Abstract
The accompanying multivariate analysis of the binding profiles of antiparkinson agents revealed contrasting patterns of affinities at diverse classes of monoaminergic receptor. Herein, we characterized efficacies at human (h)D2SHORT(S), hD2LONG(L), hD3, and hD4.4receptors and at hα2A-, hα2B-, hα2C-, and hα1A-adrenoceptors (ARs). As determined by guanosine 5′-O-(3-[35S]thio)triphosphate ([35S]GTPγS) binding, no ligand displayed “full” efficacy relative to dopamine (100%) at all “D2-like” sites. However, at hD2S receptors quinpirole, pramipexole, ropinirole, quinerolane, pergolide, and cabergoline were as efficacious as dopamine (Emax≥100%); TL99, talipexole, and apomorphine were highly efficacious (79–92%); piribedil, lisuride, bromocriptine, and terguride showed intermediate efficacy (40–55%); and roxindole displayed low efficacy (11%). For all drugs, efficacies were lower at hD2L receptors, with terguride and roxindole acting as antagonists. At hD3 receptors, efficacies ranged from 33% (roxindole) to 94% (TL99), whereas, for hD4 receptors, highest efficacies (∼70%) were seen for quinerolane, quinpirole, and TL99, whereas piribedil and terguride behaved as antagonists and bromocriptine was inactive. Although efficacies at hD2S versus hD2L sites were highly correlated (r = 0.79), they correlated only modestly to hD3/hD4 sites (r = 0.44–0.59). In [35S]GTPγS studies of hα2A-ARs, TL99 (108%), pramipexole (52%), talipexole (51%), pergolide (31%), apomorphine (16%), and quinerolane (11%) were agonists and ropinirole and roxindole were inactive, whereas piribedil and other agents were antagonists. Similar findings were obtained at hα2B- and hα2C-ARs. Using [3H]phosphatidylinositol depletion, roxindole, bromocriptine, lisuride, and terguride displayed potent antagonist properties at hα1A-ARs. In conclusion, antiparkinson agents display diverse agonist and antagonist properties at multiple subtypes of D2-like receptor and α1/α2-AR, actions, which likely contribute to their contrasting functional profiles.
Although treatment of Parkinson's disease has long centered on administration of the dopamine precursor l-dihydroxyphenylacetyl acid (l-DOPA), there is increasing interest in the therapeutic use of dopaminergic agonists, both in association withl-DOPA and as monotherapy (Hughes, 1997). Inasmuch as dopaminergic agents currently used as antiparkinson agents interact principally with “D2-like” receptors, an important question concerns their comparative actions at D2 receptors (of which functionally distinct short D2S and long D2Lisoforms exist), D3 receptors, and D4 receptors. D2S versus D2L receptor isoforms differ in both their localization and their functional roles. The D2Sisoform is principally responsible for presynaptic control of dopamine release, whereas postsynaptic D2S and D2L receptors in the basal ganglia, via contrasting patterns of interaction with D1sites, differentially influence motor function; notably, blockade of D2L sites underlies the extrapyramidal motor effects of dopaminergic antagonists (Wang et al., 2000). As shown in the accompanying article (Millan et al., 2002), therapeutically used antiparkinson agents recognize D2S and D2L isoforms with similar affinity, and many antiparkinson agents also interact with dopamine D3 receptors. Although the density of striatal D3 receptors is reduced upon degeneration of nigrostriatal dopaminergic pathways, exposure to l-DOPA may induce their up-regulation, reflecting complex regulatory mechanisms involving dopamine D1 receptors and brain-derived neurotrophic factor (Quik et al., 2000; Guillin et al., 2001; Joyce, 2001). Nevertheless, the precise nature of functional interrelationships among D3, D2, and D1 receptors, and the implication of D3 receptors in the therapeutic compared with dyskinetic effects of antiparkinson agents, remain to be clarified (Joyce, 2001). The majority of antiparkinson agents also show significant affinity for D4receptors (Millan et al., 2002), but their engagement does not improve motor function; furthermore, antagonist properties at D4 receptors may minimize psychiatric side effects and improve cognitive function (Newman-Tancredi et al., 1997;Arnsten et al., 2000).
