Abstract
The pharmacological profiles of the novel acid pump antagonist 7-(4-fluorobenzyloxy)-2,3-dimethyl-1-{[(1S,2S)-2-methylcyclopropyl]methyl}-1H-pyrrolo[2,3-d]pyridazine (CS-526) were investigated in terms of hog gastric H+,K+-ATPase activity, gastric acid secretion, and acute gastroesophageal lesions in comparison with other proton pump inhibitors (PPIs). CS-526 inhibited H+,K+-ATPase activity in a concentration-dependent manner, with an IC50 value of 61 nM. The inhibitory effect of CS-526 on H+,K+-ATPase activity was more potent than that of any of the other PPIs examined. The inhibitory mechanism of CS-526 on H+,K+-ATPase was a competitive antagonism to the K+ binding site of H+,K+-ATPase, and it was also a reversible inhibition. In pylorus-ligated rats, intraduodenal or oral administration of CS-526 inhibited gastric acid secretion in a dose-dependent manner, and the ID50 values were 2.8 or 0.7 mg/kg, respectively. In Heidenhain pouch dogs, intrapouch administration of CS-526 inhibited histamine-stimulated gastric acid secretion in a dose- and retention time-dependent manner. In a reflux esophagitis model, intraduodenal and oral administration of CS-526 prevented esophageal lesions with ID50 values of 5.4 and 1.9 mg/kg, respectively. Lansoprazole prevented esophagitis only by intraduodenal administration (ID50 = 2.2 mg/kg). Furthermore, CS-526 inhibited acute gastric mucosal lesions. These data demonstrate that the novel acid pump antagonist CS-526 has potent antisecretory and antiulcer effects. These findings indicate that CS-526 would have a curative effect on gastroesophageal reflux disease via its potent antisecretory and antiulcer actions.
Proton pump inhibitors (PPIs) are now widely used for the treatment of acid-related diseases, such as peptic ulcer and gastroesophageal reflux (Robinson, 2001, 2005). Gastric H+,K+-ATPase is a proton pump located at the apical membrane of the parietal cells, which transport H+ into the canaliculus of parietal cells in exchange for K+. Substituted benzimidazoles, such as omeprazole, lansoprazole, and rabeprazole, inactivate the H+,K+-ATPase by covalently binding to the sulfhydryl group of H+,K+-ATPase, resulting in long-lasting inhibition of gastric acid secretion (Sachs et al., 1988). Although these derivatives exert superior healing of gastric acid-related diseases, long-term treatment with omeprazole causes bacterial overgrowth in the upper gut due to long-lasting gastric acid suppression (Larner and Lendrum, 1992). Long-lasting inhibition of gastric acid secretion also induces hypergastrinemia, resulting in the induction of gastric enterochromaffin-like cell carcinoids in rats and hyperplasia of enterochromaffin cells in humans (Lamberts et al., 1988, 1993; Lindberg et al., 1990; Herling and Weidmann, 1994; Freston et al., 1995).
Therapeutic doses of PPIs reach steady state and achieve their maximal effective levels after several days of typical dosing regimens (Tytgat, 2001). Because PPIs are activated in an acidic condition, PPIs can only inhibit H+,K+-ATPase that has already been activated and transferred to the apical membrane (Forte and Yao, 1996). The resting proton pumps are internalized in a tubular vesicle that is not transferred to the apical membrane, and they are recruited when new stimuli are achieved. PPIs cannot inhibit these resting proton pumps; therefore, the effect of PPIs on acid-related diseases is slow-onset.
Reversible acid pump antagonists (APAs), the other class of proton pump inhibitors, act by K+-competitive and -reversible binding to the gastric proton pump (Pope and Parsons, 1993). Major classes of APAs are imidazopyridine and acyl quinoline derivatives, such as SCH-28080 (Wallmark et al., 1987), BY841 (Kromer et al., 2000) and AZD0865 (Gedda et al., 2007), which were included in the former class. SK&F 96067 (Keeling et al., 1991), SK&F97574 (Pope et al., 1995), and DBM-819 (Cheon et al., 2001) were categorized into the latter class. Most of these compounds have been shown to inhibit H+,K+-ATPase in a K+-competitive mechanism without any covalent binding, thereby initiating reversible inhibition of H+,K+-ATPase activities. Moreover, APAs could bind at the K+ binding site of the nonphosphorylated resting proton pump (Munson and Sachs, 1988). Therefore, the onset of the effect by APAs is expected to be very rapid. In agreement with this expectation, Wurst and Hartmann (1996) reported that almost complete gastric acid inhibition by BY841 can be achieved within 30 min after administration of the first dosage.
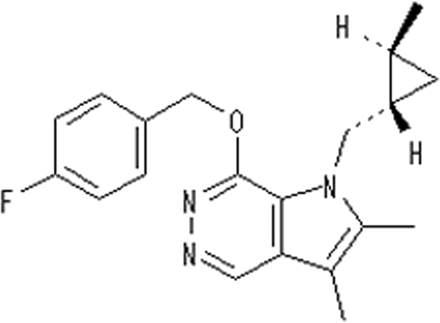
CS-526 (Fig. 1) is a novel acid suppressant that is different from PPIs and aforementioned APAs in that its chemical structure includes a pyrrolopyridazine structure. In the present study, we evaluated the inhibitory properties of CS-526 against porcine H+,K+-ATPase as an APA. The gastric acid inhibitory effects and antiulcer efficacies of CS-526 were also evaluated in comparison with those of other covalent binding PPIs, such as omeprazole, lansoprazole, and rabeprazole.
Materials and Methods
Chemicals. CS-526, R-99692, and SCH-28080 were synthesized in Ube Industries, Ltd. (Yamaguchi, Japan). Omeprazole, lansoprazole, and rabeprazole were extracted from Omepral (AstraZeneca K.K., Osaka, Japan), Takepron (Takeda Pharmaceutical Co., Ltd., Osaka, Japan), and Pariet (Eisai Co., Ltd., Tokyo, Japan), respectively.
Animals. All animal experimental procedures were performed in accordance with the Animal Experimentation Guidelines of Sankyo, Co., Ltd. (Tokyo, Japan).
In the present study, male Sprague-Dawley rats (Charles River Laboratories Japan, Inc., Kanagawa, Japan) weighing 170 to 250 g and male beagle dogs (Nosan Corporation, Kanagawa, Japan) weighing 8 to 13 kg were used.
