Abstract
The use of pharmacogenetic guidelines in personalizing treatments has shown the potential to reduce interindividual variability in drug response by enabling genotype-matched dosing and drug selection. However, other important factors, such as patient gender, may interact strongly with pharmacogenetics in determining the individual profile of toxicity and efficacy but are still rarely considered when planning pharmacological treatment. The literature indicates that males and females respond differently to drugs, with women being at higher risk for toxicity and having different plasma exposure to drugs at standard doses. Recent studies have shown that pharmacogenetic variants may have different predictive value in different sexes, as in the case of treatment with opioids, angiotensin-converting enzyme inhibitors, or proton pump inhibitors. Of particular interest is the case of treatment with fluoropyrimidines for cancer. A significant increase in toxicity has been described in female patients, with a more pronounced effect of specific DPYD and TYMS polymorphisms also noted. This manuscript reviews the major findings in the field of sex-specific pharmacogenomics.
SIGNIFICANCE STATEMENT Interindividual variability in drug response is an emerging issue in pharmacology. The genetic profile of patients, as well as their gender, may play a role in the identification of patients more exposed to the risk of adverse drug reactions or poor efficacy. This article reviews the current state of research on the interaction between gender and pharmacogenetics in addressing interindividual variability.
Introduction
It is well known that there are significant interindividual differences in drug response and toxicity in patients treated with the same therapy. The safety and efficacy of drugs depend on many factors that influence their utilization in the body (Lichtman et al., 2003; Wilkinson, 2005; Scripture and Figg, 2006). Drug-related interindividual variability factors include dosage, dosage form, and therapeutic regimen, which are responsible for drug–drug interactions. Patient-specific factors include age, gender, diet, natural physiologic cycles, pregnancy, acute illness, liver and kidney dysfunction, and other chronic diseases (Huang and Temple, 2008). One of the principal causes of variability in drug effects is the patient’s own metabolism. Approximately one-third of variability among individuals in drug response is correlated with genetic single nucleotide polymorphisms (SNPs) in genes encoding phase 1 and phase 2 enzymes.
In the lengthy and costly development of drugs, patients’ gender and genetics have been inadequately considered, resulting in biased dosing strategies for most of the available drugs. The lack of consideration of the genetic makeup of participating patients, which may lead to enrolment of patients in the clinical trial who are carriers of high-impact mutations, is emerging as a critical problem. In addition, analysis of clinical trial quality by several authors has shown that women are significantly underrepresented in pivotal phase 1, 2, and 3 clinical trials. Traditionally, studies have primarily included adult White, Caucasian males, particularly in phase I clinical trials (Fisher and Kalbaugh, 2011). The inclusion of women is central to all phases of drug development to rationalize the costs of the research and to improve drug safety and efficacy based on a personalized approach (Woodruff, 2014). Optimization of drugs in early phases of preclinical development, performed specifically on cells or mice of one sex, can lead to sex bias that is perpetuated in later phases of drug development. To get an idea of how topical this issue is, one might note, for example, that for research on SARS-CoV-2 (COVID-19), at the moment, only one of the four human cell lines used is female (Takayama, 2020).
In this context, personalized medicine or precision medicine is a model of healthcare that tailors pharmacological treatments and, more generally, medical interventions, to each individual based on the risk factors for treatment failure or for the disease (Manson et al., 2017; Mathur and Sutton, 2017).
Pharmacogenetics and Its Implementation in the Clinical Practice
Personalized medicine has gained importance in recent years in light of increasing diagnostic and informatics approaches, particularly in the genetic field. In particular, the use of genetic information has played such a critical role in personalized medicine that the term pharmacogenomics was first used in the context of genetics and then evolved into a new branch of pharmacology (Burke et al., 2014).
Pharmacogenomics and pharmacogenetics (PGx) aim to personalize therapy based on the genetic predisposition of patients (Katara and Yadav, 2019; Pirmohamed, 2001). According to many authors, adjusting dosages and medications to take genetic variants into account is the first true clinical application of genetics in the post-genomic era (Swen et al., 2007).
