Abstract
Thymic stromal lymphopoietin (TSLP), positioned at the top of the inflammatory cascade, is a key regulator that enhances allergic inflammatory responses by activating T helper type 2 cells, Group 2 innate lymphoid cells (ILC2), and myeloid dendritic cells (mDCs) via the TSLP receptor (TSLPR). We evaluated the inhibitory effects of ASP7266, a novel recombinant fully human IgG1 monoclonal antibody against TSLPR, on TSLP signaling and inflammation. The inhibitory effects of ASP7266 and the control antibody tezepelumab on TSLP and TSLPR interactions were investigated using a proliferation assay with TSLP stimulation and a chemokine production assay. The pharmacological effects of ASP7266 were investigated by examining differentiation of naive CD4+ T cells, ILC2 cytokine production, and ascaris extract–induced skin allergic reaction in cynomolgus monkeys. ASP7266 potently inhibited TSLP-induced cell proliferation and C-C motif chemokine ligand 17 production. Furthermore, ASP7266 inhibited TSLP-stimulated mDC-mediated naive CD4+ T-cell differentiation and interleukin 5 production by lineage-negative peripheral blood mononuclear cells, which can be considered ILC2 in vitro. In sensitized monkeys, ASP7266 completely suppressed ascaris extract–induced allergic skin reactions. Based on these results, ASP7266, a novel human therapeutic antibody against TSLPR, is a potential therapy for patients with allergic diseases.
SIGNIFICANCE STATEMENT TSLP, positioned at the top of the inflammatory cascade, plays a key role in various allergic diseases, including asthma, chronic rhinosinusitis with nasal polyposis, and atopic dermatitis. Here we show that the anti-TSLPR antibody ASP7266 exhibited excellent pharmacological activity in preclinical studies. Therefore, ASP7266 has the potential to be a promising treatment option for patients with allergic disorders.
Introduction
Allergic diseases, such as anaphylaxis, food allergy, asthma, allergic rhinitis, chronic rhinosinusitis with nasal polyposis (CRSwNP), and atopic dermatitis (AD), cause significant morbidity and account for a significant proportion of overall healthcare costs (Ruby Pawankar et al., 2013). T helper type 2 (Th2) cell– and Group 2 innate lymphoid cell (ILC2)-mediated type 2 inflammatory responses are implicated in a variety of allergic diseases. Targeting downstream molecules associated with type 2 inflammation, including such major type 2 cytokines as interleukin (IL)-4, IL-5, and IL-13, and products, such as IgE, represents a promising therapeutic strategy for multiple diseases. In fact, molecularly targeted drugs against type 2 cytokines have been launched for asthma, AD, and CRSwNP. However, because these drugs only partially suppress type 2 cytokine–mediated inflammation and do not suppress non-type 2 cytokine–mediated inflammation, a proportion of patients do not respond or only partially respond to such drugs. These patients represent an important unmet medical need (Gandhi et al., 2016).
Thymic stromal lymphopoietin (TSLP) is a key regulator of allergy-induced inflammation, including type 2 inflammation (Liu, 2006; Hong et al., 2020). TSLP is a cytokine derived from various cell types, such as epithelial cells, bronchial smooth muscle, or mast cells, and its production is induced by external stimuli, such as allergen exposure, viral infection, and cigarette smoke (Kato et al., 2007; Smelter et al., 2010; Wang et al., 2018). TSLP exerts its biologic effects by binding to a high-affinity heteromeric receptor complex composed of TSLP receptor (TSLPR) and IL-7 receptor α (IL-7Rα). It further enhances allergic inflammatory responses by activating various cells. Myeloid dendritic cells (mDCs) activated by TSLP express inflammatory chemokines, such as C-C motif chemokine ligand (CCL) 17 [also known as thymus and activaion-regulated chemokine (TARC)] and CCL22 (also known as macrophage-derived chemokine), which attract Th2 cells to the site of inflammation (Soumelis et al., 2002). Additionally, TSLP-stimulated mDCs strongly induce the differentiation of naive CD4+ T cells into Th2 cells, which produce the type 2 cytokines IL-4, IL-5, and IL-13, thereby causing an inflammatory reaction (Soumelis et al., 2002). Furthermore, TSLP promotes the activation and prolonged survival of ILC2, which produces large amounts of IL-5 after stimulation by TSLP, IL-25, and IL-33 and can play a role in steroid resistance (Camelo et al., 2017; Liu et al., 2018).
TSLP signaling blockade is a promising novel therapeutic strategy for allergic diseases. In transgenic mice with TSLP overexpression specifically in the lung or skin, the inflammatory response is accompanied by an increase in IgE and type 2 cytokine levels, which leads to asthmatic or AD-like symptoms (Yoo et al., 2005; Zhou et al., 2005). Conversely, knocking out TSLPR or administration of an anti-TSLPR antibody suppresses type 2 cytokine and IgE production in the blood while improving respiratory function in a mouse asthma model (Al-Shami et al., 2005; Zhou et al., 2005; Shi et al., 2008). Levels of both TSLP mRNA and protein are increased in the airways of patients with asthma compared with healthy subjects (Ying et al., 2005). Clinical trials have been conducted for the TSLP antibodies tezepelumab, CSJ-117, and MK-8226 and the TSLPR antibody RG7258. Tezepelumab has achieved the end point chosen for efficacy in a phase 2 trial targeting patients with asthma and is expected to be used as a novel therapeutic drug for asthma (Corren et al., 2017). Although a drug inhibiting TSLP/TSLPR pathways located upstream of a series of allergy signaling pathways is expected to be effective against general type 2 inflammation, no anti-TSLPR antibody to date has realized its efficacy endpoint in the context of a phase 2 trial. Accordingly, we created ASP7266, a novel recombinant full-length human IgG1 monoclonal antibody against TSLPR, to provide another therapeutic option for patients with allergic diseases.
