Abstract
Cholestasis causes hepatocyte death, possibly because of mitochondrial injury. This study investigated whether NIM811 (N-methyl-4-isoleucine cyclosporine), an inhibitor of the mitochondrial permeability transition (MPT), attenuates cholestatic liver injury in vivo. Cholestasis was induced in mice by bile duct ligation (BDL). NIM811 was gavaged (20 mg/kg) before BDL and daily (10 mg/kg) afterward. Mitochondrial depolarization, cell death, and MPT onset were assessed by intravital confocal/multiphoton microscopy of rhodamine 123, propidium iodide, and calcein. After BDL, serum alanine aminotransferase (ALT), hepatic necrosis, and apoptosis all increased. NIM811 decreased ALT, necrosis, and apoptosis by 60 to 86%. In vehicle-treated mice at 6 h after BDL, viable hepatocytes with depolarized mitochondria were 18/high-power field (hpf), and nonviable cells were ∼1/hpf, showing that depolarization preceded necrosis. Calcein entered mitochondria after BDL, indicating MPT onset in vivo. NIM811 decreased depolarization by 72%, prevented calcein entry into mitochondria, and blocked release of cytochrome c. Hepatic tumor necrosis factor α, transforming growth factor-β1, procollagen α1(I) mRNA, α-smooth muscle actin, and Sirius red staining for collagen increased after BDL but were not different in vehicle- and NIM811-treated mice. Taken together, NIM811 decreased cholestatic necrosis and apoptosis but did not block fibrosis, indicating that the MPT plays an important role in cholestatic cell death in vivo.
Cholestasis represents an interruption of bile secretion developing either acutely or chronically. Cholestatic liver disease results from hepatitis, complications of surgery, trauma, intravenous feeding, tumors, gallstones, certain medications, and idiopathic, genetic, and metabolic diseases, such as primary biliary cirrhosis and sclerosing cholangitis (Poupon et al., 2000). Accumulation of bile acid (BA) induces injury because of the toxicity of hydrophobic BA to hepatocytes and cholangiocytes (Kass, 2006). Toxic BA causes both necrotic and apoptotic cell death (Faubion et al., 1999; Gujral et al., 2004; Fickert et al., 2005). Chronic cholestatic liver diseases eventually lead to liver cirrhosis, which is a leading indication for liver transplantation.
How cholestasis induces liver injury and fibrosis remains controversial. Cytotoxic bile salts trigger hepatocyte apoptosis partly by ligand-independent activation of CD95 (Fas) death receptors (Faubion et al., 1999). In isolated hepatocytes, exposure to hydrophobic BA causes mitochondrial production of reactive oxygen species (ROS) (Sokol et al., 1995; Rolo et al., 2003). Cytotoxic BA also induces ROS formation by activation of NADPH oxidase (Reinehr and Haussinger, 2007). ROS can trigger opening of permeability transition (PT) pores in the mitochondrial inner membrane that nonspecifically transport aqueous solutes up to a molecular mass of approximately 1500 Da (Kantrow et al., 2000; Halestrap and Brennerb, 2003). Onset of the MPT collapses the mitochondrial membrane potential, leading to failure of ATP synthesis, release of proapoptotic cytochrome c, and necrotic and apoptotic cell death (Zamzami et al., 1996; Kim et al., 2003). Antioxidants and cyclosporine A (CsA), a specific inhibitor of the MPT (Halestrap and Davidson, 1990), protect cultured hepatocytes from BA-induced loss of viability (Gores et al., 1998; Yerushalmi et al., 2001; Rolo et al., 2003), implicating a role for an ROS-dependent MPT in BA hepatotoxicity. Mitochondria isolated from human livers also generate ROS and undergo MPT onset in response to BA (Sokol et al., 2005). However, whether MPT pore opening occurs in vivo during cholestasis to contribute to cholestatic liver injury and liver fibrosis remains to be determined.
NIM811 is a nonimmunosuppressive derivative of CsA that inhibits the MPT in cultured hepatocytes, isolated liver mitochondria, and liver grafts after transplantation in mice and rats (Waldmeier et al., 2002; Zhong et al., 2007; Theruvath et al., 2008). NIM811 is equipotent to CsA for inhibition of onset of the MPT in cultured cells and isolated mitochondria (Waldmeier et al., 2002; Theruvath et al., 2008). NIM811 also suppresses collagen production and proliferation of cultured hepatic stellate cells (HSCs) (Kohjima et al., 2007). Study of mitochondrial dysfunction and the MPT in vivo has been difficult. However, advances in intravital confocal/multiphoton microscopy now permit assessment of mitochondrial function and inner membrane permeability in livers of living animals (Zhong et al., 2007; Theruvath et al., 2008). Because the MPT plays an important role in both necrosis and apoptosis (Kim et al., 2003) and because MPT onset occurs in hepatocytes after exposure to BA, we used intravital confocal/multiphoton microscopy to evaluate the role of the MPT in cholestatic liver injury in vivo and to determine whether blockade of the MPT with NIM811 prevents cholestatic cell death and liver fibrosis.