Chemical structure of CS-526.
Purification of H+,K+-ATPase from Hog Gastric Mucosa. H+,K+-ATPase was prepared by the method of Im and Blakeman (1982) with some modifications. Fresh hog gastric mucosa was cut into small pieces with scissors, and it was homogenized in buffer (0.25 M sucrose, 2 mM MgCl2, 2 mM HEPES, and 1 mM EGTA, pH 7.4 with HCl) with a Waring blender and a tight-fitting Teflon-glass homogenizer. The homogenate was centrifuged for 45 min at 22,200g, and the supernatant was further centrifuged for 60 min at 100,000g. The pellets were resuspended in a buffer, and then they were layered onto a sucrose gradient. After centrifugation for 60 min at 75,000g, the white band on the 30% (w/w) sucrose was taken and centrifuged for 60 min at 100,000g. The pellets were resuspended in 8 ml of 0.25 M sucrose containing 1.3% (v/v) glycerin. This material was stored at –80°C. The protein content of the H+,K+-ATPase preparations was determined using a BCA protein assay kit (Pierce Chemical, Rockford, IL.)
Inhibitory Effect on H+,K+-ATPase Activity. The H+,K+-ATPase activity was determined as follows. An enzyme sample (20–50 μg/ml) was incubated at 37°C in 1 ml of a medium consisting of 40 mM Tris/acetic acid, pH 7.4, 2 mM MgCl2, 2 mM ATP. The H+,K+-ATPase activity was determined in the presence of 10 mM KCl. After preincubation with the compounds for 60 min, the inorganic phosphate released from ATP for 20 min was detected by the methods of Fiske and Subbarow (1925). In some experiments, H+,K+-ATPase activity was determined in the presence of dithiothreitol (DTT). Moreover, to investigate the reversibility of the inhibition on H+,K+-ATPase activity by the compounds, a washout procedure was conducted after preincubation with the compounds. The washout procedure was as follows. The incubation medium was centrifuged for 60 min at 100,000g, and supernatant was discarded. The precipitate was resuspended in freshly prepared incubation medium, and the mixture was further incubated to measure the residual activity of H+,K+-ATPase. The residual rate of activity after the washout procedure was from 61 to 91% in the vehicle treatment. In the kinetics study, we used R-99692, which is a racemic compound of CS-526, instead of CS-526. The pattern of inhibition by R-99692 was determined relative to the activation of H+,K+-ATPase activity by K+. Assays were performed in duplicate, and the data were then plotted by the method of Lineweaver and Burk (1934).
Inhibitory Effect on Na+,K+-ATPase Activity. Na+,K+-AT-Pase from canine kidney was purchased from Sigma-Aldrich (St. Louis, MO). Na+,K+-ATPase activity was determined as follows. An enzyme sample (0.1 U/ml) was incubated at 37°C in 1 ml of a medium consisting of 50 mM Tris, pH 7.5, 140 mM NaCl, 14 mM KCl, 5 mM MgCl2, and 3 mM ATP. Na+,K+-ATPase activity was determined in the absence or presence of 2.7 mM ouabain. After preincubation with the compounds for 60 min, the inorganic phosphate released from ATP for 10 min was detected by the methods of Fiske and Subbarow (1925).
Gastric Acid Secretion in Rats. The gastric acid secretion in the rats was determined using the pylorus ligation technique. Under ether anesthesia, the abdomen was incised and the pylorus was ligated. Four hours later, the animals were sacrificed by asphyxia with carbon dioxide, and the gastric contents were collected. The volume of the gastric contents was measured, and the acidity of the gastric contents was determined by titration to be pH 7.0 using 0.01 N NaOH (COMTITE-980; Hiranuma Sangyo Co., Ltd., Ibaraki, Japan). The titratable acid output was expressed as microequivalents per hour. Compounds suspended in 0.5% carboxymethylcellulose aqueous solution (w/v) were given orally or intraduodenally immediately after the ligation. The animals were deprived of food for approximately 16 to 19 h before the ligation, but they were allowed free access to water. In some experiments, compounds were given orally at 12 or 24 h before the pylorus ligation.
Gastric Acid Secretion in Heidenhain Pouch Dogs. Heidenhain pouches were prepared according to the conventional method (DeVito and Harkins, 1959) using a polyacetal fistula tube under anesthesia with pentobarbital. The postoperative animals were used for experiments at least 4 weeks after surgery.
The animals were deprived of food, but they were allowed free access to water for approximately 16 h before the experiments. After placing the animals in suspended-type dog restrainers, CS-526 or vehicle was put into the pouch and left for 180 min. Lansoprazole was orally administered. Then, 50 μg/kg/h histamine, a gastric acid stimulant, was administered by intravenous infusion at 5 ml/h, using an infusion pump, via a catheter in one of the anterior limb veins for 2 h. Gastric juice from the pouch was continuously collected every 15 min for 2 h, and then it was analyzed for volume and acidity. The acidity was determined by titration of the gastric juice against 0.01 N NaOH to pH 7.0. The titratable acid output was expressed as microequivalents per 15 min or 2 h.
Reflux Esophagitis Model. A reflux esophagitis model in rats was performed according to the method described by Goto and Kishi (1989). The rats were deprived of food, but they were allowed free access to water overnight before the following procedure was carried out. Under light ether anesthesia, the pylorus and the border between the forestomach and glandular stomach were ligated after the abdomen was incised. Six hours later, the rats were sacrificed by carbon dioxide asphyxiation. The abdominal and thoracic cavities were opened, and the entire section with the esophagus and stomach was removed. The esophagus and stomach section was inflated with 10 ml of 2% formalin aqueous solution, and it was fixed in the formalin, following which it was opened along the greater curvature. The total lesion area (LA), indicated by a black hemorrhage on the surface of the esophagus, was measured using an image analyzer (Luzex-F; NIREKO, Inc., Tokyo, Japan).
The lesions of each animal were scored according to the following criteria: 0, no lesion; 1, LA is over 0 mm2 and not more than 100 mm2; 2, LA is more than 100 mm2 and not more than 200 mm2; 3, LA is more than 200 mm2 and not more than 300 mm2; 4, LA is more than 300 mm2; 5, perforation; and 6, death from perforation. Scores of 5 and 6 are not concerned with LA.
