Abstract
Glucagon-like peptide 2 (GLP-2) is a pleiotropic intestinotrophic hormone that we hypothesized could lessen gastrointestinal inflammation associated with postoperative ileus (POI). To test this idea, the prophylactic timing and dose of a long-acting variant of human GLP-2 linked to the Fc portion of murine immunoglobulin G (IgG) (GLP-2/IgG) was optimized in a murine model of POI. Surgically treated mice received a single dose of GLP-2/IgG, IgG isotype control, or phosphate-buffered saline 1 to 48 h before small bowel surgical manipulation. The distribution of orally fed fluorescein isothiocyanate-dextran and histological analyses of myeloperoxidase-positive immune cells were determined 24 and 48 h postoperatively. TaqMan quantitative polymerase chain reaction was used to determine early changes in mRNA expression in the muscularis or mucosa. In normal mice, prolonged exposure to GLP-2 increased upper gastrointestinal (GI) transit and mucosal weight. When administered 1 or 3 h before surgery, GLP-2/IgG reduced the leukocyte infiltrate 24 and 48 h postoperatively and improved GI transit 48 h postoperatively. Surgical manipulation rapidly increased gene expression of proinflammatory cytokines and enzymes for kinetically active mediators in the mucosa and muscularis. GLP-2/IgG2a affected the expression of genes associated with mucosal inflammation and barrier function. We conclude that prophylactic treatment with a long-acting GLP-2 agonist ameliorates inflammation and improves intestinal dysmotility associated with surgical manipulation of the bowel. The action of GLP-2 is consistent with a lessening of inflammation, leading to a more rapid recovery.
Glucagon-like peptide 2 (GLP-2) is produced by a subset of enteroendocrine cells residing within the epithelium of the gastrointestinal (GI) tract (Yusta et al., 2000). It functions as a pleiotropic intestinotrophic hormone with wide-ranging effects that promote mucosal growth and intestinal homeostasis. The integrative GI responses to GLP-2 are mediated via the GLP-2 receptor, a member of the glucagon/secretin G protein-coupled receptor superfamily that is located on enteric (Bjerknes and Cheng, 2001) and vagal (Nelson et al., 2007) nerves, subepithelial myofibroblasts (Orskov et al., 2005), and a subset of intestinal epithelial cells (Thulesen et al., 2000). Activation of GLP-2 receptors regulates epithelial cell growth (Bjerknes and Cheng, 2001), reduces intestinal permeability (Estall and Drucker, 2006), increases mesenteric blood flow (Guan et al., 2006; Stephens et al., 2006), and promotes nutrient absorption (Nelson et al., 2007).
Both cytoprotective and neuroprotective properties have been attributed to GLP-2 (Wallace et al., 2008), suggesting that it may have an important role in intestinal adaptation and repair during inflammatory events. Native GLP-2 given twice daily lessened the enhanced permeability and expression of proinflammatory cytokines associated with trinitrobenzene sulphonic acid colitis (Sigalet et al., 2007). These data are supported by observations in clinical trials, leading to the conclusion that GLP-2 has protective effects in inflammatory bowel disease (Wallis et al., 2007). The importance of GLP-2 in intestinal adaptation is evidenced by Teduglutide (GATTEX, NPS Allelix; NPS Pharmaceuticals Inc., Bedminster, NJ), a GLP-2 analog developed for treatment of short bowel syndrome (Mardini and de Villiers, 2008), a disorder characterized by impaired intestinal barrier function and poor nutrient absorption. In addition, studies in rodent models of intestinal injury have shown that Teduglutide ameliorates mucosal damage, crypt cell epithelial apoptosis, intestinal lesions, and inflammation (Drucker et al., 1999; L'Heureux and Brubaker, 2003; Arthur et al., 2004). Thus the GLP-2 receptor is an attractive target for the development of pharmaceutical interventions to promote intestinal adaptation and repair.
Postoperative ileus (POI) continues to be a significant clinical problem after open abdominal surgery, extending the length of hospital stay and contributing to postoperative morbidity and complications. Disturbance to the bowel during abdominal surgery elicits multifactorial responses within the bowel wall that inhibits intestinal smooth muscle contractility. The mechanisms controlling the initiation and maintenance of POI involve a complex array of immune cell interactions, central inhibitory reflexes, and neuroimmune interactions (Boeckxstaens and de Jonge, 2009). The cited review describes a number of potential targeted approaches for the pharmacologic management of ileus, including prokinetics, μ-opioid receptor antagonists, inhibitors of early signaling inflammatory events, inhibitors of prostaglandin and nitric-oxide synthesis, and the early and enhanced induction of anti-inflammatory mediators. Although these targeted approaches to the pathways involved in ileus show efficacy, it is attractive to explore whether the integrated intestinal effects of GLP-2 in promoting intestinal homeostasis would reduce the severity of POI.
Native GLP-2 has a short biological half-life (approximately 7 min systemic) because of its rapid proteolytic degradation by dipeptidyl peptidase IV (Hartmann et al., 2000). Dosing in preclinical models requires multiple daily injections to maintain adequate systemic exposure. Modification of the 33-amino acid sequence of the natural peptide has produced a few synthetic agonist analogs of GLP-2 with enhanced resistance to dipeptidyl peptidase IV cleavage. For example, Teduglutide is a synthetic recombinant human GLP-2 variant with an alanine to glutamine substitution (A2G GLP-2) at the second amino acid residue from the N terminus of the native peptide, a modification that leads to a modest increase in half-life to approximately 25 min. In this study, we used the same peptide variant linked to the Fc portion of human IgG4 (GLP-2/IgG4) or mouse IgG2a (chimeric GLP-2/IgG2a). As described previously for a GLP-1 construct (Picha et al., 2008), this linkage increased half-life dramatically from minutes to days in rodents. During early evaluation of the construct for its capacity to promote mucosal growth, we observed to our surprise that the A2G GLP-2 peptide variant enhanced upper gastrointestinal transit. Based on the known positive effects of GLP-2 in models of intestinal inflammation and the unexpected finding of a prokinetic effect, we hypothesized that GLP-2 receptor agonism would reduce intestinal inflammation and dysmotility associated with POI.
