prior to dosing at multiple dosing at the first time point (week 1) to the pooled mean response immediately prior to dosing at multiple dosing over all remaining time points (weeks 2–6). The second difference compared the mean at week 2 to the pooled mean over weeks 3–6, with corresponding comparisons for each week continuing until the contrast was not statistically significant.
Stool consistency and body weight were assessed as PD endpoints. Subjects assessed their stool in the past 24 hours using the Bristol Stool Scale, ranging from constipation (type 1) to diarrhea (type 7). No stool in the past 24 hours was recorded as 0.
The study was exploratory in nature and was not powered to achieve a statistical endpoint. No sample size calculation was performed.
Population PK/PD Modeling
Pharmacokinetic and citrulline data from this study were combined with PK and citrulline data obtained from the first-in-human study (Bolognani et al., 2019) and used to create a PK/PD model. Details regarding the PK/PD model can be found in Appendix 1 of the Supplemental Materials (Pharmacokinetic/Pharmacodynamic Model Description).
Results
Forty-four healthy subjects were screened, 24 were randomized into the study, and 23 completed all study drug administrations per protocol (Supplemental Fig. 1). One subject in the apraglutide 10-mg arm was discontinued for safety reasons after the third dose (see further details in Safety section), and one subject in the placebo arm discontinued because of personal reasons during the follow-up period. Baseline and demographic characteristics were similar between treatment arms (Supplemental Table 1).
Noncompartmental Pharmacokinetic Analysis
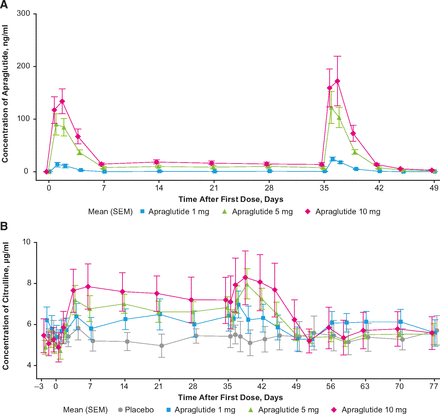
A noncompartmental PK analysis was conducted and the estimated PK parameters are described in Table 1. After the sixth dose, apraglutide displayed a mean estimated clearance (CL/F) of 16.5 to 20.7 l/day, which was constant across the three dose levels. For the 5- and 10-mg doses, the mean volume of distribution (Vz/F) was 55.4 and 105.0 liters, and mean terminal half-life (t1/2) was 72.96 and 76.32 hours, respectively. Vz/F and t1/2 could not be assessed for the 1-mg dose because there were only three data points above the LLOQ per subject. Mean ± SD maximum concentrations in the 1-, 5-, and 10-mg treatment arms were 24.2 ± 9.2 ng/ml, 124.8 ± 72.1 ng/ml, and 182.4 ± 97.6 ng/ml, respectively. Except for the 1-mg treatment arm (in which all trough concentrations [Ctrough] were below the LLOQ 7 days after the first dose), mean Ctrough levels 7 days after the first dose were comparable to mean Ctrough 7 days after the sixth dose (7.9 ± 3.5 ng/ml vs. 7.6 ± 3.4 ng/ml for the 5-mg treatment arm and 14.5 ± 5.5 ng/ml vs. 13.7 ± 4.8 ng/ml for the 10-mg arm). A statistical steady-state analysis per dose level performed using the Helmert approach revealed no statistically significant difference between Ctrough after the first dose and the pooled Ctrough after doses 2 and 6. Therefore, the time to reach steady state could not be determined based on this analysis. Visual inspection of the data suggests that steady state was reached after the first dosing at all investigated dose levels. The apraglutide concentration over time for the three dose levels is displayed in Fig. 1A.
Apraglutide plasma pharmacokinetic parameters after six weekly subcutaneous administrations of 1, 5, or 10 mg apraglutide
Changes in (A) plasma apraglutide concentration (ng/ml) and (B) plasma citrulline concentration (µg/ml) after subcutaneous administrations of apraglutide 1, 5, or 10 mg or placebo. Weekly subcutaneous administrations of 1, 5, or 10 mg apraglutide were scheduled on days 1, 8, 15, 22, 29, and 36. Arithmetic mean per dose level is displayed on linear scale ± S.E.M. Apraglutide values below the limit of quantification (<1 ng/ml) were set to 0. Ctrough levels of citrulline were obtained weekly from days 7 to 35 and more frequently from days 0 to 7 and from day 36 onward. Note that citrulline elevations reached steady state at day 7.