Materials and Methods
Animals. Male BALB/c mice (8–9 weeks) were gavaged with NIM811 (Novartis, Basel, Switzerland; 20 mg/kg) or an equal volume of vehicle containing 8.3% polyethoxylated castor oil (Sigma-Aldrich, St. Louis, MO) and 8.3% ethanol at 2 h before surgery. Mice underwent bile duct ligation (BDL) or sham operation under ether anesthesia. In brief, the common bile duct was double ligated near the liver and transected between ligatures. NIM811 (10 mg/kg) or vehicle was gavaged daily after surgery. All animals received humane care in compliance with institutional guidelines. Animal protocols were approved by the Institutional Animal Care and Use Committee.
Clinical Chemistry and Histology. At times indicated in the figure legends, mice were anesthetized with pentobarbital (80 mg/kg i.p.), and blood was collected from the inferior vena cava. Livers were harvested, and slides were prepared as described elsewhere (Zhong et al., 2003). In sections stained with hematoxylin and eosin, 10 random fields per slide were captured in a blinded manner using a Universal Imaging Image-1/AT image acquisition and analysis system (Molecular Devices, Sunnyvale, CA) with an Axioskop 50 microscope (Carl Zeiss Inc., Thornwood, NY) and a 10× objective lens. Necrotic areas were quantified by image analysis using IP Lab version 3.7 software (BD Biosciences, San Jose, CA) by dividing necrotic areas by total cellular area of the images. Some slides were stained with 0.1% Sirius red (Polysciences, Warrington, PA) and fast green FCF (Sigma-Aldrich) to evaluate liver fibrosis, and Sirius red-positive area was quantified as described previously (Zhong et al., 2003). In other experiments, liver tissue was frozen and stored at -80°C for later mRNA analysis. Serum alanine transaminase (ALT) was measured using a kit from Pointe Scientific, Inc. (Canton, MI).
Intravital Confocal and Multiphoton Microscopies. Rhodamine 123 (Rh123; Sigma-Aldrich) was used to monitor mitochondrial polarization after cholestasis. Rh123, a cationic fluorophore, is taken up by polarized mitochondria and is released when mitochondria depolarize (Emaus et al., 1986). Propidium iodide (PI) was used to label nuclei of nonviable cells. At 6 h after BDL or sham operation, mice were anesthetized with pentobarbital (80 mg/kg i.p.) and connected to a small animal ventilator via a respiratory tube (20-gauge catheter) inserted into the trachea. Rh123 (2 μmol/mouse) and PI (0.04 μmol/mouse) were infused via polyethylene 10 tubing inserted into the carotid artery over 10 min. Preliminary studies showed that carotid injections yielded reliable and uniform labeling of hepatocytes by fluorophores without disturbance to hepatic blood supply.
MPT pore opening was assessed using calcein acetoxymethyl ester (AM), which is cleaved by esterases in the cytosol to form green-fluorescing calcein-free acid. Because mitochondria are normally impermeant to calcein, calcein is retained in the cytosol, leaving mitochondria as nonfluorescent voids (Nieminen et al., 1995). These voids disappear after onset of the MPT as calcein, a 623-Da solute, enters the mitochondrial matrix space through PT pores (Nieminen et al., 1995). Because of the expense of calcein-AM and the potential for its de-esterification before entry into the liver, calcein-AM (1 mg/mouse) was injected slowly into the rectal vein at 6 h after BDL or sham operation. To prevent biliary excretion of calcein, an anion channel inhibitor, bromosulfophthalein (6.6 μmol/mouse) was injected into the rectal vein 5 min before calcein-AM.
For intravital confocal microscopy, laparotomized mice were put in a prone position over a coverslip mounted on the stage of a CARV spinning disk confocal microscopic system (Atto Bioscience, Rockville, MD) with a 40× water immersion objective lens (Zeiss C-Apochromat, 1.2 numerical aperture) using excitation wavelengths of 488 and 555 nm, respectively, and a multiwavelength emission filter to detect Rh123 and PI fluorescence. The respirator was turned off for ∼5 s to eliminate breathing movement artifacts during image acquisition. Ten random fields were imaged per mouse. Bright green punctate Rh123 fluorescence represented cells with polarized mitochondria, whereas dimmer diffuse Rh123 fluorescence represented cells with depolarized mitochondria. Nonviable PI-positive cells, indicated by red nuclear fluorescence, were counted in the same fashion. Image analysis was performed in a blinded manner.