In an in vitro study, we demonstrated the binding and competitive inhibitory activity of ASP7266 against TSLPR. In addition, we confirmed the inhibitory effect of ASP7266 on TSLP-induced cell proliferation and cytokine production for both CD4+ T cells and ILC2. Using a sensitized cynomolgus monkey model, we also showed the inhibitory effect of ASP7266 on skin allergic reactions.
Materials and Methods
Materials
ASP7266 is a recombinant human mAb consisting of two heavy chains (containing 448 amino acids) and two light chains (containing 214 amino acids) (Sato et al., 2015). CHO cells (CHOK1SV: Lonza, Basel, Switzerland) were used to produce ASP7266, and the protein was subsequently purified through a series of filtration and chromatography steps. For clinical use, ASP7266 was purified from the supernatant of cells from a CHO cell–derived master cell bank developed under Good Manufacturing Practice. Tezepelumab was produced based on the World Health Organization Drug Information (World Health Organization, 2016) using CHO cells (CHOK1SV: Lonza). Human IgG1 (isotype control) was expressed using CHO cells (CHOK1SV: Lonza), mutant TSLP with an alanine insertion/C-terminal FLAG-tag (human TSLP) was expressed using CHO cells (ExpiCHO Expression System; Thermo Fisher Scientific, Uppsala, Sweden; A29133), cynomolgus monkey TSLP with a C-terminal FLAG-tag and rat TSLPR-Fc fusion protein were expressed using HEK 293 cells (FreeStyle 293 Expression System; Thermo Fisher Scientific; K900001), and cynomolgus monkey TSLPR-Fc fusion protein was expressed using HEK 293 cells (Expi293 Expression System; Thermo Fisher Scientific; A14635). AS3287906 and AS3287907 (anti-idiotypic antibodies for ASP7266) were affinity-purified from the culture supernatants of hybridoma cells fused to lymphocytes isolated from the lymph nodes of Lpr mice (C3H/HeJJms Slc-lpr/lpr, Japan SLC, Inc., Shizuoka, Japan) that had been sensitized with ASP7266 and SP2/0 myeloma cells (American Type Culture Collection, Washington, DC, NW; CRL-1581).
Animals
The nonhuman primate holding facility of Astellas is approved from the Ministry of Health, Labor Standards and the Ministry of Environment. All animal care and experimental procedures were performed according to the Institute for Laboratory Animal Research Guide for Care and Use of Laboratory Animals, 8th edition and approved by the Institutional Animal Care and Use Committee of Astellas Pharma Inc., which has been awarded Accreditation Status by the Association for Assessment and Accreditation of Laboratory Animal Care International. The animals were treated in a humane manner with all efforts being made to minimize the number of animals selected for study. Cynomolgus monkeys (Macaca fascicularis, male, 3–5 years old) were purchased from Hamri Co., Ltd. (Ibaraki, Japan). After transportation, monkeys were reared in individual cages for 14 days for quarantine and acclimatization. Each monkey was housed in a stainless steel cage with a perch (cage size: 640 × 650 × 1240 mm) under a 12:12-hour light-dark cycle and controlled temperature (25 ± 2°C) and humidity (55 ± 10%). Monkeys were fed standard laboratory food (approximately 100 g/animal/day; PS-A, Oriental Yeast Co. Ltd, Tokyo, Japan) and given fruit (bananas, apples, raisins) and gummies as supplements. Monkeys were given free access to tap water filtered through a 5-µm filter. In addition, monkeys had access to plastic toys in their cages (Dura-Chews, Certified, Bio-Serv, Flemington, NJ).
Binding Activity of ASP7266 to TSLPR
Binding activity of ASP7266 to recombinant human TSLPR-Fc fusion protein (R&D Systems, Inc., Minneapolis, MN; 981-TR-050) was measured using the Gyrolab xP workstation (Gyros Protein Technologies AB). Final concentrations of TSLPR-Fc ranged from 0.0488 to 25 nM in Tris-buffered saline (TBS)–0.05% Tween 20 solution. Various concentrations of TSLPR-Fc and 0.38 nM ASP7266 were mixed in a 96-well plate and incubated for 48 hours at 4°C. The anti-idiotype antibody (AS3287907) used as a capture antibody was labeled with biotin using the Biotin-Labeling Kit-NH2 (DOJINDO LABORATORIES; LK3) and prepared to 20 µg/ml with TBS. Biotin-labeled AS3287907 was added to Gyrolab Bioaffy 1000 (Gyros Protein Technologies AB; P0004253) containing beads coated with streptavidin. Next, the mixture comprising TSLPR-Fc and ASP7266 was added on the Gyrolab Bioaffy 1000. Another anti-idiotype antibody (AS3287906) used as a detection antibody was fluorescently labeled using the Alexa Fluor 647 Monoclonal Antibody Labeling Kit (Thermo Fisher Scientific; A20186) and prepared to 1 µg/ml with 50% Rexxip F/TBS–0.05% Tween 20. The KD value was calculated by converting the obtained fluorescence intensity to a percentage value. The geometric mean KD value of four trials and 95% confidence interval (CI) were also calculated.
