Abstract
Secondary alcohol metabolites and reactive oxygen species mediate cardiomyopathy induced by cumulative doses of antitumor anthracyclines, such as doxorubicin and epirubicin. Epirubicin exhibits a defective conversion to both toxic species, thereby inducing cardiotoxicity at doses higher than equiactive to doxorubicin; however, the gain in cardiac tolerability seems to be marginal compared with the magnitude of the metabolic defects of epirubicin. Cardiomyopathy may occur independent of toxic metabolites if a given anthracycline tends to accumulate in the heart; therefore, we characterized whether epirubicin showed an unusual accumulation in human myocardial strips incubated in plasma. Epirubicin exhibited a higher uptake and reached myocardial levels 2 times higher than those of doxorubicin. Epirubicin also showed a unique metabolization to doxorubicinolone, the product of epirubicin deglycosidation and carbonyl reduction. In diffusing from the strips to plasma, doxorubicinolone caused membrane permeation effects that augmented epirubicin elimination. Experiments with purified doxorubicinolone showed that the efflux of 1 mol doxorubicinolone promoted the concomitant elimination of as many as ∼40 mol epirubicin. Doxorubicinolone could also diffuse from plasma back to the strips, causing a permeation effect that promoted epirubicin reuptake; however, this reverse process was slower and less potent. On balance, doxorubicinolone efflux diminished the epirubicin to doxorubicin accumulation ratio to ∼1.5. These results suggest that the cardiac tolerability of epirubicin is limited by its accumulation in the heart and that such accumulation would be even higher in the absence of doxorubicinolone formation and efflux. These results may also serve guidelines for developing noncardiotoxic anthracyclines.
The anthracyclines doxorubicin and epirubicin are widely used to treat human tumors, but both of them can cause cardiomyopathy and congestive heart failure (CHF) upon chronic administration. Cumulative doses of doxorubicin associated with 5% risk of CHF have been approximated to 400 to 450 mg/m2 (Swain et al., 2003). Epirubicin is ∼1.5 times less active than doxorubicin against tumors, which is explained by its glucuronidation in liver and improved body clearance (Innocenti et al., 2001); however, it is interesting that epirubicin has long been known to induce CHF at cumulative doses ≥2 times higher than doxorubicin. Therefore, the cardiotoxicity of epirubicin is approximated to ∼66% of that of doxorubicin (Ewer and Benjamin, 2006),
Anthracycline-related cardiotoxicity correlates with the peak plasma concentration (Cmax) of anthracyclines, their passive diffusion in the heart, and their conversion to toxic metabolites or byproducts (Gewirtz, 1989; Minotti et al., 2004). Clinical studies did not show remarkable differences between the Cmax values of doxorubicin and those of equiactive doses of epirubicin (Gianni et al., 1997; Grasselli et al., 2001); however, preclinical studies showed that the two anthracyclines exhibited different patterns of formation of toxic species in the heart.
Doxorubicin is composed of an aglycone and a sugar. The aglycone (doxorubicinone) is a tetracyclic ring system with quinone-hydroquinone moieties and a short side chain with a carbonyl group at C-13; the sugar (Daunosamine) is an amino-substituted trideoxy fucosyl moiety. One-electron reduction of the quinone moiety of doxorubicin is followed by redox coupling with oxygen and formation of noxious levels of reactive oxygen species (ROS). This process occurs primarily in mitochondria (Salvatorelli et al., 2006). On the other hand, two-electron reduction of the side-chain carbonyl group causes formation of a secondary alcohol metabolite (doxorubicinol) that is more polar than doxorubicin, accumulates in cardiomyocytes, and makes cardiotoxicity progress from acute/reversible dysfunction to chronic cardiomyopathy (Forrest et al., 2000; Minotti et al., 2004). Doxorubicinol formation is mediated primarily by cytoplasmic reductases (Salvatorelli et al., 2006).
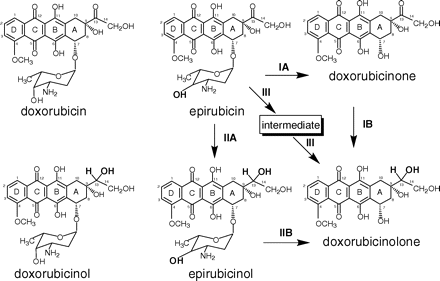
Molecules and metabolic pathways of interest in this study. Residues in bold indicate the axial-to-equatorial epimerization of the hydroxyl group at C-4′ in the aminosugar of epirubicin(ol) and the secondary alcohol moieties of doxorubicinol, epirubicinol, and doxorubicinolone. Arrows, possible pathways for formation of doxorubicinolone from epirubicin.
Epirubicin differs from doxorubicin in an axial-to-equatorial epimerization of the hydroxyl group at C-4′ in Daunosamine. Such a limited modification favored epirubicin sequestration in cytoplasmic acidic organelles like recycling endosomes, lysosomes, and vesicles of the trans-Golgi network; hence, epirubicin failed to reach mitochondria and formed essentially no ROS compared with doxorubicin (Salvatorelli et al., 2006). Epirubicin also exhibited an impaired two-electron reduction of its side-chain carbonyl group, such that the levels of formation of its secondary alcohol metabolite (epirubicinol) always averaged ≤50% of those of doxorubicinol (Salvatorelli et al., 2006, 2007). These findings were obtained by using human myocardial strips or isolated cardiac cytosol, two translational models that obviated pitfalls caused by a heterogeneous metabolization of anthracyclines in laboratory animals (Mordente et al., 2003; Menna et al., 2008). The defective conversion of epirubicin to ROS and epirubicinol in such models offered clues to explain how it caused CHF at doses higher than equiactive to doxorubicin; it also explained how epirubicin caused fewer cardiac events when combined with drugs, such as the taxanes or the anti-HER2neu antibody trastuzumab, which aggravated doxorubicin cardiotoxicity by stimulating doxorubicinol formation or by diminishing defenses against ROS, respectively (Gennari et al., 1999; Pagani et al., 2000; Untch et al., 2004; Suter et al., 2007).