Green fluorescence of calcein was detected in some mice by intravital multiphoton microscopy using a Zeiss LSM 510 NLO laser scanning confocal/multiphoton microscope and a 63× C-apo numerical aperture 1.2 water immersion objective lens. Two-photon excitation of calcein was performed with 720 nm of light from a Coherent Chameleon Ultra laser, and emission was collected through a 500- to 550-nm bandpass emission filter. Images of approximately 10 random liver fields were collected from each mouse.
Immunohistochemical Detection of α-Smooth Muscle Actin and Cytochrome c. α-Smooth muscle actin (SMA) was detected immunohistochemically as described elsewhere (Zhong et al., 2003). Immunohistochemical staining of cytochrome c was performed using an InnoGenex Mouse-on-Mouse Iso-IHC kit (InnoGenex, San Ramon, CA) according to the manufacturer's instructions after applying primary antibody at 1:200 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at room temperature for 30 min.
Detection of Apoptosis. Apoptosis was assessed by terminal deoxynucleotidyltransferase dUTP nick-end labeling (TUNEL) using an In Situ Cell Death Detection Kit (Zhong et al., 2007). TUNEL-positive and -negative cells were counted in a blinded manner in 10 randomly selected fields using a 40× objective lens.
Activation of caspase-3 was assessed by Western blotting using an antibody specific for cleaved caspase-3. Liver tissue was harvested at 3 days after BDL when TUNEL peaked and was homogenized in 0.1% Triton X-100 buffer containing protease and phosphatase inhibitors. The extract was centrifuged at 14,000g for 15 min at 4°C, and aliquots of supernatant (40 μg of protein) were separated on 4 to 12% SDS-polyacrylamide gel electrophoresis gels, transferred onto nitrocellulose membranes, and immunoblotted with primary antibodies (Affinity Bioreagents, Golden, CO) specific for cleaved caspase-3 and actin (Cell Signaling Technology Inc., Danvers, MA; 1:1000 and 1:3000, respectively, overnight at 4°C). Horseradish peroxidase-conjugated secondary antibodies were applied, and detection was by chemiluminescence (ECL; GE Healthcare, Piscataway, NJ).
Detection of TNFα, TGF-β1, and Procollagen α1(I) mRNA by Quantitative Real-Time PCR. Total RNA from liver tissue was isolated with TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Single-stranded cDNAs were synthesized from RNA (2 μg) isolated from liver tissue using a Bio-Rad iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA). Primer sets were designed and synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). The primer sequences are listed in Table 1. PCRs were performed in triplicate with a reaction mixture containing 2× IQSYBR Supermix (Bio-Rad), cDNA template, and 0.1 nM of the forward and reverse primers. Real-time PCR was conducted using an iCycler iQ Multi-Color Real-Time PCR Detection System (Bio-Rad). iTaq DNA polymerase was activated at 95°C for 10 min, and 40 cycles of amplification at 95°C for 30 s and 62°C for 30 s were performed. Data were analyzed with MyiQ software. The abundance of mRNAs was normalized against the hypoxanthine phosphoribosyl-transferase (HPRT) housekeeping gene using the ΔΔCt method.
Primers for real-time PCR
Statistical Analysis. Groups were compared using analysis of variance plus Student-Newman-Keuls post hoc test. Data shown are means ± S.E.M. (four to five livers per group). Differences were considered significant at p < 0.05.
Results
NIM811 Decreases Liver Injury after Cholestasis. Histology revealed normal liver architecture after sham operation (Fig. 1, top left). After BDL, focal necrosis developed within 1 day (data not shown) and became more marked after 3 days (Fig. 1, middle left). Necrotic areas accounted for 11% of liver sections at 1 day after BDL, which increased to 20% after 3 days and 28% after 14 days (Fig. 2A). NIM811 decreased necrotic areas to 0.5, 3, and 8% at 1, 3, and 14 days, respectively (Figs. 1, bottom left, and 2A).
Serum ALT, an indicator of liver injury, averaged 58 U/l (Fig. 2B) after sham operation and was not altered by NIM811 treatment. After BDL, ALT increased to 2280 U/l after 1 day, decreasing to 1150 and 1600 U/l after 3 and 14 days (Fig. 2B). When mice were treated with NIM811, peak ALT after BDL was decreased by 60% and remained lower than the untreated group 2 weeks after BDL (Fig. 2B).