ELISA
For competitive ELISA, 384-well plates were coated with 1 μg/ml human TSLPR-Fc fusion protein. After blocking, 10 ng/ml TSLP in the presence of ASP7266 was added, and the plate was incubated for 30 minutes at room temperature. Polyclonal anti-human TSLP rabbit antibody (Abcam plc., Cambridge, UK; ab47943, RRID: AB_883272) was added as the primary antibody, and this was followed by horseradish peroxidase–conjugated polyclonal anti-rabbit IgG (H+L) goat Fab’ (Medical & Biologic Laboratories Co., Ltd., Tokyo, Japan; 458, RRID: AB_2827722, human IgG-absorbed) as the secondary antibody. After washing, the plate was reacted with TMB+ Substrate-Chromogen (Agilent Technologies, Santa Clara, CA; S1599), after which the reaction was stopped with sulfuric acid. Captured TSLP was detected using an Infinite M200 PRO Multimode Microplate Reader (TECAN; Molecular Devices, LLC., San Jose, CA) by calculating the difference in absorbance at 450 nm and 570 nm.
To evaluate the crossreactivity of ASP7266, 384-well plates were coated with 1 µg/ml human (R&D Systems, Inc.; 981-TR-050), mouse (R&D Systems, Inc.; 546-TR-050), cynomolgus monkey, or rat TSLPR-Fc fusion protein. After blocking, ASP7266 was added, and the plate was incubated for 1 hour at room temperature. Biotinylated anti-human κ light chain goat IgG (Immuno-Biologic Laboratories, Gunma, Japan; 17249, RRID: AB_529234) was added as the primary antibody, and this was followed by Pierce High Sensitivity Streptavidin–horseradish peroxidase (Thermo Fisher Scientific; 21130). After washing, captured antibody was detected by reacting with BM Chemiluminescence ELISA Substrate (POD) (Roche, Basel, Switzerland; 11582950001).
Proliferation Assay
Ba/F3 cells used in this study were maintained in RPMI 1640 (Merck, Darmstadt, Germany; R8758-500ML) medium supplemented with 10% FBS (Cytiva, Tokyo, Japan; 30070.03), 50 units ml−1 penicillin/streptomycin (Thermo Fisher Scientific; 15070-063), and mouse IL-3 culture supplement [Becton Dickinson and Company (BD), Franklin Lakes, NJ; 354040]. Cells were seeded into a 96-well plate and cultured at a concentration of 1 × 105 cells/ml. Transfected human TSLPR- and IL-7Rα–expressing Ba/F3 cells were incubated with ASP7266, tezepelumab, or control IgG in the presence of human TSLP for approximately 48 hours. AlamarBlue cell viability reagent (Life Technologies Corporation, Carlsbad, CA; DAL1100) was added to each well and incubated with the cells. Fluorescence (at an excitation wavelength of 540 nm and emission wavelength of 590 nm) was measured using the TECAN microplate reader 6 hours after addition of AlamarBlue cell viability reagent (Thermo Fisher Scientific; DAL1025).
Chemokine and Cytokine Production Assay of Peripheral Blood Mononuclear Cells
Human peripheral blood mononuclear cells (PBMCs) (Lonza, 4W-270, 270A) were stimulated with human TSLP or TSLP/IL-25/IL-33 in the presence of ASP7266 and tezepelumab for 5 or 7 days in an incubator humidified at 5% CO2 atmosphere at 37°C. The cytokine and chemokine concentrations in the supernatant were measured using AlphaLISA (Perkin Elmer, Inc., Waltham, MA; AL267C) for IL-5 and the Human CCL17/TARC Quantikine ELISA Kit (R&D Systems, Inc; DY364) for CCL17.