Indeed, the presence of a variant in a gene encoding metabolic enzymes may increase or decrease its enzymatic activity, with significant implications for efficacy and/or the development of toxicity. Genetic variants could be related to 1) lower or higher drug exposure, 2) increased toxic metabolite concentration, 3) altered interaction with the target drug, and 4) idiosyncratic drug toxicity due to immune activation (Pinto and Dolan, 2011). An estimated 90% of the population carries at least one variant in a gene related to drug metabolism or mechanism of action, or a variant indicating a higher risk for hypersensitivity reactions (Uetrecht, 2007; Van Driest et al., 2014). The protein phenotype predicted based on the genetic profile of the encoding gene could be classified into the following groups: “Ultrarapid Metabolizers” with increased enzyme activity; “Normal Metabolizers” with a normal and weak enzyme activity; “Intermediate Metabolizers” with an intermediate enzyme functionality; and “Poor Metabolizers,” when the enzyme is completely dysfunctional (Caudle et al., 2017).
It was reported that 7% of the 1200 drugs approved by the Food and Drug Administration (FDA) are linked to a clinically effective variant, and these drugs account for 18% of approximately 4 billion prescriptions in the United States (Budnitz et al., 2006; Relling and Evans, 2015; Caraballo et al., 2017). Consequently, the inability to obtain appropriate therapy, even when PGx information is used, means the so-called “therapeutic odyssey” for the patient. This condition is characterized by the constant search for the most effective therapy, ineffective treatments, frequent visits to the doctor, alternative treatment regimens based on the use of dietary supplements, phytotherapeutics, or polytherapeutic agents, the deterioration of the patient’s condition, and the failure of treatment. PGx testing before treatment could curb this phenomenon and many of the above cases could be identified a priori to avoid side effects or therapeutic inefficacy (Lazaridis, 2017).
Currently, the slow clinical adoption of PGx is primarily due to barriers that delay its introduction into clinical practice. The lack of standardized PGx guidelines and their difficult interpretation by clinicians who have been resistant to PGx information are just some of these obstacles (Swen et al., 2007; Dunnenberger et al., 2015). Currently, authoritative consortia worldwide have developed guidelines that can help clinicians translate available genetic test results into clinical decisions. The Royal Dutch Association for the Advancement of Pharmacy-Pharmacogenetics Working Group, Clinical Pharmacogenetics Implementation Consortium, the Canadian Pharmacogenomics Network for Drug Safety, and additional scientific consortia are working to facilitate the incorporation of PGx testing into clinical practice and to translate genetic information into prescribing recommendations (Clinical Pharmacogenetics Implementation Consortium, https://cpicpgx.org) (Swen et al., 2008, 2018; Caudle et al., 2014; Abdullah-Koolmees et al., 2021). The PharmGKB website, a National Institutes of Health-funded resource, collects international guidelines and provides information on how genetic variations affect drug response, making this knowledge accessible to clinicians and researchers. To date, 249 clinical guidelines by either Clinical Pharmacogenetics Implementation Consortium, the Royal Dutch Association for the Advancement of Pharmacy-Pharmacogenetics Working Group, or other consortia are available for 147 drugs (https://www.pharmgkb.org).
Since the early 2000s, PGx clinical programs with implementation models have been reported in various contexts of healthcare, using both preventive testing or at the point-of-care (Luczak et al., 2021). In this context, the European Union-funded Ubiquitous Pharmacogenomics (U-Pgx) study tested the impact of implementing PGx guidelines on the safety of a list of 43 commonly prescribed drugs at seven clinical sites in Europe as part of a randomized and prospective clinical trial (PREemptive Pharmacogenomic testing for prevention of Adverse drug Reactions [PREPARE]) (Cecchin et al., 2017; Swen et al., 2023).