Apoptosis in liver sections was detected by TUNEL (Fig. 1, right column). TUNEL was rare in livers from sham-operated mice (0.13 cells/hpf) (Figs. 1, top right, and 2C). TUNEL did not increase at 1 day after BDL but increased markedly to 18 and 17 cells/hpf at 3 and 14 days after BDL, respectively (Figs. 1, middle right, and 2C). Increased TUNEL was almost completely blocked by NIM811 (Figs. 1, bottom right, and 2C). Occurrence of apoptosis was further confirmed by activation of caspase-3. Cleaved caspase-3 was barely detectable after sham operation but increased markedly at 3 days after BDL (Fig. 2D). This effect was largely blocked by NIM811 (Fig. 2D).
Taken together, these data show that NIM811 protects against hepatocellular injury and death at the early and late stages of cholestasis. Cell killing after BDL involved both necrosis and apoptosis, with necrosis occurring earlier than apoptosis. Specific inhibition of the MPT with NIM811 effectively prevented necrosis and apoptosis caused by cholestasis.
Mitochondrial Permeability Transition Occurs after Bile Duct Ligation: Prevention by NIM811. Previous studies showed that mitochondria of isolated hepatocytes generate ROS when exposed to hydrophobic BA, followed by MPT onset (Sokol et al., 1995; Rolo et al., 2003). Because mitochondrial depolarization is an immediate consequence of the MPT, we investigated whether mitochondrial depolarization occurs after cholestasis in vivo. At 6 h after BDL, Rh123, a green-fluorescing fluorophore taken up by polarized mitochondria, and PI, a red-fluorescing fluorophore staining nuclei of nonviable cells, were infused, and intravital confocal fluorescent microscopy was performed. Overlays of green and red images allowed identification of viable cells with polarized mitochondria (no red nuclear fluorescence, intracellular punctate mitochondrial green fluorescence), viable cells with depolarized mitochondria (no red fluorescence, no or diffuse green fluorescence), and nonviable cells (red nuclear fluorescence). In nonviable cells, mitochondria were consistently depolarized.
NIM811 mitigates hepatic necrosis and apoptosis after bile duct ligation. Livers were harvested 3 days after sham operation (Sham) or BDL. Hematoxylin and eosin (H+E) staining (left column) was performed to evaluate necrosis. TUNEL (right column) was performed to evaluate apoptosis. Arrows identify necrotic areas. White bar, 100 μm; black bar, 50 μm.
NIM811 decreases liver injury after bile duct ligation. Livers and blood were collected at 0, 1, 3, and 14 days after BDL. Necrosis (A) was quantified by image analysis of 10 random fields per liver. Blood was collected for ALT measurement (B). TUNEL-positive cells were counted in a blinded manner in 10 randomly selected fields using a 40× objective lens (C). *, p < 0.05 versus BDL of corresponding time point (n = 4–5 per group). Caspase-3 activation 3 days after BDL was evaluated by Western analysis of cleaved caspase-3, as illustrated in blot images representative of four experiments per group (D).
In sham-operated mice, green Rh123 fluorescence was punctate in virtually all hepatocytes, indicating mitochondrial polarization (Fig. 3, top left), and red PI labeling of nuclei was undetectable (Fig. 3, top left). At 6 h after BDL, many hepatocytes contained depolarized mitochondria that did not accumulate Rh123 (Fig. 3, top right). Other hepatocytes showed diffuse but relatively bright Rh123 fluorescence, possibly indicating the recent depolarization of mitochondria and release of mitochondrial Rh123 into the cytosol. Occasionally, some parenchymal and nonparenchymal cells (NPCs) were also labeled with PI (<1 cell/hpf, Fig. 3, top right). Most hepatocytes with depolarized mitochondria excluded PI, whereas PI-labeled hepatocytes never contained polarized mitochondria. These results suggest that at 6 h after BDL, mitochondrial depolarization occurred in many hepatocytes and that mitochondrial depolarization preceded hepatocyte death. After NIM811 treatment, mitochondrial depolarization decreased from 18 cells/hpf after vehicle to 5 cells/hpf (Fig. 3, bottom). PI-labeled cells were not different between the NIM811-treated and untreated groups because cell death was rare at this early stage of cholestasis.