Isolation and Stimulation of mDC and T Cells
This study was approved by the Astellas Research Ethics Committee, and written informed consent was obtained from all participants. mDCs, defined as CD11c+, HLA-DR+, and lineage marker− cells, were isolated from the peripheral blood of three healthy volunteers whose sex information was withheld by the Astellas Research Ethics Committee. PBMCs were isolated from peripheral blood using a Vacutainer CPT Cell Preparation Tube with Sodium Heparin (BD; 362753). Subsequently, mDCs were enriched from among PBMCs by negative depletion using a Dynabeads human DC enrichment kit (Thermo Fisher Scientific; 11308D). mDCs were subsequently isolated using BD FACS Aria II (BD). Immediately after isolation, mDCs were cultured in RPMI 1640 supplemented with 10% FBS, 1% pyruvate, 1% HEPES, and 1% penicillin-streptomycin (culture medium). TSLP (final concentration: 15 ng/ml) was added to the mDC suspension (1.5 × 104 cells/ml) in the presence of ASP7266 (final concentration: 30 μg/ml) in a 96-well plate. Subsequently, mDCs were incubated for 1 day at 5% CO2 atmosphere at 37°C. Naive CD4+ T cells were isolated from the blood of the same three healthy volunteers. After isolating PBMCs using a Vacutainer CPT Cell Preparation Tube with Sodium Heparin (BD; 362753), naive CD4+ T cells were isolated from among PBMCs using Naive CD4+ T Cell Isolation Kit II (Miltenyi Biotec GmbH.; 130-094-131). A 100-μl aliquot of the naive CD4+ T cell suspension (5 × 105 cells/ml) was seeded into a 96-well plate and incubated for 1 day in a 5% CO2 atmosphere at 37°C. The cultured mDCs were washed and then cocultured with isolated allogeneic naive CD4+ T cells in round-bottomed 96-well culture plates at 37°C/5% CO2. After 6 days of coculture, cells were collected by centrifugation. The cells were then suspended in culture medium containing soluble anti-CD28 antibody (2 ng/ml) and transferred to a plate coated with anti-CD3e antibody. The plate was incubated for 1 day in a 5% CO2 atmosphere at 37°C. The cytokine concentration in the supernatant was measured using Milliplex (Merck) for IL-4, IL-5, IL-13, and tumor necrosis factor-α (TNF-α). These processes were performed in duplicate in three independent experiments.
Flow Cytometric Analysis of TSLP-Induced IL-5 Production in Human PBMCs
PBMCs were thawed according to the manufacturer's protocol. Cells (9.0 × 105 cells) were cultured in RPMI-1640 supplemented with 10% FBS and 1% penicillin-streptomycin. ASP7266 (final concentration: 10 μg/ml) was added and incubated with the cells for 30 minutes at 37°C/5% CO2 in an incubator. The cells were subsequently stimulated with a combination of the following cytokines: 10 ng/ml of recombinant human IL-25 (R&D Systems, Inc.; 1258-IL/CF), 10 ng/ml of recombinant human IL-33 (BioLegend, San Diego, CA; 581806), and 10 ng/ml of TSLP; this was before further incubating at 37°C/5% CO2 for 7 days. After 7 days of culture, in preparation for intracellular cytokine staining, GolgiPlug (Brefaldin A; BD; 555029) and GolgiStop (Monensin; BD; 554724) were added according to the manufacturer's instructions. The cells were subsequently stimulated with 50 ng/ml phorbol 12-myristate 13-acetate (Merck; P8139) and 1 µg/ml ionomycin (Merck; I0634) for 4 hours. For cell surface staining, cells were labeled with an anti-human lineage cocktail (CD3, CD14, CD16, CD19, CD20, CD56; BioLegend; 348803). Dead cells were excluded based on staining with Zombie NIR Dye (BioLegend; 77184). After staining, cells were permeabilized using the Cytofix/Cytoperm kit (BD; 554715) and stained to determine cytokine production using an anti–IL-5 antibody (BioLegend; 500904, RRID: AB_315139). Sample acquisition was performed using flow cytometry (FACS verse; BD). Data were analyzed using FlowJo V10 software (FlowJo, RRID: SCR_008520).
Chemokine Assay Using Peripheral Blood
This study was approved by the Astellas Research Ethics Committee, and written informed consent was obtained from all participants. Peripheral blood was donated by healthy volunteers whose sex information was withheld by the Astellas Research Ethics Committee. Peripheral blood was collected from a vein using a syringe attached to a needle. Heparin was immediately added as an anticoagulant to the collected blood.
Human whole blood was stimulated with human TSLP (final concentration: 10 ng/ml) in the presence of ASP7266 for 24 hours at 37°C/5% CO2. Cynomolgus monkey whole blood was stimulated with cynomolgus monkey TSLP (final concentration: 10 ng/ml) in the presence of ASP7266 for 24 hours at 37°C/5% CO2. Total RNA was purified from human and monkey PBMCs using a RNeasy Plus Mini Kit (Qiagen, Limburg, Netherlands). First-strand cDNA was synthesized from total RNA using a High Capacity cDNA Reverse Transcription Kit with RNase Inhibitor (Thermo Fisher Scientific). Quantitative polymerase chain reaction was performed for each cDNA template on a QuantStudio 12K Flex Real-Time PCR System (Thermo Fisher Scientific) using validated TaqMan Gene Expression Assays (Thermo Fisher Scientific) for human CCL17 (assay ID: Hs00171074_m1, reporter: FAM) and human endogenous reference gene actin beta (ACTB, assay ID: Hs99999903_m1, reporter: VIC).