Materials and Methods
Age-matched male or female CD-1 mice were used in our studies. The experiment protocols in animals were reviewed and approved by Centocor and Johnson and Johnson Pharmaceutical Research and Development Institutional Animal Care and Use Committees. Mice were maintained on a 12-h light/dark cycle with access to rodent chow and water ad libitum.
Potency and Serum Half-Life.
The activities of A2G GLP-2, GLP-2/IgG4, and GLP-2/IgG2a peptides were evaluated in vitro at eight concentrations (0.03–3000 nM) by using LANCE cAMP assay (PerkinElmer Life and Analytical Sciences, Waltham, MA) in human embryonic kidney 293 cells stably transfected with a mutated form of human GLP-2 receptor. Serum levels of chimeric GLP-2/IgG2a in mice were detected based on the presence of allotype alleles that are distinct antigenic determinants on homologous proteins specified by allelic forms of immunoglobulin genes. Serum was collected from untreated control CD-1 mice given chimeric GLP-2/IgG2a (4 mg/kg i.v.) and surgically treated mice 48 h after intestinal manipulation, where chimeric GLP-2/IgG2a (4 mg/kg) was given 1 h before surgery. Cell culture plates (US Biological, Swampscott, MA) were coated with 2 μg/ml mouse anti-mouse IgG2aa allotype-specific (IgH-1a; clone 8.3) antibody (50 μl/well; BD Biosciences, San Jose, CA) diluted to 2 μg/ml in 50 μl of phosphate-buffered saline (PBS). Plates were then treated for 1 h with 200 μl/well blocking solution [PBS, 0.05% Tween, 1% bovine serum albumin (Sigma-Aldrich, St Louis, MO)]. After washing, purified human GLP-2 peptide conjugated to murine IgG2aa, murine IgG2aa alone, or sera from treated mice was diluted in blocking solution. Detection was by 1 μg/ml of an in-house-generated mouse anti-N-terminal human GLP-2 peptide. The signal was amplified with streptavidin-horseradish peroxidase diluted 1:8000 in blocking solution. Optical densities of tetramethylbenzidine substrate reaction product were determined (SPECTRAmax Plus 384; Molecular Devices, Sunnyvale, CA) with SoftMax Pro software (Molecular Devices). Plates were read at 450 nm (excitation) minus 650 nm (emission), and GLP-2/IgG2a was quantified from standard curves generated from known quantities of the construct. We confirmed that this assay detected intact chimeric GLP-2/IgG2a but not murine IgG2aa alone or the human construct GLP-2/IgG4 (all added at 20 μg/ml and serially diluted 1:4 down to 0.02 ng/ml in the blocking buffer).
Intestinotrophic Effects.
The intestinotrophic effects in vivo of A2G GLP-2 peptide and GLP-2/IgG4 construct were determined in female CD-1 mice. Mice were randomly assigned (n = 7 per group) to receive daily subcutaneous injections of A2G GLP-2 peptide (50 μg in a volume of 500 μl), GLP-2/IgG4 (1.6, 16, and 160 μg in a volume of 500 μl), or equivalent volume of PBS control for 10 days. In a separate study, GLP-2/IgG4 (4 mg/kg) was administered as a single dose on day 1 only, daily for 10 days, once every 2 days, or on days 1 and 7. On day 11, animals were euthanized by CO2 inhalation, and a segment of small bowel was harvested from approximately 6 cm distal to the ligament of Treitz to approximately 4 cm proximal to the cecum. The length of the unstretched bowel was measured, after which the bowel was opened longitudinally and rinsed with ice-cold PBS. The mucosa was removed by scraping with a glass slide, and mucosal scrapings were collected and weighed.
Postoperative Ileus Model.
Age-matched male CD-1 mice were anesthetized with inhaled isoflurane, and the abdomen was opened by midline laparotomy. The small intestine was eventrated and then gently compressed along its entire length by using moistened sterile cotton applicators. This procedure was designed to simulate running of the bowel, which is performed commonly during abdominal surgery in the clinical setting. The bowel was repositioned in the abdominal cavity, and the incision was closed with two layers of continuous sutures. The duration of the procedure was approximately 15 min, and the animals moved freely about their cage within 20 min of anesthetic withdrawal.
Upper Gastrointestinal Transit.
In an initial study, upper GI transit was determined 30 min after oral gavage of a test meal consisting of carmine red 6% (w/v) in 0.5% methylcellulose, Methocel (Dow Chemical, Midland, MI) and 99.5% deionized water. After CO2 euthanasia and laparotomy, the small bowel was removed intact from the pyloric sphincter to the ileocecal junction. The distance traveled by the marker was measured and reported as the percentage of the total length of the unstretched bowel.