Although undoubtedly less damaging than ROS or secondary alcohol metabolites, anthracyclines may cause cardiotoxicity by direct mechanisms if they reach unusually high levels in the heart (Jensen et al., 1984; Marcillat et al., 1989; Tokarska-Schlattner et al., 2006). This factor halted the clinical development of some investigational anthracyclines in the face of their defective conversion to toxic species (Menna et al., 2008). By having said that epirubicin lacked ROS formation and formed much less alcohol metabolite than doxorubicin did, one would expect that its cardiotoxicity ranked even less than 66% compared with doxorubicin. Therefore, we considered that the cardiac tolerability of epirubicin might be limited by its tendency to accumulate in the heart, such that the benefits of a defective conversion of epirubicin to ROS and epirubicinol would be partly counterbalanced by direct interactions of epirubicin with cardiomyocytes.
The present study aimed at characterizing how epirubicin compared with doxorubicin in terms of uptake, elimination, and net accumulation in human myocardial samples. This information would be of value in clinical settings and in the search for new generation noncardiotoxic anthracyclines.
Materials and Methods
Chemicals. We used doxorubicin, doxorubicinol, epirubicin, epirubicinol, doxorubicinone, and doxorubicinolone (Fig. 1). Doxorubicin(ol) and epirubicin(ol) were obtained through the courtesy of Nerviano Medical Sciences (Milan, Italy). Doxorubicinone and doxorubicinolone (13-dihydroxydoxorubicinone) were prepared by us through the thermoacid hydrolysis of doxorubicin and doxorubicinol, respectively (Menna et al., 2002). AL1576 was from Alcon Laboratories, Inc. (Fort Worth, TX). Quercetin, bafilomycin (type A1), and all other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Human Myocardial Strips. Small myocardial samples were obtained from the lateral aspect of excluded right atrium of patients undergoing aortocoronary bypass grafting (66.7 ± 1.6 years, n = 48). All of the samples were routinely disposed of by the surgeons during cannulation procedures; hence, the patients were not exposed to any unjustified loss of tissue. Myocardial samples were dissected to obtain thin strips (∼2 × 10 mm, <0.1 g) that were washed with ice-cold 0.3 M NaCl and incubated in 2 ml of fresh human plasma added with test compounds. Plasma was prepared before each experiment by 1500g centrifugation of blood anticoagulated with sodium citrate. Throughout this study, blood samples were obtained from three male and two female healthy donors (37 ± 5 years). We previously reported that strips incubated in plasma retained their viability and functions throughout the incubation time, as shown by: 1) bidirectional movements of anthracycline molecules across plasma and the strips always followed rigorous polarity/apolarity rules; 2) the strips properly responded to inhibitors of mitochondrial electron transport and of many other enzymes; and 3) the strips never released myoglobin, troponin T, or MB-creatine kinase during the course of incubations (Salvatorelli et al., 2006, 2007). Thus, the system was tailored to probe anthracycline pharmacokinetics without confounding effects caused by an acute damage and perturbances of membrane permeability and drug metabolization. All of the incubations were carried out in a gently shaking Dubnoff metabolic bath under an air atmosphere.
Time Courses of Anthracycline Uptake and Efflux. Anthracycline diffusion in cardiomyocytes equilibrates with anthracycline efflux and secondary rounds of anthracycline reuptake (Riganti et al., 2005; Menna et al., 2007). We developed experimental conditions that allowed independent measurements of efflux and (re)uptake. In brief, myocardial strips were incubated in plasma added with 10 μM doxorubicin or epirubicin, similar to the Cmax values determined after clinical infusions of equiactive doses of 60 mg/m2 doxorubicin or 90 mg/m2 epirubicin (Gianni et al., 1997; Grasselli et al., 2001). After 30 min, the strips were switched to conditions of “stimulated efflux” or “efflux-reuptake.” In the stimulated efflux condition, the strips were placed in fresh anthracycline-free plasma that was replaced every 30 to 60 min up to 4 h to promote the highest possible efflux of doxorubicin or epirubicin while also minimizing their reuptake. Plasma samples (200 μl) were taken at regular times and assayed for parent anthracyclines and metabolites. The values determined at each time point were added with values measured at the preceding time point; this provided a time course of cumulative anthracycline efflux, without confounding effects caused by anthracycline reuptake in the strips. In the efflux-reuptake condition, the strips were placed in fresh anthracycline-free plasma, which was left unchanged for 4 h. Plasma samples (200 μl) were taken at regular times and assayed for doxorubicin or epirubicin; this provided a time course of anthracycline efflux in equilibrium with anthracycline reuptake. Differences in plasma levels between the condition of stimulated efflux and that of efflux-reuptake gave a time course of anthracycline reuptake. Finally, we measured doxorubicin or epirubicin that had not been released from the strips under conditions of stimulated efflux. By adding this pool of nonreleaseable anthracycline with the time points of anthracycline reuptake, we could calculate the time course of doxorubicin or epirubicin uptake over the entire experiment time.
Standard Experiments of Anthracycline Distribution and Metabolism in Human Myocardial Strips. Human myocardial strips were incubated in plasma added with 10 μM doxorubicin or epirubicin. After 4 h of incubation at 37°C, the strips were washed with ice-cold 0.3 M NaCl, homogenized in a minimum volume of the same medium, and centrifuged for 90 min at 105,000g to separate soluble and whole-membrane fractions that were assayed for anthracyclines and their metabolites. Aliquots of plasma (200 μl) were also taken and assayed for anthracyclines and their metabolites. In the experiments with bafilomycin A1, the inhibitor of the H+-ATPase that acidifies cytoplasmic organelles (Bowman et al., 1988), the strips were preincubated for 1 h in 4 ml of 50 mM phosphate buffer/123 mM NaCl/5.6 mM glucose, pH 7.4, added with 300 nM bafilomycin. Next, the strips were switched to standard 4-h incubations in plasma added with anthracyclines and 90 nM bafilomycin (Salvatorelli et al., 2006).
Experiments with Doxorubicinolone[in → out]or Doxorubicinolone[out → in]. Where indicated, the strips were incubated in plasma and loaded for 1 h with 0.5 to 1 to 2.5 μM doxorubicinolone. Next, the strips were switched to standard 4-h incubations in fresh plasma added with 10 μM epirubicin. At the end of the experiments, the strips were assayed for epirubicin, epirubicinol, and doxorubicinolone that had diffused from the strips to plasma. These conditions will be referred to as experiments with doxorubicinolone[in → out]. In other experiments, the strips were coincubated with 10 μM epirubicin and purified doxorubicinolone (0.5, 1, or 2.5 μM). The latter was dissolved in a minimum volume of ethanol (≤ 5 μl), which was also included in control incubations to permit direct comparisons. At the end of 4-h incubations, the strips were assayed for epirubicin, epirubicinol, and the fraction of doxorubicinolone that had diffused from plasma to strips. These conditions will be referred to as experiments with doxorubicinolone[out → in].