Shay-Ulcer Model. The rats were deprived of food, but they were allowed free access to water for 2 days before the following procedure was carried out. Under ether anesthesia, the midline abdomen was cut open by incision, and the pylorus was ligated. Seventeen hours after the ligation, the rats were sacrificed by carbon dioxide asphyxiation. The abdominal cavity was opened, and the stomach was removed. The stomach was inflated with 10 ml of 1% formalin aqueous solution, and it was fixed in formalin, after which it was opened along the greater curvature. The total LA indicated by black hemorrhagic spots on the surface of the forestomach was measured using the image analyzer.
The lesions of each animal were scored according to the following criteria: 0, no lesion; 1, LA is more than 0 mm2 and not more than 10 mm2; 2, LA is more than 10 mm2 and not more than 20 mm2; 3, LA is more than 20 mm2 and not more than 30 mm2; 4, LA is more than 30 mm2; 5, perforation; and 6, death due to perforation. Scores of 5 and 6 are not concerned with LA.
Acute Gastric Mucosal Lesions in Rats. The effects on ammonia-(NH3-), acidified ethanol-(HCl/EtOH-), and indomethacin (IND-)-induced acute gastric mucosal lesions (AGML) were examined in rats. The rats were deprived of food, but they were allowed free access to water overnight before the following procedure was carried out. In NH3-or HCl/EtOH-AGML, the rats were orally given 1 ml of 2% ammonia or 60% ethanol with 150 mM hydrochloric acid and left for 1 h. In IND-AGML, the rats were administered indomethacin subcutaneously at a dose of 25 mg/kg and left for 7 h.
Thereafter, the rats were sacrificed by carbon dioxide asphyxiation, and the lesion areas (redness of mucosa with NH3-induced lesions; black hemorrhagic band, or spot with HCl/EtOH- and IND-AGML-induced lesions) formed on the surface of the gastric mucosa were measured after fixation in formalin using an image analyzer. All lesion area was measured by specified pair of observers (F. Inaba and Y. Morikawa-Inomata) who were unaware of the treatment.
Statistical Analysis. Microsoft Excel (Microsoft, Redmond, WA) and SAS System for Windows (SAS Institute, Cary, NC) were used for the statistical analysis. The mean ± S.E. was calculated for each group. The concentration or dose at which the compound exerted 50% inhibition (IC50 or ID50) was calculated from a concentration (dose)-inhibition rate curve prepared from the relation of the individual inhibition rate at each concentration (dose) level versus the logarithmic concentration (dose) by the least-squares method. The effects of the compounds on gastric acid secretion and AGML were compared with those of corresponding vehicle-treated group by a Student's t test or a Dunnett's multiple comparison test. In the reflux esophagitis and Shay-ulcer model, the effect of CS-526 was compared with that of the control by a nonparametric Dunnett's test. Last, the effect of CS-526 on AGML was compared with that of PPIs by an analysis of the covariance or a two-way analysis of the variance.
Results
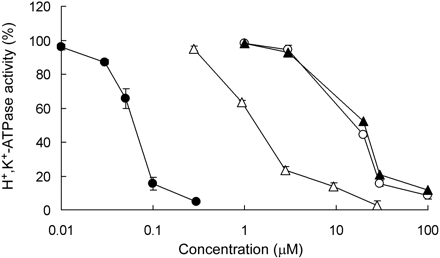
Effect of CS-526 and PPIs on Hog Gastric H+,K+-ATPase and Canine Kidney Na+,K+-ATPase Activity. The inhibitory effects of CS-526, omeprazole, lansoprazole, and rabeprazole on hog gastric H+,K+-ATPase activity are shown in Fig. 2. CS-526 inhibited H+,K+-ATPase activity in a concentration-dependent manner. The IC50 value of CS-526 was 61 nM (n = 3). Omeprazole, lansoprazole, and rabeprazole also inhibited H+,K+-ATPase activity in a concentration-dependent manner, and the IC50 values were 11, 12, and 1.7 μM, respectively (n = 3).
The IC50 values of CS-526, omeprazole, lansoprazole, and rabeprazole on canine kidney Na+,K+-ATPase activity are shown in Table 1. The IC50 values were 10.4, 718.6, 278.2, and 53.7 μM, respectively. The ratio of the IC50 value of CS-526 on Na+,K+-ATPase against H+,K+-ATPase was higher than that of any other PPIs (CS-526, 170.5; omeprazole, 65.3; lansoprazole, 23.2; and rabeprazole, 31.6).
IC50 values for CS-526, omeprazole, lansoprazole, and rabeprazole on H+,K+-ATPase and Na+,K+-ATPase
The ratio of IC50 values of CS-526 and other PPIs on Na+,K+-ATPase against H+,K+-ATPase was calculated by following equation: IC50 value of Na+,K+-ATPase/IC50 value of H+,K+-ATPase.
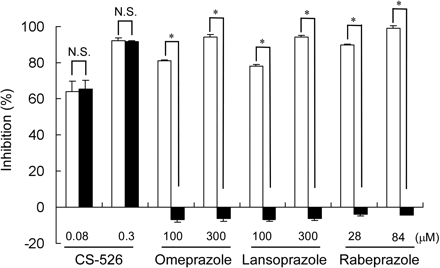
Effect of DTT on H+,K+-ATPase Inhibitory Effect of CS-526 and PPIs. The effects of DTT on hog gastric H+,K+-ATPase activity inhibition by CS-526 and PPIs are shown in Fig. 3. The inhibition rate of CS-526 at concentrations of 0.08 and 0.3 μM in the absence of DTT was 63.7 ± 6.1 and 92.0 ± 1.5%, respectively. In the presence of 100 μM DTT, the inhibition rate of CS-526 was not affected (65.4 ± 5.0% at 0.08 μM and 91.6 ± 0.5% at 0.3 μM). In contrast, the inhibitory effect of PPIs on H+,K+-ATPase activity disappeared in the presence of DTT (omeprazole DTT (–), 81.1 ± 0.7% at 100 μM and 94.2 ± 1.5% at 300 μM; omeprazole DTT (+), –6.9 ± 1.6% at 100 μM and –6.3 ± 1.7% at 300 μM; lansoprazole DTT (–), 78.0 ± 0.8% at 100 μM and 94.3 ± 0.6% at 300 μM; lansoprazole DTT (+), –6.7 ± 1.1% at 100 μM and –6.1 ± 1.0% at 300 μM; rabeprazole DTT (–), 89.7 ± 0.6% at 28 μM and 99.1 ± 1.2% at 84 μM; and rabeprazole DTT (+), –3.7 ± 1.4% at 28 μM and –4.4 ± 0.1% at 84 μM).