In all subsequent POI studies, upper GI transit was determined by calculating the geometric center (GC) from the weighted average distribution of a nonabsorbable fluorescent marker along the entire GI tract. Fluorescein isothiocyanate (FITC) conjugated to dextran (70,000 molecular weight; FITC-dextran) was dissolved at a concentration of 5 mg/ml in 0.5% Methocel)/99.5% (w/v) deionized water. FITC-dextran was administered orally by gavage (volume = 0.1 ml/10 g body weight, to a maximum of 0.250 ml). Animals were returned to their home cages for 45 min and then euthanized by inhaled isoflurane with exsanguination. The entire GI tract from the lower esophageal sphincter to the terminal colon was harvested intact and divided into stomach, small bowel (10 segments of equal length), cecum, and colon (three segments of equal length). Each segment was opened and minced, and the tissue plus luminal contents were added to 2-ml tubes containing 1 ml of PBS. The contents were vigorously vortexed, and the solid material was pelleted by centrifugation. Aliquots of the supernatant were loaded in duplicate onto a 96-well plate, and the fluorescent signal for each bowel segment was determined by using a fluorescence plate reader (Cytofluor; excitation wavelength 530 nm and emission 590 nm). GC was calculated as: Σ(S1 × 1 + S2 × 2 +…S11 × 11), where S is the fraction of the total signal detected in each of the 15 segments (Miller et al., 1981).
Myeloperoxidase Histochemistry.
The magnitude of the inflammatory cell infiltrate into the intestinal muscularis was quantified by histological analysis. Muscularis whole mounts were prepared from the midjejunum collected 24 h after surgery. Segments of intestine were opened along the mesenteric border, stretched to 150% of the length and 250% of the width, and fixed in 100% ethanol for 1 h. After washing in PBS, the mucosa was removed by fine dissection, and the remaining tissue was treated with Hanker-Yates reagent (Polysciences, Warrington, PA) for detection of polymorphonuclear neutrophils exhibiting myeloperoxidase (MPO) activity. Tissues were mounted on glass slides, cover-slipped, and inspected by light microscopy at a magnification of 200×. The number of MPO-positive immune cells infiltrating the intestinal muscularis was determined as the average number of cells counted in five to six adjacent optical fields centered between the mesenteric and antimesenteric borders.
Gene Expression in Small Bowel Mucosa and Muscularis Externa.
Age-matched male CD-1 mice were divided into experimental groups as follows: naive controls (untreated) and two groups treated with either PBS or chimeric GLP-2/IgG2a 3 h before anesthesia and surgical manipulation of the small bowel. Animals were euthanized, and tissue was harvested 0.5, 1, 3, and 6 h postoperatively. The small bowel was removed from the ligament of Treitz to the ileocecal junction, placed immediately into ice-cold PBS, and cut into segments. Bowel segments were flushed with PBS and mounted on a glass rod where the muscularis externa was stripped from the underlying mucosa. Muscularis and mucosal tissues were snap-frozen in liquid nitrogen and stored at −80°C.
RNA was isolated from frozen tissues by using the RNeasy Lipid mini protocol (QIAGEN, Valencia, CA). Briefly, 1 ml of QIAzol reagent (QIAGEN) was added per 100 mg of frozen tissue and homogenized for approximately 20 s at maximum speed with a rotor-stator homogenizer. After sitting at room temperature for 5 min, 1 ml of lysate from muscularis tissue or 200 μl of lysate from mucosal tissue plus an additional 800 μl of QIAzol reagent were extracted with 200 μl of chloroform. After centrifugation at 12,000g for 15 min at 4°C, RNA was purified from the upper aqueous phase by the RNeasy mini protocol with DNase digestion on the column per the manufacturer's instructions. RNA quality and quantity were assessed with a LabChipGX (Caliper Life Sciences, Hopkinton, MA). Reverse transcription of RNA was performed with the high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions, with 1 μg of RNA in 30 μl. Gene expression for markers of inflammation, matrix integrity, and barrier function was determined by TaqMan analysis using custom low density arrays (Applied Biosystems), with 100 ng of reverse-transcribed RNA in 100 μl of 1× TaqMan Universal PCR Master Mix (Applied Biosystems) loaded per slot. Alternatively, individual reactions were set up with 20 ng of reverse-transcibed RNA in 10 μl of 1× TaqMan Universal PCR Master Mix. Real-time PCR was performed in duplicate by using the comparative Ct method on the ABI Prism 7900HT Sequence Detection System (Applied Biosystems) according to the manufacturer's instructions. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the endogenous control. Data are reported as fold change relative to nonoperated, PBS-treated control mice.
Statistical Analysis.
The statistical analyses performed are noted with the corresponding data, which are reported as mean ± S.E.M. with P < 0.05 being considered statistically significant. In general, data were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni post hoc testing for multiple comparisons. In cases where two groups were compared, an unpaired Student's t test was performed. For the analysis of mRNA expression at different times after surgery in control and experimental groups a two-way ANOVA was performed followed by Bonferroni post hoc tests.
Results
In cell-based cAMP assays, the A2G GLP-2 peptide (EC50 = 0.58 nM) was approximately 4-fold more potent than GLP-2/IgG4 and chimeric GLP-2/IgG2a (EC50 approximately 2.3 nM). This reduced potency in the GLP-2/IgG4 construct was countered by an increased serum half-life compared with the A2G GLP-2 peptide, because a single intravenous dose (1 mg/kg) in a Cynomulgus monkey exhibited a serum half-life of 5.4 ± 0.9 days when serum samples up to 28 days were analyzed by enzyme-linked immunosorbent assay (ELISA) for full-length peptide.