High-Performance Liquid Chromatography Assays. Plasma and soluble or membrane fractions of myocardial strips were extracted with a 4-fold volume of 1:1 CHCl3/CH3OH. The organic phases were combined to obtain an extract that was analyzed by reversed-phase high-performance liquid chromatography in a Hewlett Packard 1100 system (Hewlett Packard, Palo Alto, CA). The extracts were loaded onto a Macrosphere RP 300 C-18 column (250 × 4.6 mm, 5 μm; Alltech Associates, Deerfield, IL), operated at 25°C and eluted at the flow rate of 1 ml/min for a total 25-min run time (15-min linear gradient from 100% 50 mM NaH2PO4, pH 4.0, to 65-35% CH3CN-50 mM NaH2PO4, followed by a 10-min isocratic elution with 65-35% CH3CN-50 mM NaH2PO4). The retention times were 14.2 (doxorubicinol), 14.8 (epirubicinol), 15.2 (doxorubicinolone), 15.5 (doxorubicin), 16.1 (epirubicin), and 17.4 (doxorubicinone) min. To improve separation of doxorubicinolone from doxorubicin, the extracts from doxorubicin experiments were also loaded onto an HP Zorbax CN column (250 × 4.6 mm, 5 μm; Hewlett Packard), operated at 25°C, and eluted at the flow rate of 1.5 ml/min for a total 20-min run (15-min linear gradient from 50 mM NaH2PO4 to CH3CN-25 mM NaH2PO4, pH 4.0). The retention times were 10.9 (doxorubicinol), 11.6 (doxorubicinolone), 12.1 (doxorubicin), and 13.7 (doxorubicinone) min. Anthracyclines were detected fluorimetrically with excitation at 477 nm and emission at 560 nm. There were differences in the fluorescence yields of doxorubicin(ol), epirubicin(ol), and doxorubicin(ol)one; hence, each analyte was quantified against standard curves prepared by subjecting that analyte to comparable chromatographic procedures (Salvatorelli et al., 2007). Detection limits were at least ∼2 times below the lowest levels of analyte recovery from myocardial strips or plasma. Within- and between-days coefficients of variation were <3 and <10%, respectively. In the experiments with myocardial strips, all of the values (nanomoles per gram of tissue) were expressed as micromolar equivalents upon considering that the cardiac tissue has a density very similar to that of water; in the experiments with plasma, the values (nanomoles per milliliter) were normalized to the weight of the strips and then expressed as micromolar equivalents (Salvatorelli et al., 2007).
Other Assays and Conditions. Anthracycline metabolism was reconstituted in isolated NADPH-supplemented human cardiac cytosol as described in previous reports (Salvatorelli et al., 2006, 2007). In kinetic experiments, Km values were determined with anthracyclines at 0.5 μM to 1 mM, and their metabolites were measured at the end of 4-h incubations; Vmax values were determined with anthracyclines at 50 μM, and their metabolites were measured over the linear phase of the reaction (usually 30-60 min). In kinetic experiments, the values were means of two experiments with >85% agreement. In all of the other experiments, the values were means ± S.E. of at least three experiments, Data were analyzed by paired or unpaired Student's t test, and differences were considered significant when p was <0.05. Other details are given under Results, legends to figures, and Table 1.
Anthracycline content, distribution, and metabolization in human myocardial strips
Human myocardial strips were incubated in plasma with 10 μM anthracyclines, as described under Materials and Methods. Values were means ± S.E. of 36 or 30 experiments with doxorubicin or epirubicin, respectively. Values in parentheses indicate experimental ranges.
Results
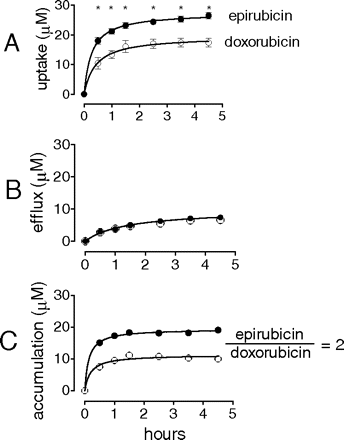
Theoretical versus Experimental Accumulation of Epirubicin in Human Myocardial Strips. Human myocardial strips were briefly loaded with anthracyclines in plasma and then switched to conditions of stimulated efflux or efflux-reuptake that allowed to calculate the maximal theoretical capability of human myocardial strips to incorporate or release anthracyclines. The uptake of epirubicin was more rapid than that of doxorubicin (t½ =∼15 versus ∼21 min) and reached higher levels at all of the time points examined (Fig. 2A). Anthracycline uptake equilibrated with anthracycline efflux, which was slower than uptake (t½ = ∼60 min) and was similar for doxorubicin and epirubicin (Fig. 2B). By correcting uptake for efflux we could calculate a theoretical time course of anthracycline accumulation in human myocardium. The strips accumulated more epirubicin than doxorubicin, with the final ratio of total epirubicin/total doxorubicin being 2 (∼20 μM epirubicin versus ∼10 μM doxorubicin) (Fig. 2C).
We next examined a broad panel of myocardial strips that had been exposed to 10 μM doxorubicin or epirubicin for 4 h to generate a dynamic equilibrium among anthracycline uptake, metabolization, and efflux. In comparison with the aforesaid conditions of maximal theoretical accumulation, total doxorubicin remained unchanged, but total epirubicin decreased from ∼20 to ∼15 μM (Table 1). The higher levels of epirubicin versus doxorubicin were caused primarily by a better distribution of epirubicin to the membrane fraction of the strips; of note, the concentration of total epirubicin in the strips formally exceeded that added in plasma, but a similar result was obtained with many samples exposed to doxorubicin (see also Table 1). This denoted the ability of the cardiac tissue to accumulate anthracyclines (Olson et al., 1988). The experiments reported in Table 1 collectively confirmed that epirubicin showed a tendency to accumulate over doxorubicin in human myocardial strips; however, the decrease in total epirubicin suggested that there were salvage pathway(s) that limited its theoretical capability to accumulate in human myocardium.