Several antiparkinson drugs display pronounced affinities for hα2A-, hα2B-, and hα2C-ARs (Millan et al., 2002). This is of note in light of the importance of adrenergic mechanisms in the etiology and treatment of Parkinson's disease (Brefel-Courbon et al., 1998; Bezard et al., 2001). In addition to their postsynaptic localization, α2A-ARs are expressed as inhibitory autoreceptors on adrenergic neurons (Nicholas et al., 1997;Millan et al., 2000a,b). Furthermore, α2A-ARs exert an inhibitory influence upon ascending serotonergic pathways, frontocortical and subcortical dopaminergic pathways (Kable et al., 2000; Millan et al., 2000a,b) as well as corticolimbic cholinergic and glutamatergic pathways (Horn et al., 1982; Tellez et al., 1997; Boehm, 1999). Correspondingly, α2A-ARs fulfill an important role in the control of motor function, mood, and cognition (Arnsten, 1997; Kable et al., 2000; Millan et al., 2000b). Furthermore, α2B-ARs are enriched in the thalamus, a structure interlinked with the basal ganglia and involved in the disruption of motor function in Parkinson's disease, whereas α2C-ARs are concentrated in the striatum itself (Nicholas et al., 1997; Bezard et al., 2001). Gene knockout studies have indicated a modulatory influence of central α2C-ARs, complementary to α2A-ARs, upon motor and cognitive function (Kable et al., 1999; Bjorklund et al., 2000). Although the precise significance of individual α2-AR subtypes remains unclear, there is evidence that α2-AR antagonist properties may be useful in the management of Parkinson's disease. First, in rats sustaining unilateral 6-hydroxydopamine lesions of the substantia nigra pars compacta (SNPC), α2-AR agonists and antagonists, respectively, inhibit and enhance amphetamine-induced rotation (Mavridis et al., 1991). Second, in primates displaying Parkinson's disease-like symptoms after treatment with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, α2-AR antagonists increase locomotor activity and reduce dyskinesias induced by l-DOPA (Brefel-Courbon et al., 1998; Bezard et al., 2001). Third, after enhancement of adrenergic transmission by blockade of α2A-AR autoreceptors, noradrenaline (NA) may (independently of α2-ARs) exert neuroprotective actions at dopaminergic neurons in the SNPC (Troadec et al., 2001). Fourth, small-scale clinical studies indicate that the α2-AR antagonist idazoxan improves motor performance in Parkinson's disease patients receivingl-DOPA (Brefel-Courbon et al., 1998).
Several antiparkinson agents also interact with hα1A-, hα1B-, and hα1D-ARs (Millan et al., 2002). Although the relevance of α1-ARs to management of Parkinson's disease is less apparent than for their α2-AR counterparts, they modulate ascending serotoninergic and dopaminergic transmission and play an important role in motor control (Millan et al., 2000a; Spreng et al., 2001;Stone et al., 2001). Indeed, α1-AR antagonists interfere with the induction of rotation by antiparkinson agents in rats sustaining unilateral lesions of the SNPC (Mavridis et al., 1991). Furthermore, frontocortical α1-ARs are implicated in the control of cognitive function (Arnsten, 1997). The perturbation of cardiovascular function associated with pronounced activation or blockade of α1-ARs should also be accentuated (Guimarães and Moura, 2001).
The above-mentioned observations exemplify the importance of characterizing functional actions of antiparkinson agents at subtypes of “hD2-like” receptor and hα1/2-AR. However, studies have been restricted to a few ligands at poorly characterized native sites compared with defined classes of (cloned) human receptor (see Discussion). Knowledge of the comparative agonist/antagonist profiles of antiparkinson agents remains, thus, fragmentary. The present study expanded, therefore, the multivariate analyses of binding profiles presented in the preceding article (Millan et al., 2002) in evaluating efficacies of diverse antiparkinson agents at cloned hD2S, hD2L, hD3, and hD4 dopamine receptors, and at hα2A-, hα2B- hα2C-, and hα1A-ARs, stably expressed in a common cellular system, Chinese hamster ovary (CHO) cells.
Materials and Methods
Determination of Drug Efficacies at hD2-Like Receptors and at hα2-AR Subtypes by [35S]GTPγS Binding.
Efficacies at CHO-expressed recombinant hD2S, hD2L, hD3, and hD4(hD4.4 isoform) receptors, and at CHO-expressed hα2A-, hα2B-, and hα2C-ARs were determined by measuring the influence of drugs alone and, where appropriate, in interaction with DA or NA upon [35S]GTPγS binding. The protocols used have been described in detail previously (Newman-Tancredi et al., 1997, 1999a,b; Millan et al., 2001). Briefly, the concentration of [35S]GTPγS was 0.1 nM (hD2S, hD2L, and hD4), 0.2 nM (hα2A-AR, hα2B-AR, and hα2C-AR) or 1.0 nM (hD3). The pH was 7.4 in each case and the temperature 22°C. Incubation time was 40 min for hD2S, hD2L, and hD3 sites, 20 min for hD4sites, and 60 min for hα2-AR subtypes. The buffer contained 20 mM HEPES, 100 or 150 mM NaCl, 3 μM GDP, and 3 or 10 mM MgCl2. Membranes were incubated with the antiparkinson agent alone and/or with DA (3 μM-hD2S, 10 μM-hD2L, and 1 μM-hD4) or NA (10 μM for each subtype) for 15 min before the addition of [35S]GTPγS. Agonist efficacies were expressed as a percentage of the effect observed with maximally effective concentrations of DA (10 μM) or NA (10 μM). Experiments were terminated by rapid filtration through GF/B filters (Whatman, Maidstone, UK) using a 96-well cell harvester (Packard Instrument Company, Inc., Downers Grove, IL), and radioactivity was determined by liquid scintillation counting.