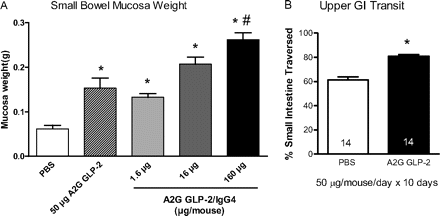
The reported protective effects of GLP-2 in inflammation models led us to test the hypothesis that a long-lived GLP-2/IgG4 construct would ameliorate inflammation in murine POI. Initial experiments evaluated the trophic effects of the A2G GLP-2 peptide and the GLP-2/IgG4 construct on the intestinal mucosa to determine appropriate doses of GLP-2/IgG construct in mice. As expected, daily administration of A2G GLP-2 peptide (50 μg/mouse) for 10 days increased mucosal wet weight (Fig. 1A). GLP-2/IgG4 increased mucosal weight dose-dependently, with the highest dose (160 μg/mouse; approximately 4 mg/kg) eliciting a 4-fold increase in mucosal weight above baseline. Unexpectedly, daily administration of A2G GLP-2 peptide increased upper GI transit (Fig. 1B) and a similar increase in mucosal weight to that shown in Fig. 1A (data not shown).
A, small intestinal weight of mucosa after daily administration of PBS, A2G GLP-2 peptide (50 μg/day), or GLP-2/IgG4 construct (1.6–160 μg) for 10 days. Data are mean ± S.E.M. n = 7 per group. *, P < 0.05 compared with PBS; #, P < 0.05 compared with peptide alone by ANOVA and Bonferroni's multiple comparisons test. B, daily administration of human A2G GLP-2 peptide increased upper GI transit (calculated by determining the leading edge of a carmine red meal 30 min after oral administration). Numbers in columns are animals per group. *, P < 0.05 by unpaired t test.
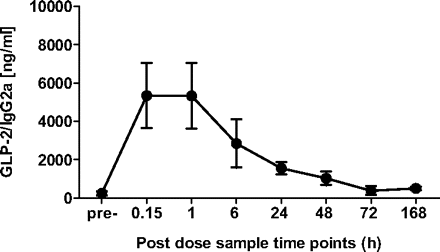
Based on these findings, a dose of 4 mg/kg GLP-2/IgG4 was selected for further characterization for both improvements in inflammation and gastrointestinal transit in the mouse surgical model of POI. However, initial experiments using the POI surgical model suggested a potential confounding effect caused by nonself-discrimination against human IgG4 in this model of inflammation. Therefore, we generated a chimeric construct derived by linking human A2G GLP-2 to murine IgG2a (chimeric GLP-2/IgG2a). After a single intravenous dose (4 mg/kg), full-length intact chimeric GLP-2/IgG2a in excess of 1 μg/ml was detected by ELISA for at least 48 h by using antibodies specific for mouse allotype IgG2aa and mouse anti-N-terminal GLP-2 (Fig. 2). In a separate group of mice, GLP-2/IgG4 (4 mg/kg) increased mucosal weight to the same extent (0.20 ± 0.02 g versus PBS 0.09 ± 0.01 g; P < 0.05) whether administered daily or every other day for 10 days. Further increasing the length of time between dosing (treating only on days 1 and 7) did not result in an increase in mucosal weight above control; therefore the biological duration of action of GLP-2/IgG4 in mice was at least 48 h. Based on the duration of effect on mucosal growth combined with the detection of serum levels of chimeric GLP-2/IgG2a out to 48 h, the construct was subsequently administered subcutaneously only once to naive mice or operated mice before surgery and intestinal manipulation.
Serum levels of intact chimeric GLP-2/IgG2a detected by ELISA using antibodies specific for mouse allotype IgG2aa and mouse anti-N-terminal GLP-2 0.15 to 168 h after a single intravenous dose of 3 mg/kg (n = 3 mice per time point).
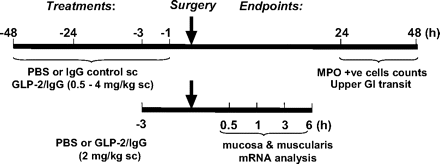
Because of the evidence from a model of ischemia-reperfusion injury suggesting that the efficacy of GLP-2 was affected by the timing of GLP-2 pretreatment (Zhang et al., 2008), we tested different pretreatment times at 1, 3, 24, or 48 h before surgery (Fig. 3). Cellular infiltrate into the intestinal muscularis (MPO+ cell counts) and upper GI transit were determined at two time points: 24 h postoperatively to determine whether chimeric GLP-2/IgG2a pretreatment inhibited the peak postoperative manifestations of ileus and 48 h postoperatively to determine effects on the recovery phase.
Summary of the protocols used to illustrate the different treatments and times relative to surgery, involving laparotomy and small intestinal manipulation, and their endpoint measures. The effects of chimeric GLP-2/IgG2a at a range of concentrations (0.5–4.0 mg/kg) were compared with isotype control (IgG2a) and/or PBS given before laparotomy (1, 3, 24, and 48 h). The distribution of FITC-dextran (upper GI transit) was determined by calculating GC, and quantification of the numbers of MPO+ cells infiltrating small bowel muscularis whole mounts were performed 24 or 48 h after surgery (n ≥ 5 per group). Chimeric GLP-2/IgG2a (2 mg/kg, s.c.) or PBS was administered 3 h before surgery for mRNA analysis of muscularis externa and mucosal layers of small intestine harvested at 0.5, 1.0, 3.0, and 6 h postsurgery (n = 5 per group).