Effect of CS-526 (closed circles), omeprazole (open circles), lansoprazole (closed triangles), and rabeprazole (open triangles) on hog gastric H+,K+-ATPase activity. The enzyme was preincubated for 60 min with different concentrations of CS-526 or other PPIs. Activity without compounds was taken as 100%. Each point represents the mean ± S.E. of three different experiments.
Effect of DTT on H+,K+-ATPase inhibition by CS-526, omeprazole, lansoprazole, and rabeprazole. The inhibitory effects of CS-526, omeprazole, lansoprazole, and rabeprazole on H+,K+-ATPase activity were measured in the presence (closed columns) and absence (open columns) of 100 μM DTT. Activity without compounds was taken as 100%. Results are expressed as the mean ± S.E. of three different experiments. A t test was performed for a comparison of the effects in the presence or absence of DTT. *, P < 0.05; N.S., not significant.
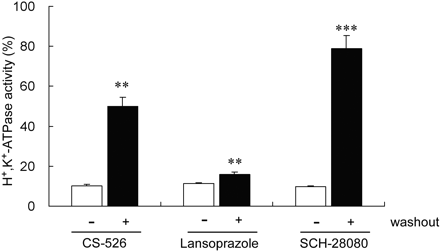
Effect of Washout on Inhibitory Effect Induced by CS-526 and Other PPIs. The inhibitory effects of CS-526, lansoprazole, and SCH-28080 on hog gastric H+,K+-ATPase activity with or without a washout procedure are shown in Fig. 4. CS-526 at a concentration of 0.3 μM inhibited H+,K+-ATPase activity, and the relative enzyme activity was 10.1 ± 1.0% (n = 5) (Fig. 4). Then, we examined the effect of a washout procedure on the inhibitory effect of CS-526. The inhibitory effect of CS-526 on H+,K+-ATPase activity was attenuated with the washout procedure (washout (–); 10.1 ± 1.0% versus washout (+); 50.2 ± 4.2% (n = 5). Lansoprazole (100 μM) also inhibited H+,K+-ATPase activity, and the relative enzyme activity was 11.2 ± 0.6% (n = 5). However, the inhibitory effect of lansoprazole on H+,K+-ATPase activity after the washout procedure was still low (15.9 ± 1.0%; n = 5), although it was significantly different (P = 0.0047) from the value without the washout procedure. SCH-28080 is well known as a reversible H+,K+-ATPase inhibitor (Beil et al., 1986; Keeling et al., 1989; Pope and Parsons, 1993). SCH-28080 (10 μM) inhibited H+,K+-ATPase activity, and the relative enzyme activity was 9.8 ± 0.5% (n = 5). But, the inhibitory effect of SCH-28080 on H+,K+-ATPase activity disappeared as a result of the washout procedure (relative enzyme activity, 78.9 ± 6.6%; n = 5).
Inhibitory effect of CS-526, lansoprazole, and SCH-28080 on H+,K+-ATPase activity with and without a washout procedure. The inhibitory effect of CS-526, lansoprazole, and SCH-28080 on H+,K+-ATPase activity were measured with (closed columns) and without (open columns) a washout procedure. The enzyme was incubated with the compounds for 60 min at 37°C. After centrifugation at 100,000g for 60 min, the precipitates were resuspended in a new medium, and the enzyme activity was measured. Results are expressed as the mean ± S.E. of five different experiments.
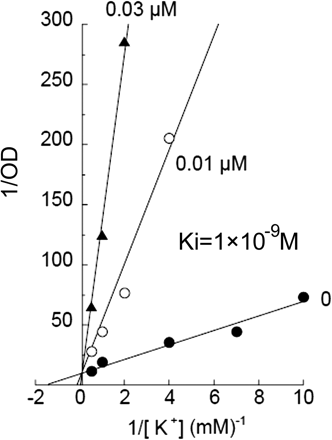
Kinetics of Inhibitory Effect Induced by CS-526 on H+,K+-ATPase Activity. K+ is transported actively from the luminal side to the cytosolic side of the gastric vesicle in exchange for H+, i.e., K+ acts as a substrate of the ATPase. We studied the effect of the K+ concentration in medium against the inhibitory activity of R-99692, which is a racemic compound of CS-526. Figure 5 shows the Lineweaver-Burk (double-reciprocal) plots between the H+,K+-ATPase activity (optical density) and K+ concentration. The plots show that the mechanism of inhibition on H+,K+-ATPase by R-99692 is a competitive type against the K+ concentration, with Ki value of 1 × 10–9 M, indicating that R-99692 competitively binds at the K+ binding site of H+,K+-ATPase.
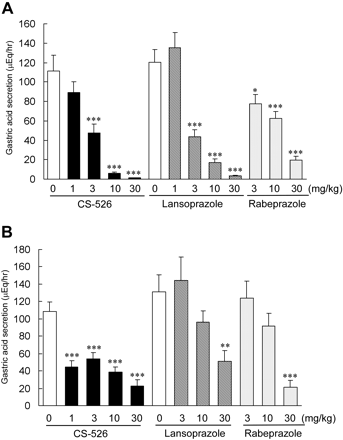
Effect of CS-526 on Gastric Acid Secretion in Pylorus-Ligated Rats. The antisecretory effects of CS-526 and other PPIs by intraduodenal administration on pylorus-ligated rat gastric acid secretion are shown in Fig. 6A. CS-526, lansoprazole, and rabeprazole dose-dependently inhibited gastric acid secretion in pylorus-ligated rats. The ID50 values were 2.8, 4.1, and 7.1 mg/kg, respectively (n = 4–11). In oral administration of these compounds after the pylorus ligation to avoid transfer of the compounds into the duodenum, each compound also inhibited the gastric acid secretion in a dose-dependent manner (Fig. 6B). However, the potency of the inhibitory effect by oral administration was different from that by intraduodenal administration. The ID50 values of CS-526, lansoprazole, and rabeprazole were 0.7, 21, and 13 mg/kg, respectively (n = 7–12). The inhibitory effects of lansoprazole and rabeprazole by oral administration were diminished in comparison with those by intraduodenal administration.