Determination of Drug Efficacies at hα1A-ARs by [3H]Phosphatidylinositol ([3H]PI) Depletion.
The efficacies of drugs alone, and in interaction with NA, were determined in CHO-expressed hα1A-ARs as described previously (Millan et al., 2001). Briefly, cells were labeled with 2 μCi/ml of [3H]myoinositol (10–20 Ci/mmol) for 24 h. Cells were washed and then incubated at 37°C for 30 min with the drug alone in Krebs-LiCl buffer: 15.6 mM NaH2PO4 pH 7, 120 mM NaCl, 4.8 mM KCl, 1.2 mM MgSO4, 1.2 mM CaCl2, 0.6% (w/v) glucose, 0.04% (w/v) bovine serum albumin, and 10 mM LiCl. In the absence of NA, ∼40,000 dpm was typically detected, compared with ∼25,000 in the presence of a maximally effective concentration of NA (30 μM). Drug efficacies were expressed as a percentage of the effect observed with a maximally effective concentration of NA (30 μM). For antagonist studies, cells were preincubated for 15 min with drug before the addition of NA (10 μM) and incubation continued for 30 min. Membranes were recovered by rapid filtration through GF/B filters (Whatman) using a 96-well cell harvester (Packard Instrument Company, Inc.), and the [3H]PI content was determined by scintillation counting (Millan et al., 2001).
Data Analyses.
[35S]GTPγS and [3H]PI isotherms were analyzed by nonlinear regression using the program PRISM (GraphPad Software, San Diego, CA).KB values for inhibition of DA- or NA-stimulated [35S]GTPγS binding at hD2-like or hα2-ARs, and of NA-induced [3H]PI depletion at hα1A-ARs, were calculated as described previously (Lazareno and Birdsall, 1993; Newman-Tancredi et al., 1999a,b) according to the equation KB= IC50/{[(2 + (agonist/EC50)nH)nH−1] − 1}, where IC50 is the inhibitory concentration50 of the antagonist, agonist is DA or NA concentration, EC50 is effective concentration50 of DA or NA alone, andnH is Hill coefficient of the DA or NA stimulation isotherm. EC50 values for NA at hα2A-, hα2B-, hα2C-, and hα1A-ARs were 354, 316, 302, and 329 nM, respectively. EC50 values for DA at hD2S, hD2L, hD3, and hD4 receptors were 350, 340, 11, and 100 nM, respectively. Protein concentrations were determined by use of a bichinconic acid kit (Sigma, St. Quentin Fallavier, France). Pearson product-moment correlation coefficients were calculated for pEC50 values determined herein compared with pKi values determined in the accompanying article (Millan et al., 2002).
Drugs.
Pramipexole dihydrochloride, piribedil hydrochloride, and ropinirole were synthesized by Servier Institut de Recherches (Paris, France). Lisuride maleate and terguride were donated by Schering (Berlin, Germany). Bromocriptine, (−)-quinpirole HCl, pergolide methanesulfonate, and TL99 (6,7-dihydroxy-N,N-dimethyl-2-aminotetralin) were purchased from Sigma/RBI (Natick, MA). Apomorphine hydrochloride was purchased from Sigma. Roxindole methanesulfonate was donated by Merck (Darmstadt, Germany) and talipexole by Boehringer Ingelheim GmbH (Ingelheim, Germany). Cabergoline was obtained from Farmitalia Carlo Erba (Rueil-Malmaison, France). Quinelorane dihydrochloride was a gift from Eli Lilly & Co. (Indianapolis, IN).
Results
Drug Actions at hD2S Receptors.
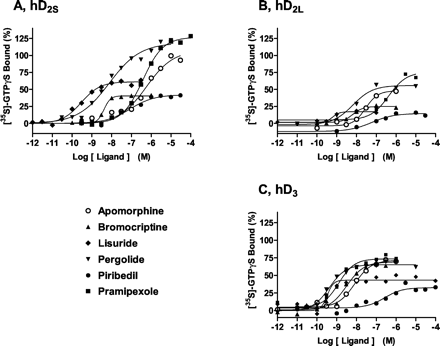
At hD2S receptors (Bmax = 1.4 pmol/mg), at a maximally effective concentration (10 μM), DA enhanced [35S]GTPγS binding by ∼2.5-fold; it displayed a pEC50 value of 6.5 (Fig.1; Table1). Quinpirole, pramipexole, quinelorane, pergolide, and cabergoline behaved as highly efficacious agonists at hD2S receptors in stimulating G protein activation ([35S]GTPγS binding) to a degree at least as marked as that of DA (Emax defined as 100%). TL99, talipexole, and apomorphine also showed high efficacies, whereas other ligands displayed less pronounced efficacies, ranging from 40% for terguride to 55% for lisuride. Roxindole showed very low efficacy. Drug potencies for stimulation of [35S]GTPγS binding (pEC50 values) at hD2S receptors correlated well (r= 0.82, P < 0.05) with their pKi values determined in competition binding experiments (data not shown; Millan et al., 2002).