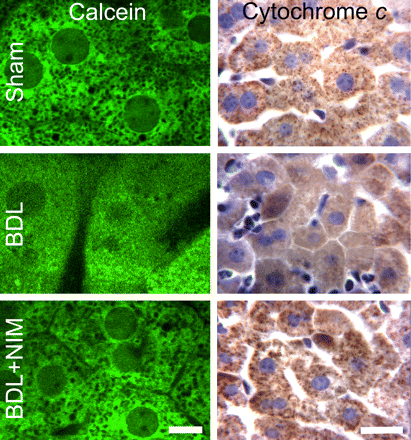
Calcein was used to monitor onset of the MPT by intravital multiphoton microscopy. Calcein loaded into the cytosol but did not permeate mitochondria. Thus, calcein fluorescence outlined individual mitochondria as dark voids in the hepatocytes of sham-operated mice (Fig. 4, top left). These voids disappeared at 6 h after BDL, indicating movement of calcein into mitochondria through opened PT pores after onset of the MPT (Fig. 4. middle left). This loss of calcein holes cannot be caused by premature cell death because cell death would cause loss of all calcein fluorescence. NIM811 effectively prevented calcein permeation into mitochondria in vivo after BDL (Fig. 4, bottom left). These findings showed directly that NIM811-sensitive mitochondrial inner membrane permeabilization occurred in vivo because of cholestasis.
Protection of NIM811 against mitochondrial depolarization after bile duct ligation. At 6 h after surgery, Rh123 and PI were infused, and intravital confocal microscopy was performed. Bar, 20 μm. Viable cells with polarized mitochondria, viable cells with depolarized mitochondria, and nonviable cells were counted in 10 random fields of each liver (bottom right panel). a, p < 0.05 versus sham; b, p < 0.05 versus BDL (n = 4–5 per group).
NIM811 Prevents Cytochrome c Release after Cholestasis. The MPT causes large-amplitude mitochondrial swelling, outer membrane rupture, and release of cytochrome c from the intermembrane space into the cytosol, which can trigger caspase activation and apoptosis (Zamzami et al., 1996; Kantrow et al., 2000). In accordance, we investigated cytochrome c release after BDL with and without NIM811 treatment. Cytochrome c detected immunohistochemically was punctate in hepatocytes of liver slides from sham-operated mice, consistent with mitochondrial localization (Fig. 4, top right). After BDL, cytochrome c staining became diffuse, indicating release of cytochrome c into the cytosol (Fig. 4, middle right). Release of cytochrome c after BDL was largely prevented by NIM811.
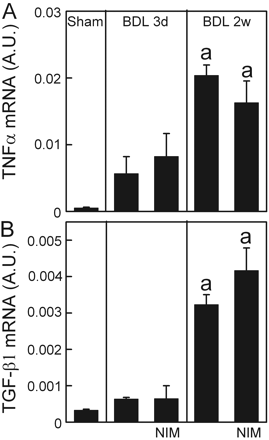
NIM811 Does Not Prevent Inflammatory and Profibrogenic Cytokine Formation after Bile Duct Ligation. Cell death after cholestasis may cause inflammation that subsequently leads to liver fibrosis. The proinflammatory cytokine, TNFα, plays an important role in liver fibrosis (Friedman, 1999). Therefore, we investigated whether NIM811 prevents TNFα expression after BDL. TNFα mRNA increased 11-fold at 3 days after BDL and 38-fold at 2 weeks in vehicle-treated mice (Fig. 5A). Surprisingly, NIM811 did not prevent these increases of TNFα expression after BDL despite protection against cell death (Fig. 5A).
NIM811 prevents onset of the mitochondrial permeability transition and cytochrome c release after bile duct ligation. At 6 h after surgery, calcein-AM was infused, and intravital multiphoton microscopy was performed (left column). Bar, 5 μm. In the right column, cytochrome c was detected by immunohistochemistry. Bar, 20 μm.
TGF-β increases in a variety of inflammatory processes and is an important promoter of hepatic fibrosis (Friedman, 1999). In accordance, we detected TGF-β expression after BDL. TGF-β1 mRNA increased 2-fold at 3 days after BDL and 10-fold after 2 weeks in vehicle-treated mice (Fig. 5B). Again, NIM811 did not prevent increases of TGF-β1 expression after BDL (Fig. 5B).
NIM811 Does Not Prevent Hepatic Fibrosis after Bile Duct Ligation. To evaluate the effects of NIM811 on liver fibrosis after BDL, liver sections were stained with Sirius red for collagen. No fibrosis was observed in livers from shamoperated mice (Fig. 6A, top left). In contrast, hepatic fibrosis developed within 2 weeks after BDL (Fig. 6A, middle left). Sirius red-positive area increased from 1% in sham-operated mice to 21% at 2 weeks after BDL (Fig. 6B, left). When mice treated with NIM811 were subjected to BDL, histology revealed similar fibrosis as vehicle treatment (Fig. 6A, bottom left). Sirius red-positive area was 23% at 2 weeks after BDL (Fig. 6B, left).