Ascaris-Induced Skin Allergic Reaction in Monkeys
Eighteen cynomolgus monkeys were selected from the 25 available monkeys. Monkeys were excluded if they had a total IgE ratio [measured using an ELISA kit (Bethyl Laboratories, Inc.; E80-108)] of 1.8 or higher compared with blank and low body weight (3.7–5.0 kg). Cynomolgus monkeys were allocated to three groups based on body weight using a randomization scheme (n = 6). The first day of sensitization to ascaris extract was designated day 0. On days 0, 7, and 14, monkeys were sensitized with ascaris extract [0.5 mg 2,4-dinitrophenylated ascaris extract (DNP-Ascaris, LSL Co., Ltd., Tokyo, Japan; LSL-LG0009) and 50 mg Aluminum hydroxide hydrate (Alum)/ml PBS] by intraperitoneal (3.6 ml/kg) and intramuscular injection (0.4 ml/kg), respectively. On days −1, 6, and 13, ASP7266 (10 mg/kg) or the formulation buffer was intravenously administered to monkeys. Skin reaction was measured on day 21. The thorax area was shaved, and ascaris extract was injected intradermally at two different sites (100 μl each) under anesthesia using 0.04 mg/kg medetomidine (Nippon Zenyaku Kogyo Co., Ltd., Fukushima, Japan), 0.3 mg/kg midazolam (Maruishi Pharmaceutical Co., Ltd., Osaka, Japan), and 0.4 mg/kg butorphanol (Meiji Seika Pharma Co., Ltd. Tokyo, Japan). Twenty minutes after the injection, wheal diameter was measured at the sites of injection. The severity of the ascaris-specific skin reaction was calculated by subtracting the mean diameter of the PBS-injected site from the mean diameter of the 100 μg/ml ascaris extract–injected site in each monkey. An ascaris extract dose of 100 μg/ml induced significant and reproducible allergic reaction in the skin. After the experiment, monkeys sensitized with ascaris extract were euthanized by incision of the abdominal aorta under anesthesia using 5mg/kg zolazepam (USpharma Ltd., Miami Lakes, FL) and 5 mg/kg tiletamine (USpharma Ltd.).
Measurement of ASP7266 Concentrations in Plasma
Monkey plasma samples were obtained to determine the ASP7266 concentration. ASP7266 in plasma or standard curve sample was added to the assay plate to bind the immobilized capture antibody (AS3287906), and a secondary antibody (biotinylated AS3287907) was added to bind captured ASP7266. Streptavidin-SULFO-TAG was subsequently added. After washing the plate, electrochemiluminescence intensity was measured using the SECTOR imager 6000.
Data and Statistical Analysis
Data were expressed as mean ± S.D. of individual experiments and calculated and shown graphically using GraphPad Prism (Version 8.0.2, GraphPad Software, San Diego, CA; RRID:SCR_002798). IC50 and 90% inhibitory concentration (IC90) values are represented as the geometric mean and 95% confidence interval. IC50 and IC90 values were calculated using non–linear regression analysis with a sigmoid-Emax model (SAS Software, SAS Institute, Inc., Cary, NC) or GraphPad Prism. Differences between two groups were compared using an unpaired two-tailed t test in GraphPad Prism. A value of P < 0.05 was considered statically significant.
Results
Binding Affinity of ASP7266 for Human TSLPR
We created ASP7266, a novel full-length human IgG1 antibody that recognizes human TSLPR, by immunizing VelocImmune mice (Macdonald et al., 2014; Murphy et al., 2014), which bear the humanized IgG loci for generation of a fully human antibody. The binding affinity of ASP7266 for human TSLPR was determined based on a kinetic exclusion assay. The geometric mean KD value of ASP7266 was 0.470 (95% CI: 0.431–0.512) nmol/l.
Inhibitory Effect of ASP7266 and Tezepelumab on TSLP/TSLPR Interaction
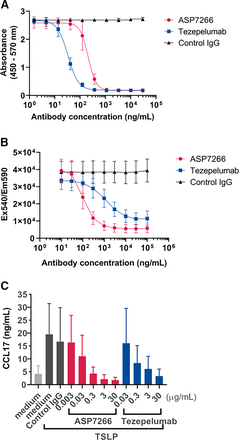
We performed competitive ELISA to evaluate the inhibitory activity of ASP7266 against the TSLP/TSLPR interaction. Because wild-type human TSLP is susceptible to enzymatic processing due to the inner amino acid sequence KKRRKRK (Comeau and Ziegler, 2010; Poposki et al., 2017), in the present study, we used mutant human TSLP, which has an alanine insertion between lysine (129) and arginine (130), to avoid degradation. This alanine insertion is not involved in the interaction with TSLPR (Verstraete et al., 2017). Competitive ELISA confirmed that ASP7266 as well as tezepelumab displayed dose-dependent inhibitory activity against the interaction between TSLP and TSLPR (Fig. 1A). Next, we evaluated the effect of ASP7266 on the TSLP/TSLPR interaction by examining Ba/F3 cell proliferation. Ba/F3 cells from a mouse pro-B cell line cotransfected with human IL-7Rα and TSLPR showed TSLP dose-dependent proliferation. ASP7266 and tezepelumab inhibited the TSLP-induced proliferation of Ba/F3 cells with an IC50 (95% CI) value of 90.7 (67.5–122) ng/ml and 518 (342–785) ng/ml, respectively (Fig. 1B; Table 1). ASP7266 more potently inhibited TSLP-induced cell proliferation than tezepelumab.