Influence of antiparkinson agents upon G protein coupling at hD2S (A), hD2L (B), and hD3 (C) receptors expressed in CHO cells. [35S]GTPγS binding was carried out as described in Table 1. Binding is expressed as a percentage of that observed with a maximally effective concentration (10 μM) of dopamine (defined as 100%). Values shown are from representative experiments performed in triplicate and repeated on at least three occasions.
Efficacies (Emax values) and potencies (pEC50 or pKb values) of antiparkinson agents at recombinant hD2S, hD2L, hD3, and hD4 receptors
Drug Actions at hD2L Receptors.
At hD2L receptors (Bmax = 2.2 pmol/mg), at a maximally effective concentration (10 μM), DA enhanced [35S]GTPγS binding by ∼1.9-fold; it displayed a pEC50 value of 6.5 (Fig. 1; Table 1). At hD2L receptors, efficacies for all ligands were markedly lower than at hD2S sites. Indeed, all ligands, except quinelorane, behaved as partial agonists. Roxindole and terguride induced no stimulation of [35S]GTPγS binding and displayed antagonist properties. The correlation coefficient for efficacies at hD2L versus hD2S receptors was 0.79 (P < 0.05). Drug potencies for stimulation of [35S]GTPγS binding (pEC50 values) at hD2Lreceptors correlated well (r = 0.93, P< 0.05) with their pKi values determined in competition binding experiments (data not shown; Millan et al., 2002).
Drug Actions at hD3 Receptors.
At hD3 receptors (Bmax = 15.6 pmol/mg), at a maximally effective concentration (10 μM), DA enhanced [35S]GTPγS binding by ∼1.6-fold; it displayed a pEC50 value of 7.8 (Fig. 1; Table 1). All drugs behaved as agonists at hD3 receptors, with efficacies varying from 30% (roxindole) and 34% (piribedil) to 88% (talipexole) and 94% (TL99). The lower efficacies of quinelorane and quinpirole at hD3 versus hD2S and hD2L sites are of note, whereas roxindole and bromocriptine showed higher efficacies at hD3 than hD2S and hD2L sites. Indeed, there was no consistent pattern of drug efficacies at hD3 relative to hD2S and hD2L receptors. Accordingly, correlation coefficients for efficacies at hD3 compared with hD2L and hD2S sites, although significant (P < 0.05), were only 0.59 and 0.49, respectively. Drug potencies for stimulation of [35S]GTPγS binding (pEC50 values) at hD3 receptors correlated significantly (r = 0.62, P < 0.05) with pKi values determined in competition binding experiments (data not shown; Millan et al., 2002).
Drug Actions at hD4 Receptors.
At hD4 receptors (Bmax = 1.4 pmol/mg), at a maximally effective concentration (10 μM), DA enhanced [35S]GTPγS binding by ∼2.2-fold; it displayed a pEC50 value of 7.0 (Table 1). Although bromocriptine did not interact with hD4receptors, agonist efficacies of the other drugs varied widely. Thus, although TL99, quinelorane, and quinpirole showed relatively high efficacies (∼70%), pergolide, talipexole, cabergoline, apomorphine, roxindole, pramipexole, and lisuride showed less marked efficacies of 32 to 56%. Piribedil displayed very low efficacy (7%) and antagonized the stimulation by DA of [35S]GTPγS binding. Terguride, which was inactive alone, similarly blocked the action of DA. On the other hand, roxindole was more efficacious at hD4 than at hD2S and hD2L receptors. Thus, there was no consistent pattern of drug efficacies at hD4 versus hD2S, hD2L, and hD3 receptors and correlation coefficients, although significant (P < 0.05), were modest: hD2S, r = 0.57; hD2L, r = 0.55; and hD3, r = 0.44. Drug potencies for stimulation of [35S]GTPγS binding (pEC50 values) at hD4receptors correlated well (r = 0.78, P< 0.05) with their pKi values determined in competition binding experiments (data not shown; Millan et al., 2002).
Drug Actions at hα2A-, hα2B-, and hα2C-ARs.