Hepatic collagen gene expression was evaluated by quantitative real-time PCR for procollagen α1(I) mRNA. At 2 weeks after BDL, procollagen α1(I) mRNA increased 19-fold compared with sham-operated mice (Fig. 6B, right). Treatment with NIM811 did not prevent the increase of procollagen α1(I) mRNA expression caused by cholestasis (Fig. 6B, right).
NIM811 does not prevent TNFα and TGF-β expression after bile duct ligation. Livers were harvested 3 days and 2 weeks after sham operation (Sham) or BDL. TNFα and TGF-β1 mRNAs were detected by quantitative real-time PCR. The abundance of TNFα and TGF-β1 mRNAs was normalized against HPRT using the ΔΔCt method. A.U., arbitrary unit. a, p < 0.05 versus sham; b, p < 0.05 versus BDL (n = 4 per group).
NIM811 Does Not Prevent Stellate Cell Activation after Bile Duct Ligation. Activated HSCs are the major source of matrix proteins in diseased liver (Friedman, 2008). In accordance, we evaluated αSMA, an indicator of HSC activation, by immunohistochemical staining after BDL. In livers from sham-operated mice, small amounts of αSMA were detected in the smooth muscle and endothelium of blood vessels (Fig. 6A, top right). At 2 weeks after BDL, αSMA increased markedly in perisinusoidal cells (Fig. 6A, middle right), indicating activation of HSCs. After BDL, αSMA increased to the same extent in livers of NIM811-treated mice as after vehicle treatment (Fig. 6A, bottom right). Taken together, these data show that NIM811 treatment did not prevent proinflammatory cytokine formation, fibrosis, and HSC activation caused by cholestasis.
Discussion
Mitochondrial Permeability Transition Plays an Important Role in Cholestasis-Induced Liver Injury in Vivo. In cholestasis, BA accumulates to cause hepatic injury. Mechanisms of BA-induced cell death are not fully understood. BA toxicity is not because of a detergent effect (Guicciardi and Gores, 2002). Several studies show that cytotoxic BA triggers hepatocyte apoptosis by activation of death receptors (Faubion et al., 1999; Fickert et al., 2005). Despite evidence of Fas and tumor necrosis factor-related apoptosis-inducing ligand-dependent apoptotic signaling in cholestatic liver injury, recent studies suggest that necrosis is the predominant form of cell death after BDL (Gujral et al., 2004; Fickert et al., 2005).
NIM811 does not prevent hepatic fibrosis and stellate cell activation after bile duct ligation. Livers were harvested 2 weeks after sham operation (Sham) or BDL. In A, Sirius red staining (left column) and immunohistochemical staining for αSMA (right column) were performed. Top, sham operation; middle, BDL; bottom, BDL plus NIM811 (NIM) treatment. White and black bars, 200 and 50 μm, respectively. In B, left, Sirius red-positive area was quantified by image analysis. In B, right, procollagen α1(I) mRNA was detected by quantitative real-time PCR. The abundance of procollagen α1(I) mRNA was normalized against HPRT using the ΔΔCt method. A.U., arbitrary unit. a, p < 0.05 versus sham; b, p < 0.05 versus BDL (n = 4 per group).
Oxidative stress and mitochondrial injury may also play a role in BA-induced cell death. Oxidative stress occurs in biliary obstructed rats (Parola et al., 1996; Pastor et al., 1997) and isolated hepatocytes and mitochondria exposed to hydrophobic BA (Sokol et al., 1995; Rolo et al., 2003). Antioxidants and overexpression of mitochondrial Mn-SOD decreased cell death after BDL (Zhong et al., 2002, 2003). ROS not only directly damage macromolecules but also alter signal transduction. For example, BA causes activation of NADPH oxidase, generating an ROS signal activating Yes-, c-Jun NH2-terminal kinase-, and epidermal growth factor receptor-dependent CD95 (Fas) tyrosine phosphorylation and formation of the death-inducing signaling complex (Reinehr and Haussinger, 2007). ROS also trigger opening of PT pores (Kantrow et al., 2000). Increased mitochondrial calpain-like protease activity during cholestasis might also induce MPT onset (Gores et al., 1998).
The MPT is an important mechanism leading to mitochondrial dysfunction and cell death (Lemasters, 2005). Despite extensive work, the exact molecular composition of PT pores remains unclear. In one model, PT pores are composed of the voltage-dependent anion channel, the adenine nucleotide translocator, cyclophilin D (CypD), and various ancillary proteins (Martinou and Green, 2001). However, recent studies using adenine nucleotide translocator and VDAC isoform knockout mice challenge this model (Kokoszka et al., 2004). An alternative model is that PT pores are formed by aggregation of misfolded and damaged membrane proteins in association with CypD and other molecular chaperones (He and Lemasters, 2002), and recent studies confirm that CypD is an important regulator of PT pore activity (Baines et al., 2005). MPT onset leads to collapse of the mitochondrial membrane potential, failure of ATP production, and necrotic cell death (Zamzami et al., 1996; Kim et al., 2003). Moreover, the MPT causes large amplitude swelling and release of cytochrome c into the cytosol, triggering apoptosis (Zamzami et al., 1996; Kantrow et al., 2000).