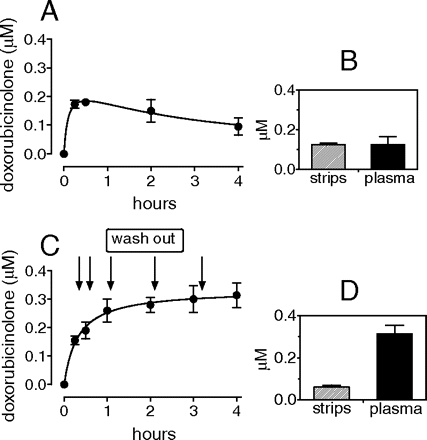
Epirubicin-Dependent Formation and Efflux of Doxorubicinolone. In agreement with previous reports, human myocardial strips were found to generate more doxorubicinol than epirubicinol (see also Table 1); however, it is interesting that the limited formation of epirubicinol was paralleled by ∼3-fold higher levels of recovery of doxorubicinolone from the soluble fraction of the strips and plasma (Table 1). Doxorubicinolone would be formed through reduction of the side-chain carbonyl group and hydrolysis of the glycosidic bond between the planar ring system of epirubicin and the aminosugar (see Fig. 1). Control experiments showed that neither plasma nor an isolated membrane fraction could convert epirubicin to doxorubicinolone, regardless of the presence or absence of NADPH as a cofactor (data not shown). Therefore, we considered the possibility that doxorubicinolone was formed in the soluble fraction of the strips and then diffused to plasma. In the first set of experiments, we measured the plasma levels of doxorubicinolone during the course of 4-h incubations of the strips with 10 μM epirubicin. The plasma levels of doxorubicinolone followed a biphasic pattern, characterized by a rapid increase in 15 to 30 min and by a slow decrease over the remaining incubation time. At the end of the experiments, the levels of doxorubicinolone in the soluble fraction of the strips were identical with those in plasma (Fig. 3, A and B). In the second set of experiments, the strips were exposed to 10 μM epirubicin, but plasma was washed out every 30 to 60 min and replaced with fresh anthracycline-free plasma to ensure that the concentration of doxorubicinolone in the strips always exceeded that in plasma. These latter conditions caused a cumulative efflux of doxorubicinolone, such that at the end of the experiments, the myocardial levels of doxorubicinolone in the strips were only marginal compared with those in plasma (Fig. 3, C and D). These experiments showed that doxorubicinolone formed in the strips and equilibrated with plasma in a gradient-dependent manner. Therefore, we could determine that the sum of doxorubicinolone in the strips with doxorubicinolone in plasma, hereafter referred to as “total doxorubicinolone,” exceeded epirubicinol by a factor of 11 ± 3. The percentage of doxorubicinolone efflux to total doxorubicinolone, hereafter referred to as “doxorubicinolone clearance,” averaged 59 ± 5%.
Theoretical anthracycline uptake and efflux in human myocardial strips. Human myocardial strips were loaded with 10 μM anthracyclines for 30 min and then switched to conditions of stimulated efflux or efflux-reuptake. The time courses of uptake and efflux were calculated as described under Materials and Methods. Accumulation was calculated by point-by-point correction of uptake for efflux. The values were means ± S.E. of three experiments (values without vertical bars had their S.E. within symbols). *, p < 0.05 for epirubicin versus doxorubicin.
Doxorubicinolone equilibration across myocardial strips and plasma. Human myocardial strips were incubated in 2 ml of plasma added with 10 μM epirubicin. Doxorubicinolone was measured in plasma during the course of 4-h incubations (A) and in plasma and strips at the end of the incubations (B). In other experiments, plasma was washed out at the time points indicated by the arrows and was replaced with 2 ml of fresh anthracycline-free plasma to promote a continuous efflux of doxorubicinolone from the strips (C). Plasma levels of doxorubicinolone were calculated by adding the levels determined at each time point with the levels determined at the preceding time point. At the end of the incubations, doxorubicinolone was measured in plasma and strips (D). Each value is mean ± S.E. of three experiments.
Doxorubicinolone could not be detected in the strips exposed to doxorubicin as if its formation was unique to epirubicin (Table 1). The same pattern was observed when the metabolism of the two anthracyclines was reconstituted in NADPH-supplemented isolated cardiac cytosol. Under highly favorable conditions that adopted 50 μM anthracyclines, epirubicin formed 0.7 ± 0.08 nmol doxorubicinolone/mg protein/4 h (n = 15), whereas doxorubicin only formed 0.07 ± 0.02 nmol (n = 10).
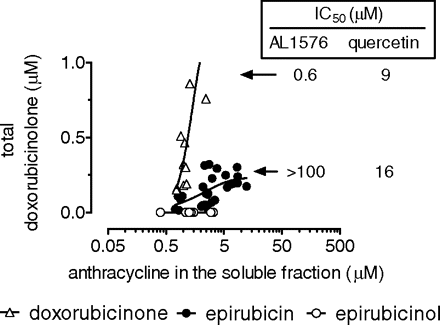
Mechanisms of Doxorubicinolone Formation. We reported that both epirubicin and purified doxorubicinone were metabolized to secondary alcohols by aldehyde reductases (Salvatorelli et al., 2007), members of the superfamily of aldo-keto reductases (Bains et al., 2008); however, doxorubicinone was a much better substrate for these enzymes compared with epirubicin, the Vmax/Km values for the formation of doxorubicinolone or epirubicinol in isolated human cardiac cytosol being 4.0 × 10-2 or 1.0 × 10-5 ml/mg protein/min, respectively. We considered that the preferred conversion of epirubicin to doxorubicinolone in myocardial strips could occur through the spontaneous or enzymatic hydrolysis of epirubicin, yielding a doxorubicinone intermediate that remained undetected because of its near-to-complete reduction to doxorubicinolone by aldehyde reductases (see pathways IA and IB in Fig. 1). To probe this possibility, we measured total doxorubicinolone formation in human myocardial strips that had been incubated with 10 μM epirubicin or purified doxorubicinone and increasing concentrations of the potent inhibitor of human aldehyde reductase, AL1576 (Barski et al., 1995). Other strips were incubated with increasing concentrations of quercetin, inhibitor of human carbonyl reductase (Holleran et al., 2004); this was done because some reports indicated that secondary alcohol metabolites could be formed also by carbonyl reductases, members of the superfamily of short-chain aldehyde dehydrogenases (Blanco et al., 2008). Figure 4 shows the results of experiments in which epirubicin and purified doxorubicinone reached similar levels in the soluble fraction of the strips, a condition that allowed direct comparisons between the two anthracyclines. Purified doxorubicinone predictably formed much higher levels of total doxorubicinolone than epirubicin did; interestingly, however, the conversion of doxorubicinone to doxorubicinolone was inhibited primarily by AL1576, whereas the conversion of epirubicin to doxorubicinolone was inhibited by quercetin only. Thus, doxorubicinolone formation did not proceed through a doxorubicinone intermediate liable to reduction by aldehyde reductases.