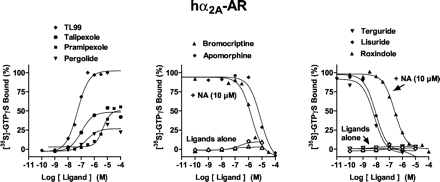
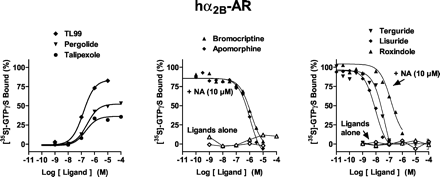
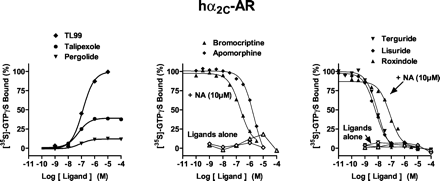
At hα2A-, hα2B-, and hα2C-ARs, a maximally effective concentration of NA (10 μM) increased [35S]GTPγS binding by 7.2-, 6.6-, and 2.7-fold, respectively; pEC50 values were 6.2, 6.5, and 6.5, respectively (Figs. 2,3 and 4; Table 2). Antiparkinson agents differed markedly concerning their functional activities at hα2A-, hα2B-, and hα2C-ARs. TL99 behaved as a high-efficacy agonist at each subtype of hα2-AR, whereas talipexole behaved as a partial agonist at each subtype. On the other hand, pergolide showed pronounced efficacy at hα2B-ARs, intermediate efficacy at hα2A-ARs, and low efficacy at hα2C-ARs. Pramipexole revealed partial agonist properties at hα2A-ARs; actions were not evaluated at hα2B- and hα2C-ARs owing to its low affinities at these sites (Millan et al., 2002). Apomorphine showed low efficacy only at hα2A-ARs, whereas high concentrations of quinelorane and quinpirole revealed weak partial agonist actions at hα2A- and hα2B-ARs, respectively. Agonist pEC50 values for stimulation of [35S]GTPγS binding corresponded well to their respective pKi values as defined in competition binding assays (Millan et al., 2002). In view of its high affinity and low efficacy at hα2A-AR subtypes, apomorphine was further evaluated in interaction with NA and shown to behave as an antagonist. Furthermore, several other drugs also reversed NA-stimulated [35S]GTPγS binding. For drugs behaving as antagonists, pKB values correlated well (P < 0.05) with their respective pKi values derived from competition binding studies (data not shown; Millan et al., 2002): hα2A-ARs, r = 0.94; hα2B-ARs, r = 0.86; and hα2C-ARs, r = 0.87.
Influence of antiparkinson agents upon G protein coupling at hα2A-adrenoceptors expressed in CHO cells. [35S]GTPγS binding was carried out as described in Table 1. Binding is expressed as a percentage of that observed with a maximally effective concentration (10 μM) of noradrenaline (defined as 100%). Values shown are from representative experiments performed in triplicate and repeated on at least three occasions.
Influence of antiparkinson agents upon G protein coupling at hα2B-adrenoceptors expressed in CHO cells. [35S]GTPγS binding was carried out as described in Table 1. [35S]GTPγS binding is expressed as a percentage of that observed with a maximally effective concentration (10 μM) of noradrenaline (defined as 100%). Values shown are from representative experiments performed in triplicate and repeated on at least three occasions.
Influence of antiparkinson agents upon G protein coupling at hα2C-adrenoceptors expressed in CHO cells. [35S]GTPγS binding was carried out as described in Table 1. [35S]GTPγS binding is expressed as a percentage of that observed with a maximally effective concentration (10 μM) of noradrenaline defined as 100%). Values shown are from representative experiments performed in triplicate and repeated on at least three occasions.
Efficacies (Emax values) and potencies (pEC50 or pKb values) of antiparkinson agents at recombinant hα2A-, hα2B-, and hα2C-adrenoceptors
Drug Actions at hα1A-ARs.
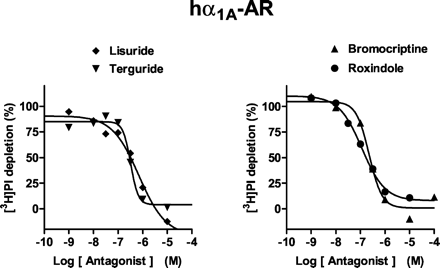
Ligands that exhibited significant binding affinity at hα1A-ARs (pKivalues ≥6.0; Millan et al., 2002) were evaluated in a functional test of phospholipase C activation, depletion of membrane-bound [3H]PI (Fig. 5; Table 3). In this procedure, NA itself revealed a pEC50 value of 6.51. No compound stimulated phospholipase C activity when tested alone, indicating an absence of agonist properties. In contrast, in order of decreasing potency, roxindole, bromocriptine, lisuride, terguride, cabergoline, and piribedil all reversed the stimulation of [3H]PI hydrolysis induced by noradrenaline (10 μM), demonstrating antagonist properties. pKB values at hα1A-ARs correlated well (r = 0.96, P < 0.05) with pKi values obtained from competition binding assays (data not shown; Millan et al., 2002).