Sex Medicine in the Context of Personalized Medicine
Among all drug classes, women are almost 2-fold more likely than men to experience adverse drug reactions (ADRs) and at increased risk of hospitalization for an ADR (Zucker and Prendergast, 2020; Madla et al., 2021; Zucker et al., 2022). Many drugs administered to both sexes at the same dose should be re-evaluated for gender-specific dose adjustment, such as the representative case of the sedative-hypnotic zolpidem, for which gender-specific dose adjustment was required after decades in which post marketing reports highlighted that women were reporting cognitive deficits when receiving the standard dose of drug for a male (Zucker et al., 2022). In the United States, between 1997 and 2000, among eight of 10 drugs were withdrawn from the market because of very severe side effects encountered mostly in women (Schiebinger et al., 2016). Sex medicine is an innovative approach to precision medicine that aims to improve care and treatment of both men and women, overcoming milligram/kilogram-based or “one size fits all” drug administration, which often leads to treatment errors (Baggio et al., 2013; Wagner et al., 2019). Sex differences affecting virtually all body areas correlate with protection from or susceptibility to cancer, cancer progression, and response to therapy (Dong et al., 2020; Zucker and Prendergast, 2020).
Patient sex could influence both pharmacokinetic (PK) and pharmacodynamic drug pathways, leading to sex differences in ADRs (Soldin and Mattison, 2009; Soldin et al., 2011; Dong et al., 2020). For most of the FDA-approved drugs studied (88%) for which sex-specific data were available PK, increased blood concentrations and longer elimination times were found in women in more than 10 therapeutic categories, and these PKs were strongly associated with sex differences in ADRs. The FDA reviewed 300 new drug applications between 1995 and 2000, and only 54% included an analysis of gender. In addition, 8% of these drugs presented a difference of approximately 40% in pharmacokinetics when comparing males and females, yet gender was not accounted for in dosing recommendations (Anderson, 2005). The lack of published sex-specific data regarding PK for many different drugs raises concerns that other drugs could present PK differences according to sex but data are not made publicly available (Zucker and Prendergast, 2020; Zucker et al., 2022).
Women are more prone to overdose due to lower volume of distribution, higher body fat percentage (13.5–16.5 kg in women), larger free fraction of the drug, and slower xenobiotic clearance. Body composition parameters that could affect drug distribution include body fluids composition and distribution that volume is greater in males than females, resulting in different responses to the drug. Plasma protein binding has also been shown to vary between the two sexes due to the influence of estrogens, which increase the concentration of serum binding globulins. The use of oral contraceptives, pregnancy, or menopause status are cited as sex-specific conditions that affect the PK and pharmacodynamics of various drugs. Another important difference between the two sexes is related to the composition of the gut microbiota (Mueller et al., 2006; Wen et al., 2008). In addition, lifestyle factors such as use of tobacco or excessive alcohol consumption, diet, and physical inactivity, which are known to have a direct impact on drug response, differ greatly between men and women, as reported in several studies. In addition, women tend to take a greater number of medications than men, leading to increased drug–drug interactions.
Sex hormones have secondary effects that up- or down-regulate genes contributing to exacerbate gender differences in treatment outcomes (Pinsonneault and Sadée, 2003). Transcriptional processes promoting the expression of absorption, distribution, metabolism, and excretion genes may be activated by estrogens by binding specific nuclear receptors causing a significant difference in the activity of phase I and phase II metabolic enzymes by sex. The phase I enzymes CYP3A4 and CYP2D6 are more active in females, whereas CYP1A family enzymes have higher metabolic activity in males. These differences result in sex-specific changes in exposure to various drugs, including clomipramine, clozapine, olanzapine, acetaminophen, codeine, diazepam, fentanyl, statins, and tamoxifen. Phase 2 enzymes such as UDP-glucuronosyltransferases and methyltransferases have been found to be more active in males than females and directly affect the clearance of ibuprofen, acetaminophen, azathioprine, dopamine, oxazepam, and levodopa (Yang et al., 2012; Franconi and Campesi, 2014).