Substrate dependence and inhibitor studies of doxorubicinolone formation in myocardial strips. Human myocardial strips were incubated in 2 ml of plasma added with 10 μM epirubicin or purified doxorubicinone or 50 μM epirubicinol. After 4 h, the soluble fraction of the strips was assayed for the levels of the three anthracyclines and formation of total doxorubicinolone (strips + plasma). As indicated, the strips were also treated with AL1576 or quercetin to measure IC50 values for inhibition of total doxorubicinolone formation.
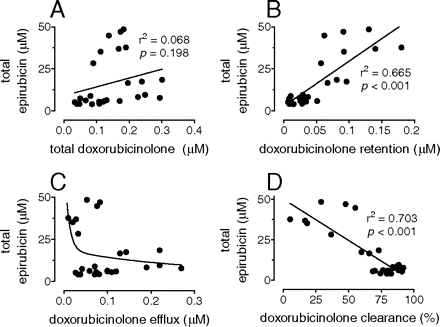
Total epirubicin versus doxorubicinolone efflux or clearance. Human myocardial strips were incubated in 2 ml of plasma added with 10 μM epirubicin. After 4 h, the myocardial levels of total epirubicin (membrane fraction + soluble fraction) were measured and plotted against those of total doxorubicinolone (strips + plasma) (A), doxorubicinolone retention (doxorubicinolone in the strips) (B), doxorubicinolone efflux (C), and doxorubicinolone clearance [100 × (doxorubicinolone efflux/total doxorubicinolone)] (D).
We next considered that doxorubicinolone could be formed through epirubicinol hydrolysis (pathway II in Fig. 1). Two lines evidence argued against this possibility. First, the myocardial formation of epirubicinol was potently inhibited by AL1576 but much less potently by quercetin (IC50 = 1.5 μM for AL1576 versus 40 μM for quercetin). Because AL1576 lacked effect on the conversion of epirubicin to doxorubicinolone (see Fig. 4), these results suggested that doxorubicinolone did not originate from epirubicinol formation and hydrolysis. Second, we conducted experiments in which the strips were directly exposed to epirubicinol to see whether it formed measurable levels of doxorubicinolone. These experiments were done with up to 50 μM epirubicinol, which was needed to overcome its limited diffusion and to ensure that it reached levels comparable with those achieved by 10 μM epirubicin or purified doxorubicinone in the soluble fraction of the strips. Under such defined conditions, epirubicinol did not form doxorubicinolone (see also Fig. 4). By having excluded pathways IA and IB and II, we tentatively envisioned a pathway III in which epirubicin formed an intermediate that underwent rapid and complete conversion to doxorubicinolone (see also Fig. 1). The components of such unique quercetin-inhibitable pathway will require ad hoc characterizations. Doxorubicinolone formation was highly favored over epirubicinol formation. In isolated cytosol, the Vmax/Km value for the conversion of epirubicin to doxorubicinolone was 2.1 × 10-4, i.e., 1 order of magnitude higher than that determined for epirubicinol.
Doxorubicinolone Clearance and Myocardial Levels of Epirubicin. At the end of standard 4-h incubations, the myocardial levels of total epirubicin did not correlate with those of total doxorubicinolone (Fig. 5A) but showed opposite patterns of correlation with doxorubicinolone in the strips (referred to as “doxorubicinolone retention”) or doxorubicinolone efflux. Total epirubicin increased and correlated linearly with doxorubicinolone retention (Fig. 5B); conversely, total epirubicin showed an inverse relation with doxorubicinolone efflux (Fig. 5C). Such a latter relation could only tentatively be fit in a two-phase exponential decay (R2 = 0.143), presumably because doxorubicinolone formation (and, hence, doxorubicinolone efflux) varied across several strips preparations (see also Fig. 5C). Therefore, we plotted epirubicin levels against doxorubicinolone clearance, which normalized doxorubicinolone efflux from a given strip to doxorubicinolone formation in that particular strip. Under these latter conditions, there was a linear and highly significant inverse relation between total epirubicin and doxorubicinolone clearance (Fig. 5D). In either case, minor increases in doxorubicinolone efflux or clearance always resulted in much greater decrements of total epirubicin in the strips.
The apparent effect of doxorubicinolone clearance on diminishing total epirubicin was caused primarily by an inverse relation of doxorubicinolone clearance with epirubicin levels in the membrane fraction of the strips (Fig. 6A). In contrast, plotting doxorubicinolone clearance against epirubicin levels in the soluble fraction of the strips showed that epirubicin first increased and then returned to or below its baseline, according to a reasonably well fit bell-shaped pattern (R2 = 0.6174). The peak of epirubicin in the soluble fraction occurred at a doxorubicinolone clearance of ∼37% (Fig. 6B).
We previously reported that bafilomycin inhibited epirubicin protonation-sequestration in cytoplasmic acidic organelles; by doing so, bafilomycin also suppressed a driving force (“ion trapping”) that otherwise stimulated the diffusion of more and more epirubicin from plasma into the soluble fraction of the strips (Salvatorelli et al., 2006). Here, bafilomycin caused no effect on the inverse relation between doxorubicinolone clearance and epirubicin levels in the membrane fraction (Fig. 6C); however, bafilomycin abolished the bell-shaped fluctuations of epirubicin in the soluble faction and made epirubicin levels remain almost stable over the whole range of doxorubicinolone clearance (Fig. 6D).