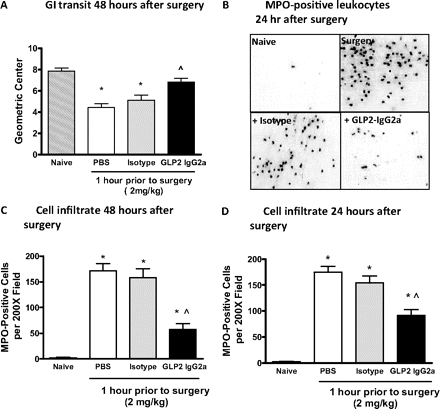
The effects of GLP-2/IgG2a administered 1 h before abdominal surgery and induction of POI are shown in Fig. 4. GLP-2/IgG2a treatment did not have a significant effect on GI transit 24 h postoperatively (data not shown), but by 48 h postoperatively transit was improved to the point where it was not significantly different from that of naive mice (Fig. 4A). Digital photomicrographs of representative muscularis whole mounts collected 24 h postoperatively show that MPO+ cells within the small bowel muscularis of surgically manipulated animals are markedly reduced in number in animals treated with GLP-2/IgG2a compared with PBS and isotype controls (Fig. 4B). These data are summarized in Fig. 4, C and D, where a significant reduction in the MPO+ cellular recruitment in GLP-2/IgG2a-treated animals was observed at both the 48- and 24-h postoperative time points (Fig. 3, C and D). A similar reduction in cellular influx was observed when a higher GLP-2/IgG2a dose (4 mg/kg) was given and responses were evaluated 48 h postoperatively (isotype pretreated = 217 ± 41 MPO+ cells per 200× field; GLP-2/IgG2a pretreated = 62 ± 14; P < 0.05). In these mice, terminal blood draws were collected and plasma levels of 1913 ± 387 ng/ml for the fully intact GLP-2/IgG2a (n = 8) were detected at 48 h, indicating that the construct exhibited good plasma exposure at this time point.
A, chimeric GLP-2/IgG2a (2 mg/kg) administered 1 h before surgical manipulation of the small bowel improves upper GI transit 48 h postoperatively. B, digital images demonstrate that the influx of MPO+ immune cells within small bowel muscularis whole mounts is reduced by chimeric GLP-2/IgG2a pretreatment, whereas IgG2a isotype (2 mg/kg s.c.) and PBS were without effect. C and D, chimeric GLP-2/IgG2a (2 mg/kg s.c.) significantly reduced the inflammatory cell infiltrate 48 h (C) and 24 h (D) postoperatively. *, P < 0.05 compared with naive controls; ⋀, P < 0.05 compared with isotype and PBS by ANOVA and Bonferroni's post test. Data are mean ± S.E.M. n = 5 to 14 per group.
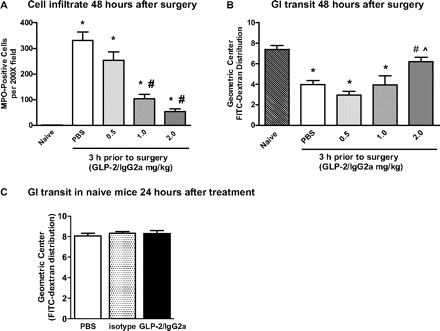
GLP-2/IgG2a given 3 h before surgery at a range of doses (0.5–2.0 mg/kg) dose-dependently decreased MPO+ immune cells at both postoperative time points, with improvements in upper GI tract transit occurring only at the 48-h postoperative time point (Fig. 5, A and B). Significant reductions in cellular infiltrate were obtained with GLP-2/IgG2a at doses of 1 and 2 mg/kg; however, an improvement in GI motility was obtained only at the 2 mg/kg dose. One explanation for the absence of a positive effect on transit at the 24-h postoperative time point might be GLP-2-mediated inhibition of GI transit, for example, caused by stimulation of vagal afferent reflexes (Nelson et al., 2007) Therefore, we tested the effects of chimeric GLP-2/IgG2a administration on upper GI transit in naive mice (Fig. 5C). The construct had no effect on GI transit measured 24 h after administration, indicating that the absence of improved GI transit 24 h after surgery could not be explained by a GLP-2-mediated inhibitory effect.
A and B, the dose-related effect of chimeric GLP-2/IgG2a (0.5–2.0 mg/kg) administered 3 h before intestinal manipulation on MPO+ immune cells in small bowel whole mounts (A) and upper GI tract transit (B) measured 48 h postoperatively. Data are mean ± S.E.M. n = 5 to 8 per group. *, P < 0.05 compared with naive; #, P < 0.05 compared with control (PBS); ⋀, P < 0.05 compared with 0.5 mg/kg GLP-2/IgG2a by ANOVA and Bonferroni's test. C, in naive mice there was no direct inhibitory effect on upper GI transit at 24 h after 2 mg/kg GLP-2 IgG2a administration (n = 10 per group).
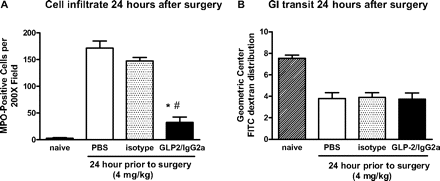
Chimeric GLP-2/IgG2a (4 mg/kg) given 24 h before surgery maintained the capacity to reduce cellular influx determined 24 h postoperatively (Fig. 6A), but again no improvement in GI transit was observed (Fig. 6B). When the time of administration of GLP-2/IgG2a (4 mg/kg) was increased to 48 h before surgery, there was no significant effect on either cellular influx 24 h postoperatively (isotype pretreated = 118 ± 16 MPO+ cells per 200× field; GLP-2/IgG2a pretreated = 90 ± 33; P > 0.05) or upper GI transit (isotype pretreated = 4.29 ± 0.50; GLP-2/IgG2a pretreated = 4.76 ± 0.60, P > 0.05).