Lineweaver-Burk plots between the H+,K+-ATPase activity and medium concentration of K+ (0.5–10 mM). The concentrations of R-99692 were 0 μM (closed circles), 0.01 μM (open circles), and 0.03 μM (closed triangles). Typical results in one of several experiments are shown.
Effect of intraduodenal (A) and oral (B) administration of CS-526, lansoprazole, and rabeprazole on gastric acid secretion in pylorus-ligated rats. Gastric contents were collected for 4 h after ligation. Results are expressed as the mean ± S.E. of acid output obtained from 4 to 12 rats. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 compared with respective control by a Dunnett's test.
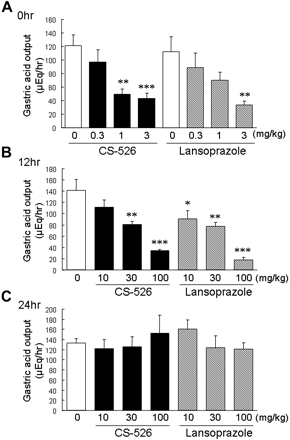
The duration of the gastric antisecretory effect of CS-526 in rats was examined in comparison with that of lansoprazole. CS-526 (orally, 0.3–3 mg/kg) and lansoprazole (intraduodenally, 0.3–3 mg/kg), administered immediately after the pylorus ligation, inhibited rat gastric acid secretion in a dose-dependent manner (Fig. 7A). The ID50 values were 1.1 and 1.4 mg/kg, respectively (n = 8–11). When the compounds were administered orally at 12 h before the pylorus ligation, the inhibitory effects of CS-526 and lansoprazole were diminished in comparison with those when administered immediately after the pylorus ligation (Fig. 7B). The ID50 values were 36 and 24 mg/kg, respectively (n = 6–9). When administered at 24 h before the pylorus ligation, the inhibition of gastric acid secretion by CS-526 and lansoprazole was not observed even at a dose of 100 mg/kg (Fig. 7C).
Effect of CS-526 and lansoprazole administered immediately (A), 12 h (B), and 24 h (C) before the pylorus ligation, on gastric acid secretion. Gastric contents were collected for 4 h after ligation. Results are expressed as the mean ± S.E. of acid output obtained from 6 to 11 rats. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 compared with respective control by a Dunnett's test.
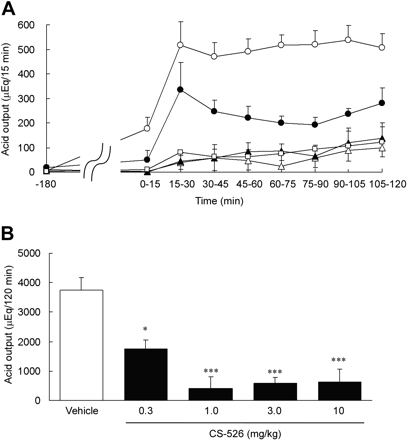
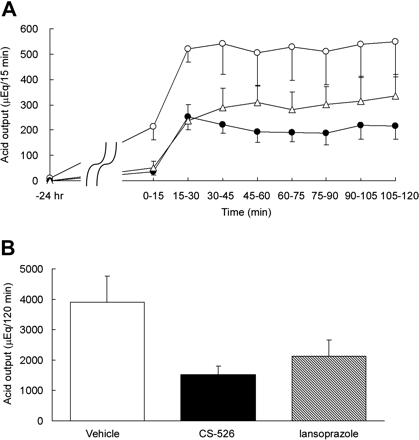
Effect of CS-526 on Gastric Acid Secretion in Heidenhain Pouch Dogs. The vehicle for the CS-526 and lansoprazole (0.4% Tween 80 + 0.5% carboxymethylcellulose) was put into the Heidenhain pouches for 180 min. Afterward, histamine caused a marked increase in gastric acid secretion, and maximum secretion was observed within 60 min with a steady state of approximately 500 microequivalents/15 min (Fig. 8A). CS-526, administered intrapouch for 180 min, dose-dependently inhibited the histamine-induced gastric acid secretion in the Heidenhain pouch dogs (Fig. 8A). The total acid output for 120 min is shown in Fig. 8B. The inhibitory effects of CS-526 on histamine-stimulated gastric acid secretion at doses of 0.3 to 10 mg/kg were statistically significant compared with the vehicle treatment (inhibition rate: 0.3 mg/kg, 52.8%; 1 mg/kg, 88.9%; 3 mg/kg, 84.2%; and 10 mg/kg, 83.5%; n = 3–6).
Change in the Antisecretory Effect of CS-526 by Retention Time into Heidenhain Pouch. Next, we investigated the effect of the retention time in the Heidenhain pouches on the antisecretory effect of CS-526 at the dose of 1.0 mg/kg. CS-526 was put into the Heidenhain pouches for 30, 60, and 180 min before histamine infusion. CS-526 inhibited the histamine-stimulated gastric acid secretion in Heidenhain pouch dogs in a time-dependent manner (Table 2). The inhibitory effect of CS-526 on the histamine-stimulated gastric acid secretion at retention times of 30, 60, and 180 min after intrapouch administration were statistically significant compared with the vehicle treatment (inhibition rate after 30 min, 53.6%; 60 min, 60.7%; and 180 min, 88.9%; n = 3–6).
Change in the antisecretory effect of CS-526 by retention time into Heidenhain pouch
CS-526 (1 mg/kg) was administered into the Heidenhain pouch for various periods before histamine infusion. Data represent the mean ± S.E. obtained from three to six dogs.
Effect of intrapouch administration of CS-526 on histamine-stimulated gastric acid secretion in Heidenhain pouch dogs. A, various concentrations of CS-526 were administered intrapouch for 180 min before histamine infusion: 0 mg/kg (open circles), 0.3 mg/kg (closed circles), 1 mg/kg (open triangles), 3 mg/kg (closed triangles), and 10 mg/kg (open squares). Each point represents the mean ± S.E. of the acid output obtained from three to six dogs. B, total gastric acid secretion for 2 h after vehicle or CS-526 administration. Each column represents the mean ± S.E. of the acid output obtained from three to six dogs. *, P < 0.05 and ***, P < 0.001 versus vehicle.