Doxorubicinolone clearance and epirubicin levels in the soluble or membrane fractions of myocardial strips. A and B, experimental conditions were as described in the legend for Fig. 5. C and D, strips were loaded and incubated with bafilomycin A1 as described under Materials and Methods.
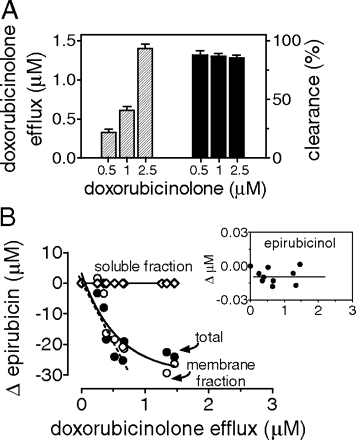
Epirubicin and epirubicinol levels in experiments with purified doxorubicinolone[in→out]. Human myocardial strips were incubated in plasma and loaded for 1 h with 0.25 to 0.5 to 1 μM purified doxorubicinolone. Next, the strips were incubated for 4 h in fresh plasma added with 10 μM epirubicin and 100 μM quercetin. A, strips and plasma were assayed for determining doxorubicinolone efflux and clearance, and the values were means ± S.E. of three experiments. B and inset, strips were assayed for epirubicin and epirubicinol, and the curves were by obtained by point-by-point plotting of individual values of epirubicin(ol) versus individual values of doxorubicinolone efflux. The values were expressed as net changes versus strips that had been exposed to epirubicin without a prior loading with doxorubicinolone.
Mechanisms of Doxorubicinolone-Dependent Epirubicin Elimination. We performed experiments with purified doxorubicinolone[in → out] or doxorubicinolone[out → in] that obviated intersample differences in doxorubicinolone formation and quantified the effects of known amounts of doxorubicinolone on total epirubicin levels. Excess quercetin was included to minimize formation of an endogenous doxorubicinolone and its interferences with doxorubicinolone added by us. At the end of experiments with doxorubicinolone[in → out], we observed a doxorubicinolone efflux that correlated with the concentrations of purified doxorubicinolone added to the strips before their incubations with epirubicin; this corresponded to an 86 to 88% doxorubicinolone clearance at each of the concentrations adopted (Fig. 7A). By comparing these samples with parallel quercetin-inhibited samples that had been exposed to epirubicin without a prior loading with purified doxorubicinolone, we determined that doxorubicinolone efflux was accompanied by a decrease of total epirubicin. This was caused by a decrease of epirubicin in the membrane fraction; there were no changes of epirubicin or epirubicinol in the soluble fraction of the strips (Fig. 7, B and inset).1 By extrapolating the linear phase of epirubicin decrease versus doxorubicinolone efflux, we determined that the efflux of 1 mol doxorubicinolone made total epirubicin decrease by ∼40 mol.
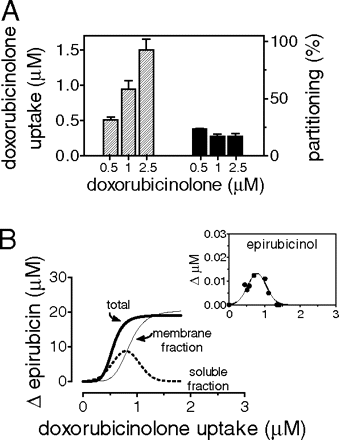
Quercetin-inhibited myocardial strips were also used in experiments with purified doxorubicinolone[out → in]. At the end of 4-h incubations, we observed a doxorubicinolone uptake that correlated with the concentrations of purified doxorubicinolone added in plasma. The net values of doxorubicinolone uptake were quite similar to those of doxorubicinolone efflux in the experiments with doxorubicinolone[in → out]; however, the percentage of doxorubicinolone that diffused from plasma to strips was much lower than the clearance of doxorubicinolone from strips to plasma (16-20% partitioning at each of the three concentrations of purified doxorubicinolone added in plasma) (Fig. 8A). Doxorubicinolone uptake caused a gradual increase of total epirubicin, 1 μM doxorubicinolone making epirubicin increase by ∼20 μM (Fig. 8B). At a doxorubicinolone uptake below 1 μM the increase of total epirubicin was slightly higher than that of epirubicin in the membrane fraction, presumably because of a transient accumulation of epirubicin and its metabolization to epirubicinol in the soluble faction; at a doxorubicinolone uptake above 1 μM, doxorubicinolone acted almost exclusively by increasing epirubicin levels in the membrane fraction, which therefore overlapped with total epirubicin in the strips (see also Fig. 8, A, B, and inset).
The experiments with doxorubicinolone[out → in] showed collectively that doxorubicinolone diffusion from plasma to the strips augmented a concomitant diffusion of epirubicin in the same direction. On balance, however, the effects of doxorubicinolone on epirubicin uptake seemed to be less robust than the effects of doxorubicinolone efflux on epirubicin elimination; whereas total epirubicin decreased almost concomitant with doxorubicinolone efflux, a converse increase of total epirubicin only occurred at a doxorubicinolone uptake ≥ 0.25 μM (see Figs. 7 and 8).