Chimeric GLP-2/IgG2a (4.0 mg/kg) administered 24 h before intestinal manipulation improves the cellular infiltrate (A) but not upper GI transit (B) in small bowel whole mounts harvested 24 h postoperatively. *, P < 0.05 compared with PBS; #, P < 0.05 compared with isotype control by ANOVA and Bonferroni's test, Data are mean ± S.E.M. n ≥5 per group.
To gain insight into the mechanisms by which the long-acting GLP-2 agonist given prophylactically decreased inflammatory infiltrate and improved GI transit, TaqMan RT-PCR was performed on mucosa, and muscularis tissues were harvested from the small bowel. Of the genes tested, 23 in the mucosa and 10 in the muscularis exhibited statistically significant changes in expression in response to either surgical manipulation of the small bowel or treatment with GLP-2/IgG2a (Table 1). The pattern of gene expression was time-dependent, with the time of peak expression varying according to the gene and its location. Surgical manipulation rapidly increased proinflammatory gene expression in both the mucosa and muscularis (Figs. 7 and 8); examples include the transcription factor early growth response gene (EGR)-1, the proinflammatory cytokines interleukin (IL)-6 and IL-1β, the chemokines monocyte chemoattractant protein (MCP)-1, macrophage inflammatory factor (MIP)-1α, and enzymes that synthesize the kinetically active mediators nitric oxide (inducible nitric-oxide synthase, iNOS) and prostaglandin E2 (cyclooxygenase-2, COX-2). Increased expression was also observed for the putative anti-inflammatory mediators IL-10 and hemoxygenase-1 (HO-1).
Genes altered by GLP-2/IgG2a treatment
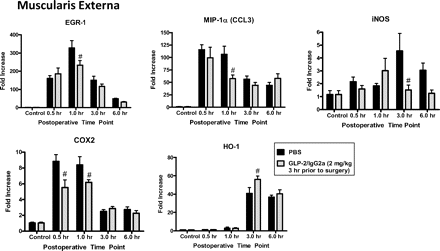
Chimeric GLP-2/IgG2a given 3 h before surgery altered mRNA levels (relative to GAPDH) of selected inflammatory-related mediators that were increased in small bowel muscularis externa 0.5 to 6 h after surgery, Data are shown as fold change over untreated controls, mean ± S.E.M. n = 5 animals per group. #, P < 0.05 compared with PBS treatment at the same time point by two-way ANOVA and Bonferroni's test.
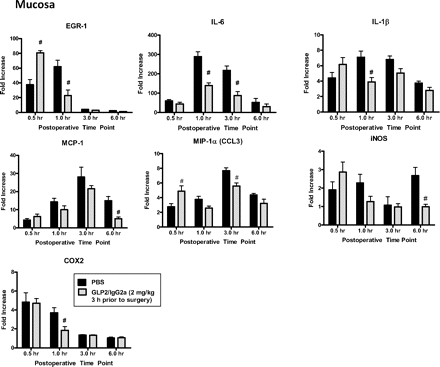
Chimeric GLP-2/IgG given 3 h before surgery altered mRNA levels (relative to GAPDH) of selected inflammatory and neurotransmitter mRNA levels in small bowel mucosa 0.5 to 6 h after surgery shown as fold change over untreated controls. Data are mean ± S.E.M. n = 5 animals per group. #, P < 0.05 compared with PBS treatment at the same time point by two-way ANOVA and Bonferroni's test.
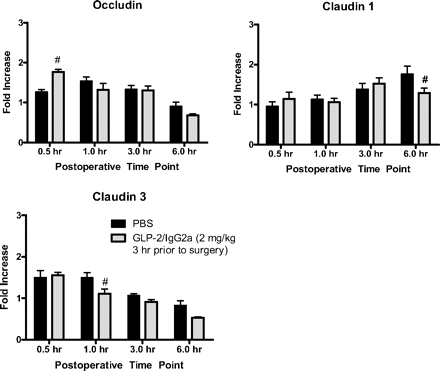
In the muscularis, pretreatment with chimeric GLP-2/IgG2a resulted in a significant, although modest, reduction in the peak increase in message for the proinflammatory mediators Egr-1, MIP-1α, iNOS, and COX2 (Fig. 7). Peak HO-1 gene expression was moderately enhanced. GLP-2/IgG2a treatment resulted in a more prominent effect on the expression of proinflammatory genes within the intestinal mucosa (Fig. 8). Treatment with GLP-2/IgG2a significantly enhanced Egr-1 expression relative to control at the 30-min time point, followed by a marked inhibition of Egr-1 expression 1 h postoperatively. Peak expression of IL-6 and iNOS was also markedly reduced. Evidence for an early reduction in COX2 gene expression was apparent at the 1-h postoperative time point. The effects of GLP-2/IgG2a treatment on IL-1β, MCP-1, and MIP-1α were less clear. Although statistically significant changes were observed at some time points, the effects were minor. The surgically induced induction of IL-10 and HO-1 (not shown) was not altered by GLP-2/IgG2a treatment in the mucosa. In general, expression of genes for tight junctional proteins (occludin, claudin 1, claudin 3) was not altered by surgical manipulation (Fig. 9), although statistically significant changes in expression were observed in GLP-2/IgG2a-treated animals.