Inhibitory activity of ASP7266 against TSLP/TSLPR interaction. (A) Inhibitory activity of ASP7266 against the human TSLP/TSLPR interaction evaluated using competitive ELISA. Data indicate mean ± S.D. for three subjects. Four-parameter logistic curve is shown. (B) Inhibitory effects of ASP7266 and tezepelumab on TSLP-induced proliferation of Ba/F3 cells. Data indicate mean ± S.D. for five subjects. (C) Inhibitory effects of ASP7266 and tezepelumab on CCL17 production in PBMCs. CCL17 measurements were conducted 5 days after stimulation with 5 ng/ml TSLP. Data indicate mean ± S.D. for five or four subjects. Medium, RPMI1640 medium without antibody. Control IgG, isotype control IgG1.
IC50 and IC90 values in TSLP/TSLPR interaction
IC50 and IC90 values were calculated using non–linear regression analysis with reactions with only medium defined as 100% inhibition, and reactions with TSLP without antibody were defined as no inhibition.
To confirm the inhibitory activity of ASP7266 and tezepelumab antibodies in human PBMCs, we assessed the effect on TSLP-induced CCL17 production as an index for the TSLP/TSLPR interaction. Stimulation with TSLP led to CCL17 production in PBMCs. Preincubation with ASP7266 and tezepelumab inhibited TSLP-induced production of CCL17 (Fig. 1C). IC50 and IC90 values of ASP7266 and tezepelumab are shown in Table 1.
Effect of ASP7266 on mDC-Mediated Differentiation of Naive CD4+ T Cells Induced by TSLP
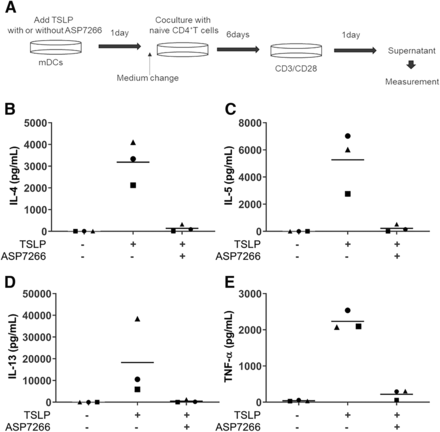
We evaluated the effect of ASP7266 on TSLP-mediated mDC activation (see Fig. 2A for experimental outline). CD4+ T cells cocultured with human TSLP-stimulated mDCs produced IL-4, IL-5, IL-13, and TNF-α (Fig. 2, B–E). However, coculturing CD4+ T cells with human TSLP-stimulated mDCs in the presence of ASP7266 markedly inhibited the production of these cytokines. These results suggest that ASP7266 inhibits TSLP-stimulated mDC-mediated differentiation of naive CD4+ T cells.
Inhibitory effects of ASP7266 on human TSLP-stimulated human mDC-mediated differentiation of naive CD4+ T cells. (A) Experimental outline. CD4+ T cells cocultured with TSLP-stimulated human mDCs produced inflammatory cytokines after CD3/CD28 stimulation. The concentration of IL-4 (B), IL-5 (C), IL-13 (D), and TNF-α (E) in the supernatant was measured using Multiplex. Horizontal bars indicate the mean of three independent experiments (each symbol) using blood from each subject.
Effect of ASP7266 on TSLP-Induced IL-5 Production in Human PBMCs
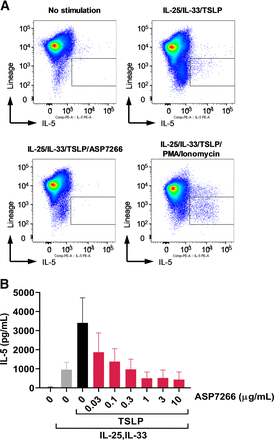
We assessed the effect of ASP7266 on TSLP-induced IL-5 production using PBMCs. After stimulation with IL-25, IL-33, and TSLP for 7 days, we confirmed that the main source of IL-5 production was lineage-negative cells, and production was completely inhibited by treatment with ASP7266 (Fig. 3A). IL-5 production from lineage-positive cells was observed only under phorbol 12-myristate 13-acetate/ionomycin stimulation (Fig. 3A, lower right). In addition, TSLP enhanced IL-25 and IL-33–mediated IL-5 protein production in PBMCs (Fig. 3B). ASP7266 dose-dependently inhibited IL-5 production in the presence of these stimuli in PBMCs (Fig. 3B). These results suggest that TSLP-induced IL-5 production in human PBMCs is mainly derived from lineage-negative cells, which can be considered ILC2 cells (see discussion).
Inhibitory effect of ASP7266 on TSLP-induced IL-5 production in human PBMCs. PBMCs were cultured for 7 days with TSLP, IL-25, and IL-33 or control medium for the no stimulation group. Cells and supernatants were collected for flow cytometry (A) and AlphaLISA (B). (A) Flow cytometric analysis of IL-5 production from lineage-negative cells (inside the box). Representative results from the same experiment are shown. (B) IL-5 protein levels from PBMCs. Data indicate mean ± S.D. for 6 subjects.