The Role of Sex Medicine in the Pharmacogenomics Era
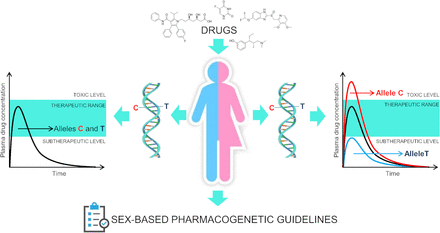
In the context of the clearly different ability of men and women to metabolize drugs, the role of PGx may become crucial. Indeed, a metabolic background already altered by sex-specific factors could enhance the effect of specific genetic polymorphisms or otherwise render them negligible (Fig. 1). Many genetic polymorphisms have been shown to exhibit a sex-specific effect (Myburgh et al., 2012). A recent genome-wide association study focused on identifying predictive markers associated with psychiatric disorders identified genetic variants that are differentially predictive in men and women. Variants in genes related to neuronal development, the immune system, and vascular function have been associated with sex-dependent effects on the onset of schizophrenia, major depressive disorder, and bipolar disorder (Blokland et al., 2022). However, there are no precise studies on the gender effects of PGx on patient treatment, as in other areas of pharmacological research.
The different background of males and females in terms of drug pharmacokinetics and pharmacodynamics interacts with the genetics of specific enzyme players, such as cytochromes and intracellular transporters, causing sex-specific pharmacogenomic associations. There is a need to develop sex-based pharmacogenomics guidelines to address this topic.
The most striking difference between males and females is sex chromosomes. Indeed, an impaired drug response was previously related to large genetic aberrations in Y chromosome, inactivation of X chromosomes, and other genetic and epigenetic alterations (Carè et al., 2018). Immune function is regulated by genes located in the X chromosome (e.g., TLR, cytokine receptors, transcription factors); therefore immune genes can be up-regulated in females due to X chromosome inactivation, which may explain the gender discrepancy in immunotherapy. Specifically, IL-2, TLR-7, TLR-8, CD-40LG, and FoxP3 are X genes related to the immune response that can lead to higher resistance to immunotherapy in females due to X escape (Irelli et al., 2020). Differential response to infection between males and females is one of the aspects related to sex differences in the immune system activation. Response to hepatitis C virus therapy was related to four genetic polymorphisms near the IL28B gene in a sex-dependent manner. Women had a better response to treatment and hepatitis C virus clearance than men (Rao et al., 2012).
Exposure to many drugs was affected by patients sex and related enzymatic activities in several cases such as irinotecan, ibuprofen, acetaminophen, dopamine, oxazepam, and azathioprine metabolized by uridine diphosphate glucuronosyl transferases; clomipramine, acetaminophen, and clozapine metabolized by CYP1A; diazepam, lovastatin, simvastatin, and fentanyl metabolized by CYP3A; and fluoxetine, codeine, tamoxifen, encainide, and flecainide metabolized by CYP2D6 (Soldin and Mattison, 2009).
The volume of distribution of drugs could be sex-dependently affected by polymorphisms in the ATP-binding cassette genes as well as their glomerular filtration (Anderson, 2008).