Chimeric GLP-2/IgG given 3 h before surgery altered mRNA levels (relative to GAPDH) of selected tight junction mRNA levels in small bowel mucosa 0.5 to 6 h after surgery shown as fold change over untreated controls. Data are mean ± S.E.M. n = 5 animals per group. #, P < 0.05 compared with PBS treatment at the same time point by two-way ANOVA and Bonferroni's test.
Discussion
This study provides the first evidence that treatment before surgery with a single dose of a long-acting GLP-2 receptor agonist reduced cellular inflammation associated with intestinal manipulation and enhanced recovery from the surgically induced delay in gastrointestinal transit. Insight into the mechanisms by which GLP-2 receptor agonism mediates these beneficial effects was obtained from examining changes in the mRNA expression profiles of genes known to be altered within the muscularis and mucosa after small bowel manipulation. GLP-2 receptor agonism did not markedly alter the resident macrophage-driven molecular inflammatory response within the muscularis, a mechanism that is normally attributed to induction of cellular inflammation in that tissue. Instead, GLP-2 may be exerting its protective effect by affecting expression of genes associated with mucosal inflammation and barrier function. Its potential therapeutic effects on inflammation and motility are discussed in the context of the known literature.
The mechanisms controlling the initiation and maintenance of POI involve a complex series of events involving altered mucosal barrier function, production of inflammatory mediators, and neuroimmune interactions (Boeckxstaens and de Jonge, 2009). In brief, disturbance to the bowel during abdominal surgery activates sympathetic inhibitory reflexes, suppressing GI motility within the first few hours after bowel manipulation. At this early time point a molecular inflammatory response is initiated via the activation of resident macrophages by the endproducts of arachidonic acid metabolism, extracellular matrix fragments, and the mucosal translocation of luminal antigen. The induction of macrophage-derived transcription factors (EGR-1, signal transducer and activator of transcription 3, nuclear factor κB), the expression of cellular adhesion molecules (intercellular adhesion molecule-1), and the production of chemokines (MCP-1, MIP-1α) elicits the recruitment of neutrophils, monocytes, and mast cells to the intestinal muscularis and mucosa, producing a cellular inflammatory response. Mediators released from mast cells and cytokines (IL-6, IL-1β), nitric oxide (iNOS derived), and prostaglandin E2 released from resident macrophages, and recruited immune cells impair neuromuscular communication, inhibit smooth muscle contractility, and enhance central inhibitory neural reflexes, resulting in suppression of GI motility over the subsequent 72 h.
The cellular inflammatory response is initiated 6 h after bowel manipulation, reaching its peak by 24 h and persisting up to 72 h. Administration of the chimeric GLP-2/IgG2a construct 3 h before surgery markedly reduced cellular inflammation within the muscularis 24 and 48 h postoperatively. This pretreatment protocol was selected to provide sufficient time for the GLP-2 construct to reach the site of action during the early molecular changes in response to surgery and provide measurable plasma exposure out to 48 h after treatment. In the intestinal muscularis, small bowel manipulation increased expression (> 100 fold) of the proinflammatory genes EGR-1, IL-6, IL-1β, MCP-1, MIP-1α, and colony-stimulating factor (CSF)-2. Increases in TNFα, cannabinoid receptor (CNR)-2, COX2, and iNOS were also noted. In general, the effects of GLP-2/IgG2a treatment were relatively modest, reducing the expression of some proinflammatory genes (e.g., EGR-1, MIP-1α, iNOS, CNR2, and COX2), while having no effect on others (e.g., IL-6, IL-1β, or MCP-1). In particular, the lack of an inhibitory effect on IL-6 and MCP-1 would tend to argue for a cellular inflammatory response, because these have been linked to the up-regulation of adhesion molecules and cellular recruitment (Turler et al., 2002; Wung et al., 2005). Expression of the anti-inflammatory molecules HO-1 and IL-10 was increased in surgically manipulated animals, but GLP-2/IgG2a treatment enhanced only HO-1 expression, which may have contributed to the reduced inflammatory response within the muscularis. GLP-2 treatment had little effect on IL-10 gene expression, consistent with the observation that the anti-inflammatory effect of GLP-2 persists in IL-10 knockout mice with colitis (Ivory et al., 2008). In conclusion, although it cannot be ruled out that the GLP-2/IgG2a construct is affecting translation of protein, the modest amelioration of the muscularis molecular inflammatory response may contribute to, but is unlikely to be, the major underlying cause of the reduced cellular inflammation.