Citrulline
Apraglutide induced a dose-dependent increase in plasma citrulline levels (Table 2; Fig. 1B). At baseline, mean ± SD citrulline levels were comparable between all four treatment arms (10 mg: 5.3 ± 1.5 µg/ml; 5 mg: 5.1 ± 0.9 µg/ml; 1 mg: 5.8 ± 1.4 µg/ml; placebo: 5.5 ± 1.0 µg/ml, corresponding to values of 30.3 µmol/l, 29.1 µmol/l, 33.1 µmol/l, and 31.4 µmol/l, respectively [note: conversion factor to µmol/l is 5.71 given that the molecular weight of citrulline is 175.2 g/mol]). Mean citrulline levels were significantly elevated compared with placebo in all apraglutide treatment arms (P = 0.0007) and remained above baseline from the first postdose time point throughout the entire treatment period (P < 0.0001). This response was statistically significant, with an increase of 1.2574 μg/ml with 5 mg apraglutide (95% CI, 0.5037–2.0112; P = 0.0025) and 1.6343 μg/ml with 10 mg apraglutide (95% CI, 0.8809–2.3878; P = 0.0002) compared with placebo. The increase versus placebo for apraglutide 1 mg was not statistically significant (0.3146 μg/ml; 95% CI, –0.4371 to 1.0663; P = 0.3910). The difference between dose groups indicates that the 5- and 10-mg doses induced significantly higher citrulline levels compared with the 1-mg dose (0.9429 μg/ml; 95% CI, 0.1768–1.7089; P = 0.0186, and 1.3198 μg/ml; 95% CI, 0.5586–2.0810; P = 0.0018), respectively, and the difference between 5- and 10-mg apraglutide doses was not statistically significant (P = 0.31).
Summary of pharmacodynamic parameters
Similar to apraglutide, the time to reach steady-state concentrations for citrulline could not be determined based on the statistical steady-state analysis performed using the Helmert approach. Analysis of the contrasts found no significant difference between citrulline Ctrough after the first dose and the pooled trough concentrations preceding doses 2 to 6. Visual inspection of the data suggested that steady state was reached after the first dose with all three dose levels, based on the observation that the mean citrulline Ctrough was numerically similar for all collected samples.
Seventy-two hours after the final dose (day 39), plasma citrulline levels peaked for the apraglutide groups. Plasma citrulline levels were 8.3 ± 2.9 µg/ml with apraglutide 10 mg, 8.0 ± 1.8 µg/ml with 5 mg, 6.2 ± 1.6 µg/ml with 1 mg, and 5.1 ± 1.5 µg/ml with placebo. Mean citrulline levels remained numerically higher compared with baseline for up to 10, 14, and 17 days after the final dose in the 1-, 5-, and 10-mg apraglutide treatment arms, respectively.
A noncompartmental model was applied to the plasma citrulline concentration-time data. After the sixth respective doses of apraglutide at 1, 5, and 10 mg, mean ± SD maximum citrulline levels (maximum response) estimated by noncompartmental analysis of the plasma citrulline concentration-time data were 7.2 ± 1.7, 8.2 ± 1.8, and 8.7 ± 3.0 μg/ml, respectively, which were achieved with a respective time to reach maximum citrulline concentration of 2.0, 3.9, and 3.9 days. The corresponding maximum citrulline level in the placebo arm was 6.4 ± 1.7 μg/ml, achieved at 16.9 days after the final dose.
Bristol Stool Scale and Body Weight
Overall, subjects treated with apraglutide versus placebo achieved a significantly harder stool consistency as demonstrated by the Bristol Stool Scale (P = 0.0189; Table 2). Compared with placebo, significantly harder stools were achieved by subjects in the 1- and 5-mg treatment arms (–0.9 [95% CI, –1.6 to –0.3], P = 0.0092; –1.0 [95% CI, –1.7 to –0.4], P = 0.0046, respectively) but not by those in the 10-mg treatment arm (Table 2). Results were visible within 1 day of the 5-mg dose and after 1 week for the 1-mg dose. A sensitivity analysis conducted using scores from subjects in the 1- and 5-mg treatment arms who occasionally reported no stools (recorded as value 0 in this analysis) showed no statistically significant treatment effects, either overall or between treatment arms. Body weight appeared to be constant throughout the study, with no statistically significant difference overall or between treatment arms (Table 2).
Safety and Tolerability
No deaths or serious AEs were reported during the study. One subject in the 10-mg treatment arm discontinued treatment after the third dose because of an itchy rash with small papules on the abdomen, corresponding with the second and third injection sites. The rash resolved spontaneously after treatment cessation.