Effect of CS-526 and Lansoprazole on Histamine-Stimulated Gastric Acid Secretion in Heidenhain Pouch Dogs. The gastric antisecretory effect of CS-526 was compared with that of the conventional proton pump inhibitor lansoprazole. Each compound was administered at the dose of 10 mg/kg. CS-526 and lansoprazole were given by intrapouch and oral administration, respectively. CS-526 and lansoprazole inhibited histamine-stimulated gastric acid secretion in Heidenhain pouch dogs, even at 24 h after administration (Fig. 9A). The inhibition rate of CS-526 and lansoprazole on histamine-stimulated total gastric acid secretion for 2 h was 61.1 and 45.8%, respectively (Fig. 9B). No difference in the gastric antisecretory effect was observed between the CS-526-treated and the lansoprazole-treated group.
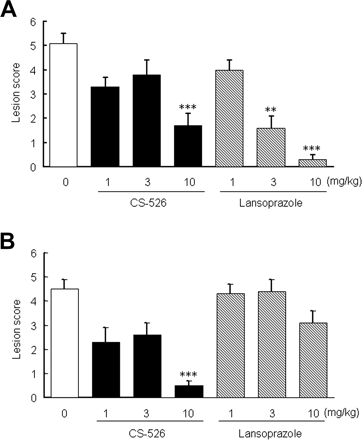
Effect of CS-526 and Lansoprazole on a Reflux Esophagitis Model in Rats. We examined the effect of CS-526 and lansoprazole on a reflux esophagitis model in rats. Six hours after the pylorus-ligation and the border between the forestomach and glandular stomach-ligation, hemorrhagic lesions and perforation of the esophagus were observed in the control group. Intraduodenal administration of CS-526 at 10 mg/kg significantly reduced the lesion scores (Fig. 10A). CS-526 also inhibited the lesion scores at 10 mg/kg by oral administration (Fig. 10B). The ID50 values of the intraduodenal and oral administration of CS-526 were 5.4 and 1.9 mg/kg, respectively (n = 9–11). Lansoprazole (3 and 10 mg/kg) significantly inhibited the lesion score by intraduodenal administration (Fig. 10A), with an ID50 value of 2.2 mg/kg. However, orally administered lansoprazole did not reduce the lesion score (Fig. 10B).
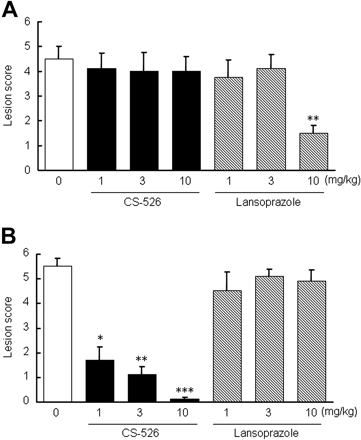
Effect of CS-526 and Lansoprazole on a Shay-Ulcer Model in Rats. We examined the effect of CS-526 and lansoprazole on a Shay-ulcer model in rats. Seventeen hours after the pylorus ligation, hemorrhagic lesions and perforation of the forestomach were observed in the control group. Intraduodenal administration of CS-526 did not inhibit lesion formation, even at the dose of 10 mg/kg (Fig. 11A). However, orally administered CS-526 significantly reduced the lesion scores from 1.0 to 10 mg/kg (Fig. 11B). The ID50 values of the intraduodenal and oral administration of CS-526 were >10 and <1.0 mg/kg, respectively (n = 10). Lansoprazole (10 mg/kg) significantly inhibited the lesion score by intraduodenal administration, with the ID50 value of 7.4 mg/kg (Fig. 11A). However, oral administration of lansoprazole did not reduce the lesion score (Fig. 11B).
Effects of CS-526 and lansoprazole on histamine-stimulated gastric acid secretion in Heidenhain pouch dogs. CS-526 (10 mg/kg) and lansoprazole (10 mg/kg) were given 24 h before histamine infusion by intrapouch (retention time at 3 h) and orally, respectively. Each point represents the mean ± S.E. of the acid output obtained from five to six dogs. Control (open circles), CS-526 (closed circles), and lansoprazole (open triangles). B, total gastric acid secretion for 2 h after vehicle or CS-526 administration. Each column represents the mean ± S.E. of lesion scores obtained from five to six dogs.
Effect of intraduodenal (A) and oral (B) administration of CS-526 and lansoprazole on reflux esophagitis model in rats. The pylorus and the border of the forestomach and glandular stomach were ligated. Compounds or vehicle was given immediately after the ligation. Rats were sacrificed 6 h after the ligation, and the lesion area was measured. Results are expressed as the mean ± S.E. of the lesion score obtained from 9 to 11 rats. **, P < 0.01 and ***, P < 0.001 compared with respective control by a nonparametric Dunnett's test.
Effect of intraduodenal (A) and oral (B) administration of CS-526 and lansoprazole on a shay-ulcer model in rats. The pylorus was ligated. Compounds or vehicle was given immediately after the ligation. Rats were sacrificed 17 h after the ligation, and the lesion area was measured. Results are expressed as the mean ± S.E. of lesion score obtained from 10 to 12 rats. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 compared with respective control by a nonparametric Dunnett's test.
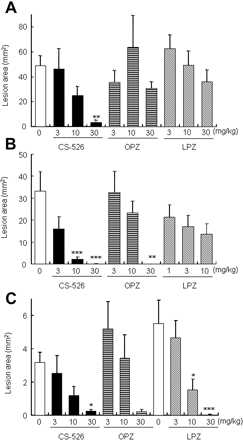
Effect of CS-526 and PPIs on Acute Gastric Mucosal Lesion Models in Rats. In an NH3-AGML model, redness of the gastric mucosa and black hemorrhagic lesions were observed macroscopically in the vehicle-treated control rats. CS-526 prevented the formation of lesions dose-dependently in the dose range of 3.0 to 30 mg/kg, with an ID50 (95% CI) value of 9.9 mg/kg (4.2–25), and it significantly inhibited lesion formation at a dose of 30 mg/kg (P = 0.0060; Fig. 12A). In contrast, omeprazole and lansoprazole did not prevent lesion formation up to the dose of 30 mg/kg (Fig. 12A). In this model, the effect of CS-526 was not significantly more potent than that of omeprazole (P = 0.1021; Table 3), but it was significantly more potent than that of lansoprazole (P = 0.0050; Table 3).