Crossreactivity of ASP7266 to Mice, Rats, and Monkeys
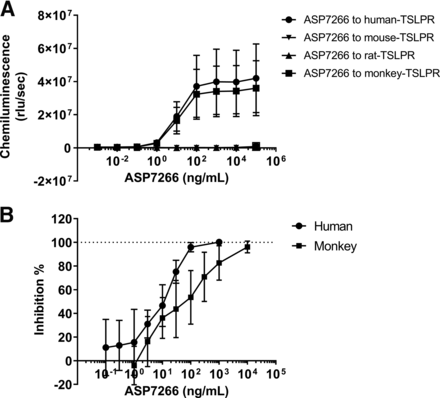
To confirm the effect of ASP7266 in other species, we compared the binding and neutralizing activity of ASP7266 against TSLPR from humans, mice, rats, and monkeys. ASP7266 bound to both human and monkey TSPLR. However, ASP7266 did not bind to rat or mouse TSLPR. Meanwhile, human IgG isotype control showed little binding to human, mouse, rat, or monkey TSLPR (Fig. 4A). These results indicate that ASP7266 shows comparable binding affinity to human and monkey TSLPR and much lower crossreactivity to mouse and rat TSLPR. We also evaluated the effects on TSLP-induced CCL17 production using monkey and human peripheral blood. ASP7266 inhibited TSLP-induced CCL17 mRNA expression. The inhibitory effect of ASP7266 was indicated by an IC50 value of 6.90 ng/ml and 55.7 ng/ml in human and monkey peripheral blood, respectively.
Cross-reactivity of ASP7266 with mouse, rat, and monkey TSLPR. (A) Binding of ASP7266 to TSLPR was determined using ELISA. Each data point represents the mean ± S.D. for four subjects. (B) Inhibitory effect of ASP7266 on CCL17 mRNA expression level in human and monkey peripheral white blood cells. Each data point represents the mean ± S.D. for six subjects.
Effect of ASP7266 on Ascaris-Induced Skin Allergic Reactions in Monkeys
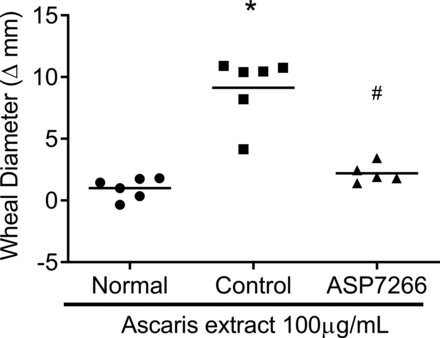
We investigated the effect of ASP7266 on ascaris-induced skin allergic reactions in monkeys because this monkey model allows for observation of antigen-specific type 2 allergic responses. The mean ± S.D. of wheal diameter in the normal, control, and ASP7266 group was 1.0 ± 0.9 mm, 9.1 ± 2.6 mm, and 2.2 ± 0.8 mm, respectively. Three doses of ASP7266 on days −1, 6, and 13 inhibited ascaris extract–induced skin allergic reactions (95% CI: −9.7 to −4.2; P = 0.0003; inhibition rate: 85%) (Fig. 5). We confirmed that the blood concentration of ASP7266 on day 14 was 1 µg/ml or higher in this model (Supplemental Fig. 1). These results demonstrate that ASP7266 inhibits type 2 allergic responses in vivo.
Inhibitory effect of ASP7266 on ascaris extract–induced skin reactions in a monkey model. Monkeys in the normal group (no sensitization) and control group (with sensitization) were administered the formulation buffer instead of ASP7266. The severity of the ascaris-specific skin reaction was determined by subtracting the mean diameter of the PBS-injected site from the mean diameter of the ascaris extract–injected site in each monkey. The horizontal line indicates the mean for six (normal and control group) or five monkeys (ASP7266-treated group). Differences between two groups were compared using an unpaired two-tailed t test. * P < 0.05 vs. normal group, # P < 0.05 vs. control group.
Discussion
TSLP plays a key role in various allergic diseases, including asthma, AD, and CRSwNP. Although an anti-TSLPR antibody is expected to be effective for the treatment of allergic diseases, no anti-TSLPR antibody has been administered to patients to date. Here, we generated ASP7266, an anti-TSLPR antibody, and evaluated its pharmacological activity in a receptor binding assay, a functional assay, and a monkey allergy model.