Overall, 18 subjects reported a total of 60 TEAEs (Supplemental Table 2). Five subjects in the 10-mg treatment arm, six in the 5-mg arm, four in the 1-mg arm, and three receiving placebo reported at least one TEAE. Most TEAEs were of mild severity and none were dose-dependent. The most frequently reported TEAEs were GI symptoms, with 19 events reported across treatment groups. No clinically relevant changes in blood chemistry, hematology, coagulation, urinalysis, vital signs, or ECGs were identified. Of the 18 subjects who received apraglutide, one in the 10-mg arm who had a negative anti-apraglutide antibody titer at baseline displayed positive anti-apraglutide antibodies with titers increasing from 320 ng/ml on day 36 to 1280 ng/ml on day 78 (the last data point), with no evidence of effects on PK or safety outcomes. Three patients had mild elevations in liver enzymes after the final administration of apraglutide that spontaneously resolved.
Pharmacokinetic/Pharmacodynamic Modeling
A one-compartmental model with zero-order absorption and linear clearance (Supplemental Fig. 2) was developed using data from this study and the first-in-human study (Bolognani et al., 2019). A model with correlation between volume of the central compartment (V1/F) and CL/F, body weight effects on CL/F and V1/F and a dose effect on the absorption duration (Tk0) best described the observations as judged by the goodness-of-fit plots and the log-likelihood value. Model diagnostics showed that the population PK/PD model described the apraglutide and citrulline observations well and without any major bias. PD observations were fitted together with the PK observations using the final PK model with its structure, residual error, covariates, and random effects models. Plasma citrulline was described well by a turnover model and a sigmoid maximal effects PD model. The population parameter estimates of the PK/PD model are shown in Table 3, and the goodness-of-fit plots of the PK/PD model are shown in Supplemental Fig. 3.
Pharmacokinetic/pharmacodynamic model population parameter estimates for a 70-kg individual receiving 5 mg (s.c.) apraglutide once weekly
Stochastic approximation used for estimation of SE; %CV computed as 100 × √(exp(SD2) – 1).
Pharmacokinetic/Pharmacodynamic Simulations
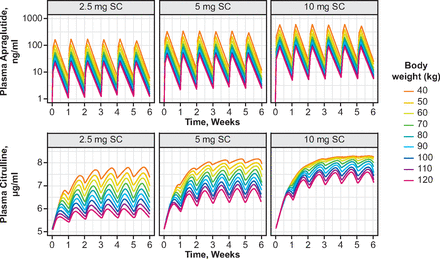
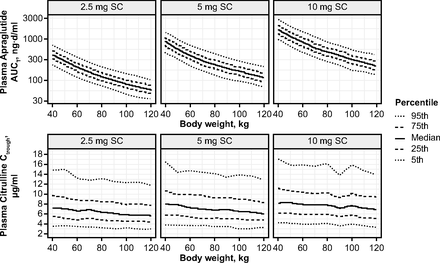
Figure 2 and Supplemental Table 3 show the individual simulated apraglutide and citrulline plasma concentration profiles for weekly subcutaneous apraglutide doses 2.5, 5, and 10 mg for subjects with various body weights. Predicted apraglutide and citrulline concentration-time profiles did not indicate any accumulation. To investigate the effect of body weight on exposure and citrulline concentration, the steady-state area under the curve at a particular time point (area under the plasma concentration-time curve to the end of the treatment period of the corresponding dosing [AUCtau] at week 6) was computed from simulated apraglutide concentration-time profiles after weekly subcutaneous administrations of 2.5, 5, and 10 mg, as well as the expected associated citrulline concentration (Fig. 3). The predicted exposure-response showed a body weight–dependent effect on apraglutide exposure. Peak citrulline concentrations were reached within 2 to 3 weeks with all apraglutide doses.
Predicted plasma levels of apraglutide and citrulline per kg body weight after weekly subcutaneous administrations of 2.5, 5, and 10 mg apraglutide. Modeled plasma apraglutide (top panel) and citrulline (bottom panel) after 2.5-, 5-, and 10-mg subcutaneous administrations in patients with a body weight varying between 40 and 120 kg.
Simulated week 6 apraglutide AUCtau and citrulline Ctrough for individuals with body weight between 40 and 120 kg treated with weekly subcutaneous administrations of 2.5, 5, or 10 mg apraglutide.