Discussion
Lack of ROS formation and limited conversion to epirubicinol render epirubicin undoubtedly less cardiotoxic than doxorubicin in many clinical settings; however, the gain in cardiac tolerability that is seen with epirubicin seems to be marginal compared with the magnitude of its metabolic defects. Because high levels of anthracyclines may be toxic per se, we explored whether epirubicin reached myocardial levels that were high enough to dissipate the benefits of its defective conversion to ROS or epirubicinol. By adopting highly controlled conditions of stimulated efflux or efflux reuptake, which explored the maximal theoretical capability of human myocardial strips to take up or release anthracyclines, we found that epirubicin could reach myocardial levels ∼2 times higher than those of doxorubicin. Epirubicin accumulation was only caused by its faster and more pronounced uptake, probably attributable to factors such as the higher lipophilicity of epirubicin versus doxorubicin (octanol/water partitioning coefficients, 2.3 versus 0.8) (Wielinga et al., 2000) and the driving force operated by the protonation-sequestration of epirubicin in cytoplasmic acidic vesicles (Salvatorelli et al., 2006).2
Previous studies of investigational anthracyclines showed that an accumulation ratio of 2:1 versus doxorubicin would eliminate the benefits of a defective formation of one toxic species or another (Menna et al., 2008). Therefore, we probed epirubicin also in standard 4-h incubations that provided a more dynamic picture of uptake in equilibrium with biotransformation and efflux. These latter conditions favored a decrease of the myocardial levels of epirubicin but not of doxorubicin; therefore, the epirubicin/doxorubicin accumulation ratio decreased to ∼1.5, a value that would be more consistent with the incomplete but measurable gain of cardiac tolerability shown by epirubicin in patients. Epirubicin accumulation was limited by doxorubicinolone formation and efflux (or clearance), and several aspects of this mechanism fit in the concept of a salvage pathway. First, doxorubicinolone formation neither occurred through sequential formation and hydrolytic deglycosidation of epirubicinol nor reflected epirubicin hydrolysis followed by carbonyl reduction of doxorubicinone by aldehyde reductases; instead, it seems that epirubicin converted to an intermediate that could not be identified because of its rapid and complete biotransformation to doxorubicinolone. Second, doxorubicinolone formation was kinetically favored over epirubicinol formation. Third, doxorubicinolone was lipophilic enough to diffuse from the strips to plasma, which was opposite to the polar character of epirubicinol and its tendency to accumulate in the strips. And finally, minor increases in doxorubicinolone efflux (or clearance) were accompanied by remarkable decrements of epirubicin in the strips.
Epirubicin and epirubicinol levels in experiments with purified doxorubicinolone[out→in]. Human myocardial strips were incubated for 4 h in plasma added with 10 μM epirubicin, 0.25 to 0.5 to 1 μM purified doxorubicinolone, and 100 μM quercetin. A, strips and plasma were assayed for the net uptake of doxorubicinolone and the percentage of doxorubicinolone partitioning from plasma to strips [100 × (doxorubicinolone in strips)/(doxorubicinolone in strips + doxorubicinolone in plasma)]. B and inset, strips were assayed for epirubicin and epirubicinol, and the curves were by obtained by point-by-point plotting of individual values of epirubicin(ol) versus individual values of doxorubicinolone uptake. The values were expressed as net changes versus strips that had been exposed to epirubicin without doxorubicinolone.
An inverse relation between the myocardial levels of epirubicin and those of doxorubicinolone efflux (or clearance) suggested that doxorubicinolone acted during the course of its transmembrane diffusion from the strips to plasma. This concept raised questions about whether doxorubicinolone efflux (or clearance) caused effects that improved epirubicin elimination from the strips or diminished epirubicin diffusion from plasma to the strips. This latter possibility was negated by experiments with doxorubicinolone[out → in]; doxorubicinolone augmented epirubicin uptake and epirubicinol formation, with such an effect depending on that fraction of doxorubicinolone that codiffused from plasma into the strips. Therefore, we suggest that doxorubicinolone diminished the myocardial levels by stimulating epirubicin elimination. In accordance, total epirubicin decreased in the experiments with doxorubicinolone[in→out], and such an effect depended on that fraction of doxorubicinolone that diffused from the strips toward plasma.
The effects of doxorubicinolone clearance on epirubicin elimination require further attention. In the standard 4-h incubations, a low to high doxorubicinolone clearance always decreased that prevailing pool of epirubicin which localized to the membrane fraction of the strips; in contrast, the levels of epirubicin in the soluble fraction increased and then decreased as doxorubicinolone clearance progressed from low to high values (see Fig. 6, A and B). We suggest that this pattern reflected epirubicin mobilization from membranes to plasma through a central cytoplasmic compartment. At a low to moderate doxorubicinolone clearance, epirubicin mobilization from membranes to cytosol overlapped with unstimulated or ion trapping-stimulated diffusion of epirubicin from plasma into the same compartment; hence, epirubicin reached its peak in the soluble fraction of the strips. All such factors were eliminated by bafilomycin or high doxorubicinolone clearance; under these latter conditions, epirubicin elimination from the soluble fraction to plasma only equilibrated with an unstimulated passive diffusion of epirubicin from plasma, such that epirubicin attained steady-state levels in the soluble fraction of the strips (see Fig. 6D). In the experiments with purified doxorubicinolone[in → out], there was the usual decrease of epirubicin in the membrane fraction but not the bell-shaped fluctuation of epirubicin levels in the soluble fraction. This could be explained by the near-to-complete clearance of doxorubicinolone that occurred in these experiments (see Fig. 7A).
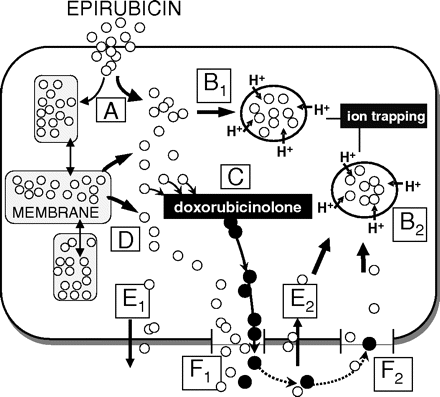
Schematic representation of the effects of doxorubicinolone on epirubicin uptake and elimination. A, epirubicin enters by passive distribution and distributes to cytosol and primarily to membranes; B1 and B2, protonation-sequestration in cytoplasmic acid organelles operates a driving force for the uptake or reuptake of epirubicin in the cytosol; C, epirubicin is metabolized to doxorubicinolone through carbonyl reduction and deglycosidation; D, epirubicin mobilizes from membranes to cytosol, overlapping with ion trapping-stimulated diffusion of epirubicin into the cytosol; E1 and E2, epirubicin elimination and reuptake may occur through passive diffusion; F1 and F2, doxorubicinolone causes a permeation effect that eliminates much greater amounts of epirubicin (F1), followed by a slow/incomplete reuptake of doxorubicinolone and a less pronounced permeation effect on the reuptake of epirubicin (F2).