Both CsA and bongkrekic acid, inhibitors of the MPT, protect cultured hepatocytes from BA-induced cell death (Gores et al., 1998; Yerushalmi et al., 2001). Mitochondria isolated from cholestatic rats also exhibit disruption of mitochondrial calcium homeostasis by BA, which might increase susceptibility of mitochondria to calcium-induced MPT onset (Rolo et al., 2002). In addition, human liver mitochondria undergo the MPT in response to hydrophobic BA (Sokol et al., 2005). These data show that BA causes MPT onset in vitro. However, whether cholestasis-induced MPT onset occurs in live animals has never been determined. Using intravital confocal/multiphoton microscopy, here we show directly that mitochondrial depolarization and inner membrane permeabilization do occur in vivo after BDL (Fig. 3). This mitochondrial depolarization is caused by MPT onset, as indicated by entry of calcein into mitochondria after BDL (Fig. 4) and blockade of this permeabilization with the consequent depolarization by the specific MPT inhibitor, NIM811 (Figs. 3 and 4). Importantly, MPT onset and mitochondrial depolarization preceded necrosis and apoptosis (Figs. 3 and 4). Moreover, inhibition of MPT onset substantially attenuated cytochrome c release from mitochondria (Fig. 4). These data show clearly that the MPT plays an important role in cholestatic liver injury in vivo.
CsA at high therapeutic serum concentrations (0.17–0.8 μM) partially inhibits the canalicular bile salt excretion pump (BSEP) and the basolateral Na+/taurocholate-cotransporting polypeptide (NTCP) (Mita et al., 2006). Effects of NIM811 on BSEP and NTCP have not yet been reported, and a contribution of inhibition of bile salt transport to NIM811 cytoprotection after BDL cannot be excluded. However, in the present work, neither partial inhibition of BSEP nor NTCP would be expected to prevent hepatocellular accumulation of toxic BA because BSEP inhibition would only serve to increase intracellular BA concentration and because incomplete inhibition of NTCP would not prevent equilibration of BA accumulation inside hepatocytes with the plasmalemmal Na+ gradient. Future studies will be needed to better characterize the effects of NIM811 on hepatocellular BA transport.
Previous work establishes a role for death receptor activation in cholestatic liver injury (Faubion et al., 1999; Gujral et al., 2004; Fickert et al., 2005), but controversy still exists as to how death receptor activation leads to mitochondrial dysfunction, cytochrome c release, and cell death. Some studies support a mechanism in which caspase 8 activation causes tBid formation, which in turn activates Bax/Bak-dependent permeabilization of the outer membrane and cytochrome c release (Zhao et al., 2003). Other findings indicate that death receptor activation causes the MPT, an inner membrane event, which leads to either ATP depletion-dependent necrosis or caspase-dependent apoptosis (Lemasters, 2005). Our findings here suggest that the MPT is the predominant event precipitating phenotypes of both apoptotic and necrotic cell death in cholestasis, at least in the mouse model of BDL. Future studies need to assess role of MPT in Fas-dependent injury, such as Jo-2 antibody stimulation.
MPT Inhibition Does Not Prevent Hepatic Fibrosis after Bile Duct Ligation. Chronic cholestatic liver diseases cause liver cirrhosis (Poupon et al., 2000). Fibrogenesis in liver is likely an active wound-healing process regulated by cytokines, chemokines, and additional signals like ROS (Friedman, 2008). Hepatocytes release fibrogenic lipid peroxides (Novo et al., 2006), and lipid peroxidation associated with hepatocyte necrosis is considered a classical inflammatory and fibrogenic stimulus (Friedman, 2008). In addition, apoptotic hepatocytes can release cytoplasmic fragments that are fibrogenic toward cultured HSCs (Canbay et al., 2003). Therefore, we expected that prevention of cell injury by NIM811 would alleviate subsequent liver fibrosis. It is striking that although inhibition of the MPT by NIM811 effectively prevented cholestatic necrosis and apoptosis of hepatocytes through the whole cholestatic process (Figs. 1 and 2), NIM811 did not attenuate liver fibrosis after BDL (Fig. 6).