Discussion
This study demonstrates a favorable safety and tolerability profile for apraglutide 1, 5, and 10 mg in healthy adult volunteers after 6 weekly subcutaneous doses and a linear PK profile with no accumulation after multiple administrations. Dose-dependent elevations in plasma citrulline levels remained above baseline for 10 to 17 days after the final apraglutide administration, suggesting that apraglutide has a lasting effect on enterocyte mass, an effect that was demonstrated in animals (Martchenko et al., 2020). This also demonstrates that apraglutide has the potential to provide therapeutic benefits via once-weekly subcutaneous dosing. Overall, results support continuing the development of apraglutide for the treatment of people with SBS-IF and GvHD using a weekly subcutaneous dosing regimen.
GLP-2 analogs have been developed with different modifications in the amino acid sequence compared with the native GLP-2 peptide; although all these analogs have different PK properties, it is not fully understood how each modification results in the observed PK differences. Hargrove et al. (2020) have published a comparison of the sequences of native GLP-2, apraglutide, teduglutide and glepaglutide, as well as the comparative PK properties.
Citrulline is an amino acid not incorporated in proteins that is predominantly produced by enterocytes in the small intestine (Barzał et al., 2014). Citrulline is released from enterocytes into the peripheral blood and is used in various metabolic pathways, including the generation of nitric oxide from arginine. Previous studies demonstrate that circulating citrulline concentrations correlate with both enteric mucosal mass and intestinal length in a range of clinical settings (Fragkos and Forbes, 2018). Furthermore, citrulline has been reported to have a relatively short half-life of approximately 1 hour in healthy volunteers after oral administration due to the high renal clearance (Moinard et al., 2008). This suggests that plasma levels of citrulline in the current study are a measurement of the mass of the intestinal mucosa, making citrulline plasma levels an ideal candidate to study the effects of trophic factors in the intestine, such as GLP-2 analogs.
Results from this study support findings from preclinical studies in healthy animals and those with SBS-IF, which demonstrate significant increases in small intestine weight in rats, mice, and pigs treated with apraglutide (Slim et al., 2019; Hargrove et al., 2020; Martchenko et al., 2020; Pauline et al., 2021). Moreover, studies in mice show that apraglutide pretreatment and coadministration reduced chemotherapy-induced GI damage and preserved cellular integrity during chemotherapy (Dimitriadou and Minden, 2022). This appears to show that apraglutide has both regenerative and protective effects on the intestinal mucosa.
Overall, the safety of apraglutide in the current study was consistent with that observed in a phase 1 study in healthy volunteers in which single and multiple ascending doses of apraglutide 2.8, 5.7, 11.4, 28.4, and 56 mg were well tolerated, with no observed serious adverse events or immunogenicity (Bolognani et al., 2019), and in two studies in which apraglutide 5 mg was well tolerated in patients with SBS (Eliasson et al., 2022a,b). The safety of apraglutide and exploratory clinical efficacy in people with GvHD are being evaluated in the phase 2 STARGAZE trial (NCT05415410).
The choice of once-weekly apraglutide doses used in this study was based on results from prior studies showing that weekly apraglutide induced significant clinical effects and consistent plasma citrulline elevations in patients with SBS and healthy volunteers (Bolognani et al., 2019; Eliasson et al., 2022a,b). Furthermore, 6 weeks of treatment was expected to be sufficient to induce the maximal trophic effect in the intestine based on prior studies for other GLP-2 analogs that had study durations of 3–8 weeks (Buchman et al., 2010; Naimi et al., 2019).
Dose-dependent increases in plasma citrulline levels were observed in this study. The mean citrulline increase after the last apraglutide administration declined more rapidly for the 1-mg dose compared with the 5- and 10-mg doses. However, previous studies showed that serum citrulline concentrations are, on average, approximately 2.1 µg/ml lower in patients with SBS-IF versus healthy controls, with substantial variability between patients and a moderate correlation between intestinal length and serum citrulline (Fragkos and Forbes, 2018). In a phase 3 study, teduglutide 0.05 mg/kg per day induced citrulline elevations of 1.56 µg/ml in patients with SBS-IF (Jeppesen et al., 2020), lower than the effects observed with 5 mg apraglutide in the current study. This suggests that 5 mg apraglutide may be an effective therapeutic dose for patients with SBS-IF. However, further studies are required to assess whether apraglutide induces the same magnitude of citrulline elevation in patients with SBS-IF compared with healthy subjects and whether citrulline elevations are associated with beneficial clinical outcomes. Moreover, apraglutide was associated with dose-dependent increases in citrulline levels in mice treated with conditioning chemotherapy (Dimitriadou and Minden, 2022).
Subjects treated with apraglutide in this study achieved harder stool consistency than those treated with placebo; however, no dose-dependent effects of apraglutide on the Bristol Stool Scale were observed. As expected from a healthy population, no significant treatment effects were observed for apraglutide on body weight, either overall or between any of the treatment groups after only 6 weeks of treatment.