The observation that doxorubicinolone[in → out] or doxorubicinolone[out → in] augmented epirubicin fluxes bidirectionally suggests that doxorubicinolone acted as a membrane-permeabilizing agent (Veldman et al., 2004). The experiments with doxorubicinolone[in → out] offered an opportunity to approximate that the efflux of 1 mol doxorubicinolone caused the elimination of as many as ∼40 mol epirubicin, which was conceptually in agreement with a permeation-like effect. Keeping this in mind and considering that in standard 4-h incubations, doxorubicinolone efflux averaged ∼0.085 μM doxorubicinolone, we can approximate that doxorubicinolone formation and efflux contributed to eliminate ∼3.4 μM epirubicin. One such approximation would agree ∼73% with the ∼4.8 μM decrease of total epirubicin that occurred when the strips were examined in standard 4-h incubations rather than in conditions of maximal theoretical accumulation (see Table 1).
The incomplete agreement of doxorubicinolone efflux with epirubicin elimination raises the possibility that other mechanism(s) contributed to diminishing epirubicin levels in human myocardium. By having demonstrated that doxorubicinolone diffused from the strips to plasma and back to the strips, we also consider that doxorubicinolone-dependent epirubicin efflux equilibrated with doxorubicinolone-dependent epirubicin uptake. The latter could only in part counteract the former; in fact, 1) doxorubicinolone diffusion from plasma to the strips was slower than doxorubicinolone efflux in the opposite direction, 2) the percentage of doxorubicinolone that diffused from plasma to the strips was only marginal compared with the clearance of doxorubicinolone from the strips, and 3) doxorubicinolone stimulated epirubicin uptake less potently than epirubicin elimination (see Figs. 3A, 7, A and B, and 8, A and B). The incomplete diffusion of doxorubicinolone from plasma to the strips and its relatively limited potency at stimulating epirubicin uptake may be ascribed to the strong binding and hydrophobic stabilization of doxorubicinolone with albumin and other plasma proteins (Sokolove and Shinaberry, 1988; Cui et al., 2008).
We previously reported that in human myocardial strips one-electron redox cycling of doxorubicin might be accompanied by formation of 7-deoxydoxorubicinolone (Salvatorelli et al., 2006).3 Although very similar to doxorubicinolone, 7-deoxydoxorubicinolone immobilizes in mitochondria (Gille and Nohl, 1997), which implies that it cannot diffuse and cause membrane permeation effects that improved doxorubicin elimination. Our present results show that human myocardial strips did not metabolize doxorubicin to diffusion-prone doxorubicinolone; hence, total doxorubicin attained similar levels under conditions of maximal theoretical accumulation or in standard 4-h incubations. It is interesting that we also noticed that purified doxorubicinolone would have no effect on the partitioning of doxorubicin across plasma and strips (data not shown). This observation argued against analog-independent gross effects of doxorubicinolone on the integrity of myocardial strips; it also denoted differences between doxorubicinolone and permeabilizing agents, such as natural double-chain lipids or digitonin, that were shown to potentiate the uptake or efflux of both doxorubicin and epirubicin in different cell types (Wielinga et al., 2000; Veldman et al., 2004). As was said before, epirubicin is appreciably more lipophilic than doxorubicin; hence, the effect of doxorubicinolone was discrete enough to only favor membrane permeation to the more lipophilic epirubicin. It was in keeping with this concept that the strips never released epirubicinol, which is appreciably more polar than epirubicin.
The effects of doxorubicinolone on epirubicin movements across myocardial strips and plasma are summarized in Fig. 9. We suggest that the permeation effect of doxorubicinolone and its prevailing role on improving epirubicin elimination are important factors for epirubicin to maintain a favorable profile of cardiac tolerability. Also of note is that anthracyclines introduce a lifetime risk of cardiac events, which may occur months or years after completing chemotherapy (Gianni et al., 2008; Menna et al., 2008; Mulrooney et al., 2008). The current thinking is that comorbidities, unfavorable life-styles, or environmental factors precipitate cardiac events by conspiring with a long-lived cardiac anthracycline reservoir. Defective conversion of epirubicin to epirubicinol certainly diminishes the chances of forming a long-lived anthracycline reservoir; however, the permeation effect of doxorubicinolone would be important to avoid an accumulation of epirubicin. On a different note, these concepts may form new guidelines in the search for noncardiotoxic anthracyclines. Pharmaceutical efforts usually are paid to eliminate or modify the quinone or carbonyl moieties that enable anthracyclines to form toxic ROS or secondary alcohol metabolites. Our results suggest that potential candidates should also be probed for their ability to form diffusible metabolites that eliminate excess anthracycline by mechanisms similar to those described for doxorubicinolone.
Acknowledgments
We thank Dr. Raimondo De Cristofaro (Catholic University School of Medicine, Rome, Italy) for helpful discussions.
Footnotes
-
↵1 In these experiments, quercetin was used high enough (100 μM) to inhibit epirubicinol formation by 60 to 70%. Therefore, the effects of doxorubicinolone[in → out] on epirubicinol levels must be intended as net changes versus strips, which also formed fewer epirubicinol in response to excess quercetin. The same concept applies to experiments with doxorubicinolone[out → in].
-
↵2 Epirubicin did not differ from doxorubicin in regard to the pKa of the protonatable NH2 residue in the sugar. Protonation-sequestration of epirubicin was only favored by its higher lipophilicity and diffusion in the acidic organelles (Salvatorelli et al., 2006).
-
↵3 7-Deoxydoxorubicinolone originates from disproportionation of two semiquinone free radicals, with regeneration of 1 mol doxorubicin and concomitant carbonyl reduction and reductive deglycosidation of another 1 mol doxorubicin (Salvatorelli et al., 2006). In the present study, 7-deoxydoxorubicinolone averaged 0.022 ± 0.005 μM and could only be recovered from the membrane fraction.
-
This work was supported by the Associazione Italiana Ricerca sul Cancro and University Campus Bio-Medico of Rome [Intramural Project “Cardio-Oncology”].
-
E.S. and P.M. contributed equally to this work.
-
doi:10.1124/jpet.108.149260.
-
ABBREVIATIONS: CHF, congestive heart failure; Cmax, peak plasma concentration; ROS, reactive oxygen species; AL1576, 2,7-difluorospirofluorene-9,5′-imidazolidine-2′,4′-dione; quercetin, 3,3′,4′,5,7-pentahydroxyflavone.
- Received December 2, 2008.
- Accepted January 13, 2009.
- The American Society for Pharmacology and Experimental Therapeutics