Many genetic polymorphisms have been associated with sex differences in drug response (Table 1). An interaction between sex and genetics of patients has been highlighted for MC1R gene in analgesic therapy. When pentazocine was administered to fair-skinned, red-haired women in comparison with red-haired men and women who did not have the variant alleles, better analgesia was associated with the presence of two variant alleles of the MC1R in their genome (Mogil et al., 2003). In a study conducted in 582 opioid-dependent patients randomized to receive methadone or buprenorphine/naloxone, no overall effect of genetic polymorphisms in OPRD1 was observed, but a specific sex-specific effect was noted for two intronic SNPs (rs581111 and rs529520) that were related to the outcome of the treatment only in women treated with buprenorphine (Clarke et al., 2014). In addition, a genotype analysis of patients treated with opioids, antipsychotics, and antiepileptic drugs for noncancer chronic pain showed that the nature of ADRs differed between males and females according to the patients genotype: OPRM1G allele and COMT-GG genotype were associated with specific side effects (in men, vomiting and depression, whereas in women, dizziness and dry skin). The sexual dysfunction incidence was the same in both sexes (Planelles et al., 2020). A recent observational study compared the use of tapentadol (n = 194) or oxycodone/naloxone (n = 175) with the prescription of other opioids for the treatment of chronic noncancer pain in 585 real-world patients. In addition to clinical endpoints of treatment outcomes, some genetic variants of the OPRM1 (rs1799971, A118G) and COMT (rs4680, G472A) genes were analyzed. In patients treated with oxycodone/naloxone, patients with the homozygous genotype COMT-472AA had a higher rate of erythema and vomiting, especially in women, again indicating a gender effect (Barrachina et al., 2022). Transcriptomics signatures were analyzed in male and female bipolar patients. Although the study did not focus on germline genetic polymorphisms, the authors showed that two genes (RBPMS2 and LILRA5) were selectively expressed in men that responded to lithium treatment whereas three genes (ABRACL, FHL3, and NBPF14) were expressed in female lithium responders (Eugene et al., 2018). About the treatment of cardiovascular disease, polymorphisms in angiotensin-converting enzyme (ACE) gene were associated with a sex-specific differential outcome after treatment with ACE inhibitors. They are more effective in women with genotype D/D compared with men with genotype D/D and more effective in men with genotype D/D than in men with genotype I/D or I/I (Ruggenenti et al., 2000). Hydrochlorothiazide response was also affected by ACE polymorphism in a sex-specific manner, and I/I women responded better than D/D men (Schwartz et al., 2002). Male patients carrying an allelic variant of ABCC2-24C > T and treated with atorvastatin for hypercholesterolemia had a significant lower response than in women carrying the same genetic variant (Prado et al., 2018).
List of studies addressing the interaction between germline genetic polymorphisms and patient sex in defining the risk of toxicity or inefficacy
More recently, CYP2C19, involved in the clearance of proton pump inhibitors (such as pantoprazole, rabeprazole, omeprazole, lansoprazole, and esomeprazole) was studied in relation to the prevalence of migraine in the large UK Biobank cohort. A higher migraine prevalence at baseline (odds ratio [OR] = 1.25, P < 0.0001) was associated to the CYP2C19 metabolizer phenotype in patients treated with proton pump inhibitors. In particular, a poor CYP2C19 metabolizer status was associated with chronic migraine-only in men, suggesting a potentially relevant role of the interaction between gender and CYP2C19 phenotype (Pisanu et al., 2021).
The Effect of Pharmacogenetics–Sex Interaction on Cancer Treatment with Fluoropyrimidines