The population PK/PD model successfully simulated the effects of different apraglutide doses on plasma citrulline levels at a population level without major bias. The model found that apraglutide has a predictable dose-dependent PK/PD profile and suggests that apraglutide exposure decreases with increasing body weight. Estimates suggest that apraglutide 5 mg leads to an approximately 2.5-µg/ml elevation in citrulline levels in a 70-kg patient and supports once-weekly subcutaneous dosing of apraglutide 5 mg as a potential treatment option for patients with SBS-IF or GvHD.
A potential limitation of this study is that citrulline levels can be affected by lifestyle factors (e.g., diet and strenuous exercise) and circadian variation (Brodan et al., 1976). As a precaution, subjects in this study were instructed not to eat citrulline-containing foods or undertake strenuous exercise within 24 hours before study visits, and all samples were taken at the same time of day after a fast of at least 8 hours to minimize diurnal variations.
Results from this study show that weekly subcutaneous administration of apraglutide for 6 weeks is well tolerated at doses up to 10 mg per week and has a linear PK profile with no accumulation after repeated doses. Dose-dependent increases in plasma levels of citrulline suggest an effect for apraglutide on intestinal mass. Furthermore, citrulline elevations were sustained above baseline for 10–17 days after the final apraglutide dose. The population PK model demonstrates that apraglutide has a predictable PK profile with a long half-life and that apraglutide plasma concentrations and the effect on plasma citrulline can be accurately described by the PK/PD model. Overall, these data support the continued development of apraglutide weekly subcutaneous injections as a potential treatment approach that is applicable for patients with SBS-IF or GvHD.
Acknowledgments
The authors thank Tanja Hoffman for her excellent study management support and Sebastian Spindeldreher for his support with bioassays. Editorial assistance was provided by ICON (Blue Bell, PA, USA), and funded by VectivBio.
Data Availability
The authors declare that all the data supporting the findings of this study are available within the paper and its supplemental material.
Authorship Contributions
Participated in research design: Bolognani, Kruithof, van Gent, Moerland, Crenn, Greig, Gal.
Conducted experiments: Kruithof, Schulthess, Machacek, Gal.
Contributed new reagents or analytic tools: Schulthess, Machacek.
Performed data analysis: Schulthess, Machacek, de Kam, Bergmann, van Gent.
Wrote or contributed to the writing of the manuscript: Bolognani, Kruithof, Schulthess, Machacek, de Kam, Bergmann, van Gent, Moerland, Crenn, Greig, Gal.
Footnotes
- Received January 18, 2023.
- Accepted May 24, 2023.
This work was supported by VectivBio AG.
This work was previously presented as a poster at the following conferences: “Development of a population pharmacokinetic (PK) and pharmacodynamic (PD) model for apraglutide using data from two randomized phase I studies” presented by Bolognani F, et al. American Society for Parenteral and Enteral Nutration–Nutrition Sciences & Practice Conference (ASPEN21); 2021 Mar 20–23; virtual meeting; and “Population model confirms predictable pharmacokinetic (PK) and pharmacodynamic (PD) profile for apraglutide - data from two randomized phase 1 studies” presented by Bolognani F, et al. Digestive Disease Week; 2021 May 21–23; virtual meeting.
↵1Current affiliation: VectivBio (Basel, Switzerland).
G.G. is a paid consultant to VectivBio. A.C.K., M.L.d.K., K.R.B., M.v.G., M.Mo., and P.G. were employees of an organization who received funding from VectivBio for the conduct of this research study. P.S. and M.Ma. were employees of an organization that received research funding for this study.
↵
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
Abbreviations
- AE
- adverse event
- ANCOVA
- analysis of covariance
- AUCtau
- area under the plasma concentration-time curve to the end of the treatment period of the corresponding dosing
- CI
- confidence interval
- CL/F
- estimated clearance
- Ctrough
- minimum concentration
- DPP-IV
- dipeptidylpeptidase-IV
- GI
- gastrointestinal
- GLP-2
- glucagon-like peptide-2
- GvHD
- graft-versus-host disease
- LLOQ
- lower limit of quantification
- PD
- pharmacodynamics
- PK
- pharmacokinetics
- PS
- parenteral support
- SBS
- short bowel syndrome
- SBS-IF
- intestinal failure associated with short bowel syndrome
- t1/2
- terminal half-life
- TEAE
- treatment-emergent adverse event
- tmax
- time to maximum concentration
- Vz/F
- estimated volume of distribution
- Copyright © 2023 by The Author(s)
This is an open access article distributed under the CC BY Attribution 4.0 International license.