One way by which the GLP-2 construct exerts anti-inflammatory effects in POI may involve early modulation of gene expression of multiple signaling pathways in the mucosa. Chimeric GLP-2/IgG2a inhibited early proinflammatory gene expression of IL-6, IL-1β, MCP-1, MIP-1α, and COX2, indicative of an overall blunting of the molecular inflammatory response. GLP-2 treatment also reduced mucosal MPO and inflammatory cytokine protein levels (interferon γ, TNF-γ, IL-1β) in ileitis/colitis (Sigalet et al., 2007). However, the positive effects of GLP-2 receptor agonism in the mucosa did not appear to involve the enhancement of anti-inflammatory mechanisms, because the surgically induced increase in IL-10 and HO-1 expression were not altered by GLP-2/IgG2a treatment. Rather, the GLP-2 construct ameliorated a prominent effect in the mucosa, which was the enhanced mRNA expression of Egr-1. In addition to its role in inflammatory events associated with POI (Schmidt et al., 2008), Egr-1 has been linked to protection against intestinal epithelial cell injury and impaired barrier function (Moon et al., 2007). Surgical manipulation of the bowel transiently increases mucosal permeability (Schwarz et al., 2002; Turler et al., 2007), which allows passage of bacterial cell wall components, enterotoxins, and intact bacteria. Native GLP-2 treatment reduces stress-induced mucosal bacteria adherence and translocation induced by enhancing intestinal epithelial barrier function (Cameron and Perdue, 2005). Proinflammatory cytokines can also alter the interactions between the tight junction proteins claudins and occludin, resulting in increased paracellular permeability (Capaldo and Nusrat, 2009). Increased occludin protein expression is reported to enhance transepithelial resistance (Balda et al., 1996), and GLP-2/IgG2a treatment resulted in a small, although significant, increase in occludin gene expression. Finally, endogenous GLP-2 in the mucosa may contribute to healing responses, as indicated by observations of increased numbers of GLP-2-positive enteroendocrine cells during and after resolution of ileits/colitis (Lomax et al., 2006). Together, these GLP-2-mediated effects may enhance epithelial barrier function, reducing translocation of luminal materials and limiting the activation of processes that drive inflammation.
The onset of cellular inflammation correlates temporally with the decline in intestinal smooth muscle contractility, with both reaching their peak 24 h postoperatively. In the present study, consistent and dose-related improvements in upper gastrointestinal motility were noted only 48 h postsurgery. The presence of dysmotility at 24 h, in the absence of a cellular infiltrate in the muscularis, has been noted previously and was attributed to a persistent molecular inflammatory response driven by the resident macrophage cell population within the muscularis (Moore et al., 2007). In the current study, GLP-2 receptor agonism had little effect on key mediators in the muscularis that are known to disrupt motility (IL-6, IL-1β, COX-2, iNOS). IL-6 and IL-1β are known to impair neuromuscular communication and decrease motor function (Natale et al., 2003). COX-2-derived prostaglandins and iNOS-derived nitric oxide have direct inhibitory effects on smooth muscle contractility (Schwarz et al., 2001; Turler et al., 2006). Thus, the failure of GLP-2/IgG2a treatment to strongly suppress this molecular response may account for the persistent intestinal dysmotility observed 24 h postoperatively.
Another factor contributing to dysmotility could be through GLP-2-mediated activation of neural inhibitory reflexes within the first few hours after bowel manipulation. Acute administration of GLP-2 peptides delays gastric emptying and slows intestinal transit within a few hours after treatment (Wojdemann et al., 1998; Shibata et al., 2001) and raises the question of whether this inhibitory effect could carry out to 24 h. However, the administration of the chimeric GLP-2/IgG2a construct in normal mice had no effect on transit at this time point. Therefore, the dysmotility seen 24 h postoperatively in manipulated mice is unlikely to be explained by a direct effect of the construct on smooth muscle or central neural-inhibitory reflexes.
The increased GI transit after longer exposure to GLP-2 in normal and POI mice is a novel finding. In POI mice this is likely to be the result of the reduced inflammatory cell infiltrate. The molecular inflammatory response within the resident macrophage population begins to decline 6 to 12 h postoperatively. This, along with the absence of an ongoing molecular inflammatory response from infiltrating cells, would be expected to result in a more rapid recovery of bowel motility. The activation of vagal cholinergic anti-inflammatory processes by long-acting GLP-2 receptor agonism may also contribute to this process, whereby efferent cholinergic activity of the vagus nerve ameliorates cellular inflammation and dysmotility associated with POI (de Jonge et al., 2005; The et al., 2007).
In summary, our findings demonstrate that prophylactic treatment with a long-acting GLP-2 receptor agonist attenuates inflammation associated with postoperative ileus and accelerates recovery from bowel stasis. The beneficial effects of GLP-2 receptor agonism is mediated by multiple functional pathways involving reduced gene expression for proinflammatory mediators and the enhanced expression of genes involved in maintaining mucosal integrity and repair. These findings extend those reporting the beneficial effects of GLP-2 in other models of gastrointestinal injury and confirm that the mechanism of action involves the induction of genes that support bowel homeostasis.
Acknowledgments
We thank Peter Bugelski and Karyn O'Neil (Centocor Research and Development) for insight and Patricia Andrade-Gordon (Johnson & Johnson Pharmaceutical Research and Development), Frederic Baribaud, Eva Emmel, and Anuk Das (Centocor Research and Development) for support.
Footnotes
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.161497.
-
ABBREVIATIONS:
- GLP-2
- glucagon-like peptide 2
- CNR
- cannabinoid receptor
- COX-2
- cyclooxygenase-2
- CSF
- colony-stimulating factor
- EGR-1
- early growth response gene
- FITC
- fluorescein isothiocyanate
- GC
- geometric center
- GI
- gastrointestinal
- HO-1
- hemoxygenase-1
- IgG
- immunoglobulin G
- iNOS
- inducible nitric-oxide synthase
- MCP
- monocyte chemoattractant protein
- MIP
- macrophage inflammatory protein
- MPO
- myeloperoxidase
- PBS
- phosphate-buffered saline
- POI
- postoperative ileus
- TLR
- Toll-like receptor
- TNF
- tumor necrosis factor
- VIP
- vasoactive intestinal polypeptide
- ANOVA
- analysis of variance
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- ELISA
- enzyme-linked immunosorbent assay
- IL
- interleukin.
- Received September 14, 2009.
- Accepted February 10, 2010.
- Copyright © 2010 by The American Society for Pharmacology and Experimental Therapeutics