Influence of antiparkinson agents upon stimulation of phospholipase C activity by noradrenaline at hα1A-receptors expressed in CHO cells. [3H]PI depletion studies were carried out as described under Materials and Methods. The antagonist actions of drugs were examined against noradrenaline (10 μM). Values shown are from representative experiments performed in triplicate and repeated on at least three occasions.
Efficacies and potencies (pKbvalues) of antiparkinson agents at recombinant hα1A-ARs
Discussion
This comprehensive comparison of the actions of 14 antiparkinson agents at eight classes of cloned “hD2-like” and hα1/hα2-AR revealed marked differences in efficacies, observations of significance to their contrasting functional profiles in experimental models and in human.
D2S and D2L Receptors.
In the only previous comparison of antiparkinson agonists at hD2S versus hD2L receptors (ropinirole, talipexole, pergolide, lisuride, and bromocriptine), no marked differences in efficacy were apparent (Gardner et al., 1997;Gardner and Strange, 1998). In the present, more extensive work, however, drug efficacies were invariably higher at hD2S sites. The reasons underlying this difference require elucidation, but it may be of pertinence that hD2S and hD2L receptors differentially interact with distinct subtypes of G protein, as implicated in their contrasting patterns of coupling to calcium channels (Wolfe and Morris, 1999). Furthermore, the expression level (2.2 pmol/mg) of hD2L sites herein was higher than that of hD2S sites (1.4 pmol/mg), whereas the inverse was true for Gardner et al. (1997) (1.3 versus 2.7 pmol/mg, respectively). In the light of these comments, it should briefly be pointed out that receptor density can play an important role in determining drug efficacy (Newman-Tancredi et al., 2001). Although receptor density (Bmax) can easily be determined at pure populations of transfected receptors (seeResults), equivalent information for defined networks of neurons is not available because Bmaxestimations in native tissue almost inevitably incorporate neurons not expressing the receptor in question. Thus, although the receptor densities of hD2S and hD2Lsites here were in the same range as previous studies (Coldwall et al., 1999; Perachon et al., 1999), they cannot be compared with certainty to cerebral populations. Furthermore, it is important to note differences in density between pre- versus postsynaptic populations of D2 (and D3) receptors (Seyfried and Boettcher, 1990), as well as the up-regulation of postsynaptic sites after damage to dopaminergic innervation (Kostrzewa, 1995; Newman-Tancredi et al., 2001), an experimental manipulation that mimics the pathology of Parkinson's disease (see below).
Although the relative degree of D2S versus D2L receptor stimulation required for optimal control of Parkinson's disease remains to be clarified, quinelorane was the only drug to exhibit efficacy equivalent to dopamine at both hD2S and hD2L receptors, in line with its high efficacy at native, rat D2receptors (Sánchez and Arnt, 1992; Newman-Tancredi et al., 2001). The relatively high efficacies of quinpirole, ropinirole, pramipexole, and talipexole at hD2S (Terasmaa et al., 2000) and hD2L sites for stimulation of [35S]GTPγS binding coincide with measures of extracellular acidification and mitogenesis (Mierau et al., 1995;Coldwall et al., 1999; Perachon et al., 1999; Alberts et al., 2000;Gilliland and Alper, 2000). The present [35S]GTPγS approach likewise revealed high efficacies at hD2S and hD2Lsites of cabergoline and TL99 (Hughes, 1997). Like bromocriptine and lisuride, piribedil displayed intermediate efficacy. This is interesting because piribedil is highly active in rodent and primate models of antiparkinson activity; furthermore, piribedil improves motor and cognitive function in patients both alone and in association withl-DOPA (Rondot and Ziegler, 1992; Maurin et al., 2001;Nagaraja and Jayashree, 2001). Interestingly, terguride failed to activate hD2L receptors, in line with its low efficacy in rodent models of hypothermia, locomotion, and drug discrimination (Arnt and Hyttel, 1990; Sánchez and Arnt, 1992). Although terguride showed weak partial agonist activity in hD2L receptor-expressing SH-SY5Y cells, its efficacy was much lower than that of quinpirole (Gilliland and Alper, 2000). Furthermore, although terguride showed modest antiparkinson activity and attenuated l-DOPA-induced dyskinesia in primates, it was effective in only a small percentage (10–20%) of Parkinson's disease patients in (subsequently discontinued) clinical trials (Filipova et al., 1988; Akai et al., 1993). Furthermore, roxindole, which likewise exhibited low efficacy at hD2L and hD2S receptors (Newman-Tancredi et al., 1999a), failed to reducel-DOPA-induced dyskinesias in Parkinson's disease patients and has not, as yet, been shown to possess antiparkinson activity in human.