ID50 values of CS-526, omeprazole, and lansoprazole on NH3-, acidified ethanol-, and IND-induced AGML
The inhibitory effect of CS-526 was compared with that of omeprazole and lansoprazole by analysis of covariance.
In HCl/EtOH-AGML, CS-526 treatment diminished the lesion area dose-dependently in the dose range of 3.0 to 30 mg/kg, with an ID50 value less than 3.0 mg/kg, and significant inhibition of lesion formation was observed at the doses of 10 and 30 mg/kg (P = 0.0004 and P = 0.0002, respectively; Fig. 12B). Omeprazole and lansoprazole also inhibited lesion formation, with ID50 (95% CI) values of 11 mg/kg (6.0–24) and 3.8 mg/kg (not determined), respectively (Table 3). In this model, the effect of CS-526 was significantly more potent than that of omeprazole (P = 0.0037; Table 3), but it was not more potent than that of lansoprazole (P = 0.1929; Table 3).
Effect of CS-526, omeprazole, and lansoprazole on ammonia-(A), acidified ethanol-(B), and indomethacin-induced (C) acute gastric mucosal lesion models in rats. Results are expressed as the mean ± S.E. of the lesion areas obtained from 8 to 11 rats. OPZ, omeprazole; LPZ, lansoprazole. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 compared with respective control by a Dunnett's test.
In IND-AGML, CS-526 treatment diminished the lesion area dose-dependently in the dose range of 3.0 to 30 mg/kg, with an ID50 (95% CI) value of 7.4 mg/kg (0.41–2.4), and a significant inhibition of lesion formation was observed at the dose of 30 mg/kg (P = 0.0135; Fig. 12C). Omeprazole and lansoprazole also inhibited lesion formation, with ID50 (95% CI) values of 18 mg/kg (9.2–150) and 6.9 mg/kg (3.9–10), respectively (Table 3). In this model, the effect of CS-526 was significantly more potent than that of omeprazole (P = 0.0467; Table 3), but it was not more potent than that of lansoprazole (P = 0.6230; Table 3).
Discussion
In the present study, CS-526 exerted a more potent inhibitory effect on hog gastric H+,K+-ATPase activity than any other PPIs, such as omeprazole, lansoprazole, and rabeprazole (Fig. 2). CS-526 and other PPIs also inhibited canine kidney Na+,K+-ATPase activity at higher concentrations than those that inhibited H+,K+-ATPase (Table 1). However, the ratio of the IC50 value of CS-526 against Na+,K+-ATPase activity to that against H+,K+-ATPase activity was highest among these PPIs values. Therefore, we concluded that CS-526 was more selective to H+,K+-ATPase than Na+,K+-AT-Pase in comparison with PPIs.
The inhibitory effect of PPIs against H+,K+-ATPase is known to be abolished by the treatment of sulfhydryl-blocker or sulfhydryl-containing compounds such as N-ethylmaleimide or DTT (Nagaya et al., 1989), which interfere with the making of the covalent bond between PPIs and sulfhydryl group of cysteine in ATPase. In this study, the inhibitory effect of PPIs was almost abolished by DTT treatment, but that of CS-526 was not affected (Fig. 3). To analyze the enzyme kinetics, the effect of CS-526 on H+,K+-ATPase activity was studied by varying the K+ concentration (Fig. 5). A Lineweaver-Burk plot analysis showed that the kinetic pattern of inhibition of CS-526 (R-99692) on H+,K+-ATPase is a competitive type with respect to the potassium ion. In another experiment, the H+,K+-ATPase activity inhibited by CS-526 was partly restored and that by SCH-28080 was fully restored by the washout procedure (Fig. 4). In contrast, we assumed that the effect of lansoprazole, an irreversible proton pump inhibitor, on H+,K+-ATPase activity would almost never be affected by the washout procedure, although a significant difference was in fact observed. These results suggest that the inhibitory manner of CS-526 against H+,K+-ATPase would be potassium-competitive and reversible without making a covalent bond, and we confirmed that CS-526 would be one of the APAs. Because H+,K+-ATPase activity was not fully recovered after the washout of CS-526, we considered that CS-526 would bind the H+,K+-ATPase tightly, at least more than SCH-28080. This relatively tight binding character of CS-526 on H+,K+-ATPase may cause long-lasting inhibition of gastric acid secretion in rats and dogs (Figs. 7 and 9).
The in vivo antisecretory effects of CS-526 were investigated in pylorus-ligated rats and Heidenhain pouch dogs. In the rat study, CS-526 inhibited gastric acid secretion by intraduodenal and oral administration after a pylorus ligation (Fig. 6). CS-526 and its metabolite were shown in the plasma after intraduodenal administration of CS-526; however, neither CS-526 nor its metabolite was observed following the oral administration after the pylorus ligation (data not shown). This means that CS-526 was not absorbed from the stomach. In the Heidenhain pouch dog model, we showed that the intrapouch application of CS-526, which is also not considered to absorb into general circulation, inhibited gastric acid secretion (Fig. 8). These results suggest that CS-526 would inhibit gastric acid secretion by binding with H+,K+-ATPase in the parietal cells via not only general circulation following absorption from the small intestine but also by direct penetration into the mucosa from the gastric lumen. Lansoprazole and rabeprazole may also directly act on parietal cells via penetration of the mucosa from the gastric lumen, because they also inhibited gastric acid by oral administration after pylorus ligation in rats. However, the potencies of lansoprazole and rabeprazole in oral administration were attenuated, as shown when their ID50 values increased 5- and 2-fold from those by intraduodenal administration, respectively, due to their acid-labile properties. Okabe et al. (1995) also reported that one of PPI (leminoprazole) inhibited gastric acid secretion by an intra-Heidenhain pouch application in dogs. In the intrapouch application of CS-526, we showed a retention time dependence of the gastric antisecretory effect in Heidenhain pouch dogs (Table 2). At least 180 min is required to achieve the maximum acid inhibition for CS-526 in this system. Because the gastric mucus gel layer covered on the surface of gastric mucosa is a physiological barrier from noxious agents and the acid secreting parietal cells that have H+,K+-ATPase are located in the lower half of the gastric pit, we supposed that it could take a relatively long time for CS-526 to penetrate the gel layer and reach its action site via physical simple diffusion.