Fluoropyrimidines (FPs), including 5-fluorouracil (5-FU) and capecitabine, are widely used in the treatment of tumors and represent an important example of gender disparity in treatment outcomes (Stein et al., 1995; Sloan et al., 2000; Mueller et al., 2013). Clearance of FPs is lower in women than in men (Milano et al., 1992), resulting in higher toxicity in women and compromising therapeutic efficacy (Mader, 2007; Wang and Huang, 2007). Zalcberg et al. (1998) reported as early as 1998 that hematologic and nonhematologic toxicities were influenced by gender after administration of 5-FU and leucovorin in patients with advanced colorectal cancer. Severe toxicities such as stomatitis, leukopenia, alopecia, and diarrhea occurred more frequently in women compared with men during 5-FU–based chemotherapy. These data were recently confirmed by Wagner et al. (2021) in an analysis of women with colon cancer who received adjuvant FP-based chemotherapy. The mechanism underlying the gender-dependent PK of 5-FU when administered as an infusion, with lower drug clearance in women, is still unclear, but the authors postulated a role in dihydropyrimidine dehydrogenase (DPD) activity (Milano et al., 1992). DPD activity has been shown to be decreased by 15% in women (Etienne et al., 1994). This may pave the way to investigate the role of gender in predicting DPYD variants in FP-related toxicity, as the proportion of toxicity cases explained by DPYD variants may be different in men and women (Amstutz et al., 2011). Lower DPD activity associated with toxicity in females could be explained by DPD enzymatic deficiency (Milano et al., 1999). Four DPYD variants (DPYD*2A, DPYD*13, DPYD_c.2846C > T, and DPYD-HapB3) have been shown to functionally affect DPD functionality and increase the risk of severe toxicity associated with FPs administration. All international guidelines now agree that pretreatment DPYD genotyping is recommended (Amstutz et al., 2018; Lunenburg et al., 2020), and recently the European Medicines Agency also published a document recommending DPD screening to increase treatment safety (https://www.ema.europa.eu/en/news/ema-recommendations-dpd-testing-prior-treatment-fluorouracil-capecitabine-tegafur-flucytosine). According to current guidelines, patients who are carriers of an allelic variant within the panel should receive a halved initial dose of FPs. If there is a remote possibility that a patient is a carrier of more than one variant allele, patients should be phenotyped (i.e., DPD activity should be determined); otherwise, treatment with FPs should be avoided.
A potential interaction between DPYD polymorphisms and patient’s sex has been investigated (Table 2). In a study by Schwab et al. (2008), it was shown that the effect of the DPYD*2A variant on toxicity associated with 5-FU can be considered gender-specific. Although a significant association with this variant was observed in male patients, no effect was seen in female patients. Similarly, Lee et al. (2014) observed a stronger effect of DPYD*2A in males compared with females, suggesting an interaction between gender and DPYD polymorphisms in the context of treatment with FPs.
List of studies addressing the interaction between germline genetic polymorphisms and patient sex in defining the risk of fluoropyrimidines-related toxicity
Other genes and the sex-specific effect of their polymorphisms have been investigated for FPs-based treatments (Table 2). In a multicenter, randomized, noninferiority phase III study conducted in patients with high-risk stage II/III colon cancer treated with 6 versus 3 months of adjuvant chemotherapy with FOLFOX-4 or XELOX (TOSCA), Ruzzo et al. (2019) analyzed how the interaction between 17 genetic polymorphisms and patient gender might affect treatment-related toxicity. An interaction of rs1801133 (MTHFR) and rs1799793 (ERCC2) on time to onset of grade 3 hematologic toxicity, of rs13181 (ERCC2) on time to onset of grade 3 gastrointestinal toxicity, and of rs11615 (ERCC1) on time to onset of grade 3 neurologic toxicity was found. The rs1799793 genotype GA (P = 0.006) and the A allele (P = 0.009) specifically decreased the time to onset of grade 3 hematologic toxicities in males, whereas the rs11615 genotype CC worsened the time to onset of grade 3 neurologic toxicities and the rs13181 G allele improved the time to onset of grade 3 gastrointestinal toxicities in females (Ruzzo et al., 2019). In this case, the effect of genes in DNA repair pathways may reflect the effect of oxaliplatin in the combination treatment that patients received.
On the other hand, the sex-specific effect on FPs toxicity observed for the MTHFR polymorphism was recently confirmed by Ioannou et al. (2022) who genotyped 313 cancer patients treated with FPs for the same MTHFR polymorphism rs1801133. They found a specific effect of the polymorphism in female patients, in whom the MTHFR c.665 CT and TT genotypes were associated with both the need for dose reduction due to toxicity and the percentage of dose reduction. Such differences were not present in male patients, again confirming that genetic polymorphisms have a stronger influence on toxicity risk in females instead of males (Ioannou et al., 2022).