Correspondingly, a certain, minimal “threshold” of efficacy may be necessary for antiparkinson properties. However, “full” agonism at the level of G protein-coupling ([35S]GTPγS binding) is not essential for robust clinical activity in Parkinson's disease because 1) efficacy is “amplified” by intracellular cascades downstream of G proteins (Cussac et al., 2002); 2) postsynaptic striatal D2 receptors (probably the D2L isoform) are hypersensitive due to loss of dopaminergic input from the SNPC (Kostrzewa, 1995; Geurts et al., 1999;Newman-Tancredi et al., 2001); and 3) submaximal efficacy is sufficient to activate highly sensitive D2S autoreceptors implicated in neuroprotective properties of dopaminergic agonists (Seyfried and Boettcher, 1990). Moreover, antiparkinson agents of intermediate efficacy may preferentially engage nigrostriatal D2 receptors implicated in the treatment of Parkinson's disease compared with other populations mediating side effects. Thus, “submaximal” efficacy at the G protein level for drugs such as piribedil or bromocriptine may be advantageous in optimizing the therapeutic index between clinical efficacy and side effects.
hD3 Receptors.
Although hD3receptors couple less efficiently to G proteins in CHO cells than their hD2S/hD2L counterparts, DA stimulated [35S]GTPγS binding in the high-expressing cell line used herein (Newman-Tancredi et al., 1999b). The substantial affinities of apomorphine, quinpirole, pramipexole, talipexole, bromocriptine, and pergolide corroborate studies of their actions in models of microphysiometry and mitogenesis (Mierau et al., 1995; Coldwell et al., 1999; Perachon et al., 1999). The high efficacy of TL99 at hD3 receptors is of note in view of its marked efficacy at hD2S/hD2L and hD4 sites, whereas the modest efficacies of terguride and roxindole at hD3 sites mimic their low efficacies at hD2L and (terguride) hD4 sites. As concerns piribedil, its intermediate efficacy at hD3 receptors resembles its actions at hD2S/hD2Lreceptors and is consistent with agonist properties in vivo at D3 autoreceptors (Millan et al., 1995). As discussed elsewhere (Joyce, 2001), the role of D3sites in the expression of beneficial and deleterious actions of antiparkinson agents remains unclear, a question of particular importance because, as shown herein, all antiparkinson agents activated D3 receptors.
hD4 Receptors.
In line with studies of CHO cells expressing the hD4.2 isoform (Gilliland and Alper, 2000) and of cloned, rat D4 sites (Gazi et al., 2000), quinpirole showed substantial efficacy at hD4 (hD4.4) receptors. This characteristic was shared by quinerolane and TL99. The agonist properties of pergolide, apomorphine, talipexole, and pramipexole at hD4 sites complement work using other measures of drug efficacy and/or other hD4 isoforms (Mieurau et al., 1995; Coldwell et al., 1999; Gazi et al., 2000; Gilliland and Alper, 2000). Like pergolide, two other ergolines, cabergoline and lisuride, similarly showed agonist properties at hD4 sites. In contrast, piribedil displayed low efficacy at hD4 receptors, whereas bromocriptine was inactive. Because bromocriptine and piribedil are clinically efficacious antiparkinson agents, these data support the argument that activation of D4 receptors is not necessary for therapeutic efficacy (Newman-Tancredi et al., 1997). Moreover, the essentially D4 antagonist properties of piribedil may limit psychiatric side effects and contribute to its improvement of cognitive function (Arnsten et al., 2000; Nagaraja and Jayashree, 2001).
hα2-ARs.
Striking differences in drug efficacies were seen at hα2-AR subtypes. In analogy to piribedil (Millan et al., 2001), lisuride, bromocriptine, and apomorphine displayed antagonist properties, observations amplifying functional studies of isolated organs and hippocampal NA release in rats (McPherson, 1984; Jackisch et al., 1985). In line with their high affinities for hα2-ARs (Millan et al., 2002), roxindole and two further ergot-related ligands, terguride and cabergoline, also manifested potent α2-AR antagonist properties. In contrast, in line with in vivo studies (at undefined α2-AR subtypes) in rodents (Horn et al., 1982; Meltzer et al., 1989; Sánchez and Arnt, 1992), TL99 displayed agonist, and talipexole partial agonist, properties at hα2A-, hα2B-, and hα2C-ARs. Extending observations of partial agonist properties at central α2-ARs in rodents (Ferrari et al., 1993), pramipexole displayed modest efficacy at hα2A-ARs. Any potential significance of this (low-potency) action in vivo, however, remains to be clarified. On the other hand, in vivo studies in rodents have revealed agonist actions of pergolide at central α2-ARs (Langtry and Clissold, 1990) and particularly pronounced agonist properties at hα2B-ARs were observed here.