It is reported that the lesions in the esophagus caused by an experimental reflux esophagitis model are prevented by the treatment of H2 receptor antagonists (H2RAs) and PPIs (Goto and Kishi, 1989; Inatomi et al., 1991). This indicates that gastric acid would make key contributions to the formation of these lesions. In the present study, CS-526 significantly inhibited esophageal lesions by oral and intraduodenal administration, with ID50 values of 1.9 and 5.4 mg/kg, respectively (Fig. 10), and these values were close to the ID50 against acid secretion of CS-526. Likewise, the effect of lansoprazole against this lesion seemed to be closely related to its acid inhibitory efficacy. Intraduodenal administration of lansoprazole significantly inhibited esophageal lesions, with an ID50 value of 2.2 mg/kg, but it did not inhibit esophageal lesions by oral administration up to 10 mg/kg (Fig. 10). Thus, it was suggested that the mechanism of the inhibitory effect by CS-526 and lansoprazole on reflux esophagitis seems to be their gastric antisecretory effect.
The acid inhibitory effect of CS-526 and lansoprazole was attenuated by time (Fig. 7, A–C), and the duration after the pylorus ligation was longer in the Shay-ulcer model (17 h) than in reflux esophagitis (6 h). As we expected, the effect of lansoprazole against Shay-ulcer was weaker than that against reflux esophagitis, as shown by the increment of ID50. However, it is strange that the effect of CS-526 in oral administration against Shay-ulcer was enormously potent; a significant effect was observed even at the dose of 1 mg/kg, and it was obviously more potent than that against the reflux esophagitis. Although the reason why such potentiation occurred was ambiguous, we speculated that CS-526 would exert cytoprotective efficacy on the forestomach in addition to its acid inhibitory effect. It is also possible that the acid-inhibitory efficacy would be maintained when CS-526 was not transported to the small intestine and it was not metabolized. If this is true, then CS-526 could have a more potent inhibitory effect on gastric acid secretion when the gastroretentive formulation was realized, and this would be one of the differentiating points for acid-stable APAs against acid-labile PPIs.
The potent effect of CS-526 on various acute gastric mucosal lesions that were caused by clinically probable stimuli was confirmed. The most remarkable point was the effect on NH3-AGML. Ammonia is produced by the urease of Helicobacter pylori in the gastric mucosa, and it is considered to be one of the pathogens for H. pylori-induced gastric mucosal injury. NH3-AGML was first described by Murakami et al. (1988a). It was reported to be inhibited by antioxidative agents (Murakami et al., 1989) and not by prostaglandins (PGs) (Murakami et al., 1988b). In this study, only CS-526 prevented NH3-AGML formation. The fact that the other PPIs could not inhibit lesion formation even at the dose of 30 mg/kg was consistent with a previous report that showed that acid suppressants (H2RAs) further aggravate NH3-AGML formation (Saita et al., 1995).
HCl/EtOH-AGML is a lesion whose severity was not dependent on the acid-secretory states of animals, because the loading of excess acid in the lumen was conducted externally. The protective effect against this AGML was observed for PGs and polyamines but not for cimetidine (Mizui and Doteuchi, 1983). Omeprazole (Nishida et al., 1994) and lansoprazole (Blandizzi et al., 1999) are reported to protect against this lesion, with ID50 values of approximately 10 mg/kg, which was consistent with the present data. A recent report showed that the mechanism of the protective effect of lansoprazole on the formation of this lesion is considered to be related to sulfhydryl compounds in the gastric mucosa, partially related to PGs, and unrelated to its inhibitory effect on acid secretion (Blandizzi et al., 1999; Natale et al., 2004).
The protective mechanisms of CS-526 against NH3- and HCl/EtOH-AGML were not clarified. However, the gastric antisecretory effect per se was not considered to be associated with the protective effect.
Indomethacin is categorized as one of the nonsteroidal anti-inflammatory drugs. Nonsteroidal anti-inflammatory drugs induce AGML and gastric bleeding, which are recognized to be serious clinical problems in rheumatoid arthritis and other painful inflammatory disorders. IND-AGML is prevented by PGs, H2RAs, and PPIs. In the present study, the preventive effect of CS-526 on IND-AGML was observed
In conclusion, our findings indicate that CS-526, a novel APA, is a reversible-type compound and that it has as potent an antisecretory effect on gastric acid secretion as that of conventional PPIs. CS-526 may act on parietal cells by two routes: one route is absorption from the small intestine and the other route is direct permeation through the gastric mucosa. Furthermore, CS-526 has a potent cytoprotective effect on acute gastrointestinal lesions. These findings indicate that CS-526 may provide significant benefits to patients with gastroesophageal reflux disease and peptic ulcers.
Footnotes
-
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
-
doi:10.1124/jpet.107.121350.
-
ABBREVIATIONS: PPI, proton pump inhibitor; APA, acid pump antagonist; CS-526, 7-(4-fluorobenzyloxy)-2,3-dimethyl-1-{[(1S,2S)-2-methylcyclopropyl]methyl}-1H-pyrrolo[2,3-d]pyridazine; DTT, dithiothreitol; LA, lesion area; NH3, ammonia; EtOH, ethanol; IND, indomethacin; AGML, acute gastric mucosal lesion(s); CI, confidence interval; H2RA, H2 receptor antagonist; PG, prostaglandin; SCH-28080, 3-(cyanomethyl)-2-methyl,8-(phenylmethoxy)imidazo(1,2-a)pyridine; BY841, 8-[(2-methoxycarbonyl-amino-6-methyl-phenyl)-methylamino]-2,3-dimethylimidazo [1,2-a]pyridine; AZD0865, 8-[(2,6-dimethylbenzyl)amino]-N-[2-hydroxyethyl]-2,3-dimethylimidazo[1,2-a]pyridine-6-carboxyamide; SK&F 96067, 3-butyryl-4-(2-methylphenylamino)-8-methoxyquinoline; SK&F 97574, 3-butyryl-4-(2-methylphenylamino)-8-(2-hydroxyethoxy)quinoline; DBM-819, 1-(2-methyl-4-methoxyphenyl)-4-[(3-hydroxypropyl)amino]-6-methyl-2,3-dihydropyrrolo[3,2-c]quinoline; R-99692, 7-(4-fluorobenzyloxy)-2,3-dimethyl-1-[(2-methylcyclopropyl)methyl]-1H-pyrrolo[2,3-d]pyridazine.
- Received February 12, 2007.
- Accepted July 11, 2007.
- The American Society for Pharmacology and Experimental Therapeutics