The dissociation of cytoprotection of NIM811 from antifibrogenesis suggests that the cell death may not play an essential role in cholestatic liver fibrosis. Although mechanisms of liver fibrosis are not well understood, the concept is generally accepted that prolonged liver injury triggers activation of HSCs and hepatic recruitment of inflammatory cells, effects likely mediated by inflammatory cytokines (Friedman, 1999). Therefore, we investigated whether NIM811 altered inflammatory cytokine production. Expression of TNFα, a major proinflammatory cytokine that is important in liver fibrosis (Friedman, 1999), increased after BDL, but this effect was not attenuated by NIM811 (Fig. 5). Likewise, NIM811 did not block expression of TGF-β, a major profibrogenic cytokine (Friedman, 1999), after BDL (Fig. 5). These unexpected findings suggest that inflammatory processes in cholestasis are not because of cell death but elicited mainly by direct effects of BA or other excretion products accumulated during cholestasis on inflammatory cytokine-producing cells, such as Kupffer cells and HSCs.
Activation of HSCs is a critical step in hepatic fibrogenesis (Friedman, 2008). Activated HSCs produce profibrogenic cytokines, such as TGF-β, synthesize collagen (Friedman, 1999; Kisseleva and Brenner, 2007; Parsons et al., 2007), and secrete inhibitors of matrix-degrading enzymes (Kisseleva and Brenner, 2007). Therefore, we investigated whether NIM811 prevented HSC activation. As expected, αSMA, a marker of HSC activation, dramatically increased after BDL (Fig. 6). Although NIM811 inhibits proliferation of cultured hepatic HSCs in vitro (Kohjima et al., 2007), NIM811 had no effect on αSMA production in vivo (Fig. 6). Thus, prevention of cell death did not block HSC activation after cholestasis, consistent with the conclusion that excess BA or other excretion products accumulated in cholestasis either activate HSCs directly or stimulate other NPCs (i.e., Kupffer cells, endothelial cells, and cholangiocytes) to release inflammatory or fibrogenic cytokines and growth factors that activate HSCs. BA maintains HSCs in an activated state (Brady et al., 1996). Other substances that accumulate during cholestasis (e.g., bilirubin, cholesterol, phospholipids, metals) may also stimulate liver fibrosis. For example, accumulated iron might stimulate ROS production to cause HSC activation. Moreover, high cholesterol activates HSCs and induces liver fibrosis (Kainuma et al., 2006). Future studies will be needed to better characterize these profibrotic mechanisms in cholestasis that are independent of hepatocellular death.
BDL represents acute and complete cholestasis that leads to overt cholangiocyte reactions. Whether independence of liver fibrosis from cell death as observed in acute BDL also occurs in cholestasis of more chronic onset or in other liver fibrosis models (e.g., CCl4, thioacetamide, ethanol) is still unknown and will also need to be the subject of future studies.
Taken together, the present study shows that the MPT occurs in vivo in hepatocytes during cholestasis and plays an essential role in necrotic and apoptotic hepatocellular death. NIM811, a specific inhibitor of the MPT, effectively prevents cell killing but does not prevent subsequent liver fibrosis. Liver fibrosis is possibly elicited by direct effects of BA on HSCs, cholangiocytes, and other NPCs.
Footnotes
-
This study was supported, in part, by the National Institutes of Health (Grants DK70844, DK073336, DK037034, and C06 RR015455).
-
The data presented here have appeared in abstract form as follows: Zhong Z, Curtin RA, Theruvath TP, Waldmeier PC, and Lemaster JJ (2006) NIM811, a mitochondrial permeability transition inhibitor, attentuates cholestatic liver injury. Gastroenterology 130:759.
-
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
-
doi:10.1124/jpet.108.143578.
-
ABBREVIATIONS: BA, bile acid; ROS, reactive oxygen species; PT, permeability transition; MPT, mitochondrial permeability transition; CsA, cyclosporine A; NIM811, N-methyl-4-isoleucine cyclosporine; HSC, hepatic stellate cell; BDL, bile duct ligation; ALT, alanine transaminase; Rh123, rhodamine 123; PI, propidium iodide; AM, acetoxymethyl ester; SMA, smooth muscle actin; TUNEL, terminal deoxynucleotidyltransferase dUTP nick-end labeling; TNF, tumor necrosis factor; TGF, transforming growth factor; PCR, polymerase chain reaction; HPRT, hypoxanthine phosphoribosyl-transferase; hpf, high-power field; NPC, nonparenchymal cell; CypD, cyclophilin D; BSEP, bile salt excretion pump; NTCP, Na+/taurocholate-cotransporting polypeptide.
- Received July 15, 2008.
- Accepted September 17, 2008.
- The American Society for Pharmacology and Experimental Therapeutics