These contrasting actions of antiparkinson agents are of considerable significance in light of evidence that blockade of α2-ARs improves motor performance, cognitive function, and perhaps mood in Parkinson's disease (Brefel-Courbon et al., 1998). Indeed, experimental and clinical studies with piribedil support the notion that “built-in” α2-AR antagonist actions may be beneficial in Parkinson's disease (Maurin et al., 2001; Millan et al., 2001;Nagaraja and Jayashree, 2001). In contrast, α2-AR agonists interfere with the facilitory influence of antiparkinson agents upon motor function (Mavridis et al., 1991; Bezard et al., 2001). Indeed, talipexole-induced stereotypy in rats (which reflects agonist properties at striatal D2 receptors) is only apparent upon prevention of its α2-AR agonist properties by coadministration of idazoxan (Meltzer et al., 1989). Similarly, TL99-induced hypomotility has been attributed to its α2-AR agonist properties (Horn et al., 1982), whereas it only elicits rotation in unilateral SNPC-lesioned rats upon cotreatment with α2-AR antagonists (Martin et al., 1983).
Nevertheless, future studies should address the significance of α2-AR subtypes in the clinical actions of antiparkinson drugs. Although α2A-ARs are certainly of key importance (Kable et al., 2000; Millan et al., 2000a), α2B- and α2C-ARs sites should not be neglected. The former are concentrated in the thalamus, a structure intimately involved in the motor deficits of Parkinson's disease, whereas α2C-ARs are enriched in the striatum itself (Nicholas et al., 1997; Bezard et al., 2001). Moreover, gene knockout studies indicate that α2C-ARs contribute to the modulation of monoaminergic transmission, cognitive function, and motor performance (Bjorklund et al., 2000; Kable et al., 2000). Although no antiparkinson agent showed agonist versus antagonist actions at distinct α2-AR subtypes, the lack of antagonist actions of piribedil at α2B- versus α2A/2C-ARs sites, and the preferential agonist actions of pergolide at α2B- versus α2A- and α2C-ARs, may prove instructive in elucidating their relevance to Parkinson's disease and its treatment.
Actions at hα1A-ARs.
Roxindole and the ergot derivatives bromocriptine, lisuride, and terguride interact with cloned hα1-ARs (Millan et al., 2002), although, with the exception of a study of bromocripine at peripheral α1-ARs (McPherson, 1984), no information on their functional activities is available. Thus, their potent antagonism of NA-induced [3H]PI depletion at cloned hα1A-ARs is of note, whereas cabergoline and piribedil showed weak antagonist properties in line with their low affinities at these sites (Millan et al., 2002). Potent blockade of hα1-ARs may be unfavorable inasmuch as α1-AR antagonists interfere with antiparkinson properties in experimental models (Mavridis et al., 1991). Although blockade of α1-ARs in the cortex and on pars reticulata GABAergic neurons may be involved, generalized sedative/motor-suppressive effects due to blockade of α1A-ARs in motor nuclei of the brainstem and the spinal cord may also be of significance (Stone et al., 2001). Blockade of segmental and peripheral α1A-ARs may also be deleterious in that it exacerbates the perturbation of cardiovascular function elicited via stimulation of spinal dopaminergic receptors (Guimarães and Moura, 2001). On the other hand, inasmuch as frontocortical α1-ARs inhibit working memory, their blockade might improve cognitive function (Arnsten, 1997), although there is currently no clinical support for this possibility. Further investigations should determine the potential importance for antiparkinson agents of actions at hα1B- and hα1D-AR subtypes (Millan et al., 2002), which likewise modulate motor, cognitive, and cardiovascular function (Guimarães and Moura, 2001; Spreng et al., 2001; Stone et al., 2001).
Conclusions
The present data reveal striking differences among antiparkinson agents concerning efficacies at multiple classes of hD2-like receptor and ha1/ha2-AR. These observations amplify receptor-binding analyses of the accompanying article (Millan et al., 2002) in demonstrating that antiparkinson drugs are heterogeneous rather than a common group of “dopaminergic agonists”. In this light, as discussed above, partial agonist and agonist properties at D2S/D2L receptors are favorable in the management of motor symptoms of Parkinson's disease, whereas blockade of α2A-ARs may improve cognitive-attentional function and mood. The present data provide a framework for additional studies of the significance of these and other subtypes of dopaminergic receptor and α1/α2-AR in the etiology of Parkinson's disease, and in the beneficial and deleterious properties of antiparkinson agents.
Acknowledgments
We thank M. Soubeyran for secretarial assistance, and Laurence Verrièle, Manuelle Touzard, Christine Chaput, Valérie Pasteau, Laetitia Marini, Nelly Fabry, and Anne Bonnard for technical assistance.
Footnotes
-
DOI: 10.1124/jpet.102.039875
- Abbreviations:
- l-DOPA
- l-dihydroxyphenylacetic acid
- AR
- adrenoceptor
- SNPC
- substantia nigra pars compacta
- NA
- noradrenaline
- h
- human
- CHO
- Chinese hamster ovary
- DA
- dopamine
- [35S]GTPγS
- guanosine 5′-O-(3-[35S]thio)triphosphate
- PI
- phosphatidylinositol
- Received June 12, 2002.
- Accepted July 22, 2002.
- The American Society for Pharmacology and Experimental Therapeutics