Although the role of the TYMS polymorphism in predicting severe adverse effects in patients treated with FPs is still debated, a higher rate of ADRs in patients with TYMS-TSER 3R/2R genotype and treated with 5-FU/capecitabine was observed in female cancer patients compared with males, possibly related to ER regulation of TS expression (Ioannou et al., 2021). TYMS genotype 2R/2R was found to have a higher prevalence in African American female patients with adverse effects compared with male patients (Khushman et al., 2021).
Conclusion and Future Perspectives
Pgx is becoming increasingly important in clinics, and several consortia have made specific recommendations for genotype-based prescribing. In this context, the impact of patient sex may be important, because it is reported to affect the clinical outcome of pharmacological treatment, in terms of both toxicity and efficacy. This is often mediated by a different absorption, distribution, metabolism, and excretion of drugs in men and women—a 40% difference between the two sexes (PK)—and by a different reactive immune system. However, sex-specific PGx recommendations that take into account both aspects have not yet been formulated. Evidence shows that women have a doubled risk to develop an ADR compared with men with higher hospitalization rates (Tharpe, 2011). There is growing evidence that the effect of genetic polymorphisms may differ in different gender contexts. There are limited and anecdotal data on the effects of gender on PGx-directed drug therapy derived from clinical investigations. These limitations could be overcome, ensuring adequate representation of both genders in clinical trials. Regulatory agencies regularly conduct analyzes to verify gender balance in recruitment, but in general the primary and secondary endpoints do not consider separate gender analyzes, particularly when addressing gender differences in PGx.
In recent years, the impact of germline genetic variants on therapeutic outcomes has been increasingly studied and is beginning to be used in clinical practice to improve drug efficacy and safety. Although there is evidence of sex differences in the efficacy and toxicity of many drugs, including chemotherapeutics, sex medicine still remains a mirage. Personalized and precision medicine should in the future assess how PGx variants might have a different role in men and women.
Authorship Contributions
Participated in research design: Cecchin, Toffoli.
Performed data analysis: Posocco, Mezzalira.
Wrote or contributed to the writing of the manuscript: Cecchin, Posocco, Appetecchia, Toffoli.
Footnotes
- Received August 14, 2022.
- Accepted March 21, 2023.
This work was supported by the Italian Drug Agency [Grant 1729 dd] (to G.T.).
No author has an actual or perceived conflict of interest with the contents of this article.
Abbreviations
- 5-FU
- 5-fluorouracil
- ABC
- ATP-binding cassette
- ABRACL
- actin-binding ρ-activating C-terminal–like
- ACE
- angiotensin-converting enzyme
- ADR
- adverse drug reaction
- CD-40LG
- cluster of differentiation 40 ligand
- COMT
- catechol-O-methyltransferase
- CYP3A4
- CYP2D6, CYP1A, CYP2C19, cytochrome P450 3A4, 2D6, 1A, 2C19
- DPYD
- dihydropyrimidine dehydrogenase (gene)
- DPD
- dihydropyrimidine dehydrogenase (protein)
- FoxP3
- Forkhead box P3
- ERCC1
- excision repair cross-complementing isoform 1
- ERCC2
- excision repair cross-complementing isoform 2
- FDA
- Food and Drug Administration
- FHL3
- four and a half LIM domains 3
- FPs
- fluoropyrimidines
- IL-2
- interleukin 2
- IL28B
- interleukin 28B
- LILRA5
- leukocyte immunoglobulin like receptor A5
- MC1R
- melanocortin 1 receptor
- MTHFR
- methylenetetrahydrofolate reductase
- NBPF14
- neuroblastoma breakpoint family member 14
- OPRD1
- opioid receptor Δ1
- OPRM1
- opioid receptor μ1
- PGx
- pharmacogenetics
- PK
- pharmacokinetics
- RBPMS2
- RNA binding protein mRNA processing factor 2
- SNP
- single nucleotide polymorphism
- TLR-7
- Toll-like receptor 7
- TLR-8
- Toll-like receptor 8
- TYMS
- thymidylate synthetase
- Copyright © 2023 by The American Society for Pharmacology and Experimental Therapeutics