These studies allowed us to evaluate the biologic and pharmacological activity of ASP7266. ASP7266 bound to TSLPR and inhibited the TSLP/TSLPR interaction and TSLP-induced proliferation of Ba/F3 cells (Fig. 1, A and B). We also tested ASP7266 in mDC functional assays, as activation of mDCs is a critical step in the pathogenesis of type 2 inflammation. In these assays, ASP7266 inhibited TSLP-induced production of the Th2-attracting chemokine CCL17 by mDCs (Fig. 1C) and the production of type 2 cytokines, such as IL-4, IL-5, and IL-13 arising through mDC-mediated differentiation of naive CD4+ T cells (Fig. 2). Currently, available antibodies, including dupilumab (anti–IL-4R antibody), mepolizumab (anti–IL-5 antibody), benralizumab (anti–IL-5R antibody), and omalizumab (anti–IgE antibody) target type 2 cytokine pathways. These drugs are administered to patients with type 2 endotype based on biomarkers, such as eosinophil count, blood IgE level, or fractional exhaled nitric oxide (Castro et al., 2018). Although these drugs attenuate the risk of asthma exacerbation, there is a need to further improve efficacy, especially in patients with non–type 2 endotype (Hanania et al., 2013). With this in mind, TSLP/TSLPR is positioned at the top of the inflammatory cascade, wherein environmental insults, such as allergens, viruses, and pollutants cause epithelial cell injury and the subsequent production of TSLP (Soumelis et al., 2002). Inhibiting the TSLP/TSLPR cascade is therefore expected to suppress both type 2 and non–type 2 inflammation. In fact, tezepelumab has been shown to be effective against both type 2 and non–type 2 asthma in a phase 2 clinical study (Corren et al., 2017). To support this hypothesis, we have shown that ASP7266 suppresses production of TNF-α, a cytokine involved in non–type 2 inflammation (Soumelis et al., 2002; Berry et al., 2006) and exacerbation of allergic diseases, such as asthma (Berry et al., 2006, 2007; Tan et al., 2016) (Fig. 2). We thus expect that ASP7266 could be effective against both type 2 and non–type 2 allergic diseases.
One important finding in this study is that ASP7266 suppressed IL-5 production from lineage-negative cells among human PBMCs activated by combined stimulation with IL-25, IL-33, and TSLP (Fig. 3). Given that IL-5–producing lineage-negative cells in this condition are defined as ILC2 (Spits et al., 2013), our findings indicate that ASP7266 can effectively attenuate ILC2 activation. Furthermore, together with the previous observation that TSLP confers steroid resistance to ILC2 (Kabata et al., 2013; Liu et al., 2018), our results suggest that ASP7266 may be an attractive treatment option for steroid-resistant inflammatory diseases, such as severe asthma, severe AD, severe eosinophilic esophagitis, and refractory CRSwNP.
Our results in the monkey model further suggest that ASP7266 may be clinically effective for the treatment of allergic diseases. ASP7266 almost completely suppressed antigen-specific skin allergic reactions in a monkey ascaris-induced allergy model (Fig. 5). Antigen-specific skin allergic reaction is a typical method used to assess inflammatory responses and one used as a diagnostic tool for allergic diseases, such as allergic rhinitis and asthma (Bousquet et al., 2012). Omalizumab, an anti-IgE antibody that has demonstrated clinical efficacy, also suppresses skin allergic reactions (Varghese and Lieberman, 2007). Likewise, we expect that ASP7266 could be clinically effective against allergic diseases, such as asthma, AD, and CRSwNP.
In conclusion, ASP7266 is a novel antibody with a unique profile that can inhibit cytokine production from both CD4+ T cells and ILC2 and completely suppress skin allergic reactions in a monkey model, suggesting that it may be effective against multiple types of inflammation. Given that antibodies against receptors are generally more effective than those against ligands (Singh et al., 2016; Dávila González et al., 2019), ASP7266 is expected to display better efficacy than anti-TSLP antibodies, such as tezepelumab. Although the clinical efficacy requires further investigation, ASP7266 has the potential to become beneficial therapeutic option for patients with allergic diseases, even when compared with existing biologics.
Acknowledgments
The authors of this manuscript would like to thank Takashi Emori for critical reading of the manuscript and valuable comments and Taku Tanaka and Miki Iida for expert technical assistance. The authors also thank Hiromu Sato and Daisuke Yamajuku for contributing new reagents.
Authorship Contributions
Participated in research design: Numazaki, Abe, Hanaoka, Imamura, Maeda, Kimura, Miyanohara, Saito, Saita.
Conducted experiments: Numazaki, Abe, Hanaoka, Imamura, Maeda, Kimura, Miyanohara, Saito.
Contributed new reagents or analytic tools: Arai.
Performed data analysis: Numazaki, Abe, Hanaoka, Imamura, Maeda, Kimura, Miyanohara, Saito.
Wrote or contributed to the writing of the manuscript: Numazaki, Abe, Hanaoka, Imamura, Maeda, Kimura, Miyanohara, Saito, Arai, Suzuki, Saita.
Footnotes
- Received April 21, 2021.
- Accepted November 1, 2021.
All authors were employees of Astellas Pharma Inc. during the conduct of the study. M.N. and K.A. are listed as inventors on a patent application covering the use of anti-human thymic stromal lymphopoietin receptor antibody described in this manuscript (publication number: WO2015020193). This study was funded by Astellas Pharma Inc.
↵
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
Abbreviations
- AD
- atopic dermatitis
- BD
- Becton Dickinson and Company
- CCL
- C-C motif chemokine ligand
- CI
- confidence interval
- CRSwNP
- chronic rhinosinusitis with nasal polyposis
- IC90
- 90% inhibitory concentration
- IL
- interleukin
- ILC2
- Group 2 innate lymphoid cell
- mDC
- myeloid dendritic cell
- PBMC
- peripheral blood mononuclear cell
- TBS
- Tris-buffered saline
- Th2
- T helper type 2
- TNF-α
- tumor necrosis factor-α
- TSLP
- thymic stromal lymphopoietin
- TSLPR
- TSLP receptor
- Copyright © 2021 by The American Society for Pharmacology and Experimental Therapeutics