Abstract
Significant differences exist in the sensitivity of mice and rats to the neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) that cannot be explained by differences in exposure to or uptake of 1-methyl-4-phenylpyridinium (MPP+) into dopamine (DA) neurons. MPP+ is also a substrate for the brain vesicular monoamine transporter (VMAT2), and sequestration into synaptic vesicles may be one mechanism of protection against MPP+ toxicity. A greater sequestration of MPP+ into vesicles of DA neurons in rats versus mice could explain the lower vulnerability of DA neurons in the rat to MPP+ toxicity. To test this hypothesis, the kinetics of uptake for [3H]MPP+ and [3H]DA as well as [3H]dihydrotetrabenazine binding to VMAT2 were compared in vesicles isolated from the striata of rats and mice. The Km value of [3H]MPP+ transport was similar in the two species. In contrast, the maximal transport rate (Vmax) was 2-fold greater in vesicles from rats than in those from mice. Likewise, theKm value for [3H]DA transport was similar in both preparations, but theVmax value was 2-fold greater in rat than in mouse vesicles. The Bmax value for [3H]dihydrotetrabenazine binding was also 2-fold greater in striatal vesicles from rats than in those from mice. Electron micrographs demonstrated that vesicles isolated from rats and mice were approximately the same size. Based on these observations, we propose that striatal vesicles from rats have more VMAT2 than vesicles from mice and that this species difference in VMAT2 density may help explain the reduced vulnerability of rat DA neurons to MPP+neurotoxicity.
The exposure of humans, nonhuman primates, and several species of animals to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) results in the loss of nigrostriatal dopamine (DA) neurons (reviewed inSonsalla and Nicklas, 1992). After entry into the brain, MPTP is converted to the neurotoxin 1-methyl-4-phenylpyridinium (MPP+) by monoamine oxidase B (Chiba et al., 1984). It is the accumulation of MPP+ via the plasmalemmal dopamine transporter (DAT) to which the selective neurotoxicity of MPTP has been attributed (Javitch et al., 1985). Inside the neuron, MPP+ can be accumulated into mitochondria (Ramsay and Singer, 1986) or into synaptic vesicles (Del Zompo et al., 1993; Moriyama et al., 1993). Although inhibition of complex I of the mitochondrial electron transport chain by MPP+ is involved in processes leading to cell death (Vyas et al., 1986), the sequestration of this neurotoxin into synaptic vesicles may provide a form of neuroprotection (Reinhard et al., 1987, 1988; Liu et al., 1992; Takahashi et al., 1997; Gainetdinov et al., 1998).
For reasons unknown to date, DA neurons in rats are relatively resistant to MPTP-induced neurotoxicity (Chiueh et al., 1984; Fuller and Steranka, 1985; Giovanni et al., 1994a,b; Zuddas et al., 1994). Based on studies with MPTP in various strains of mice, we proposed that the extent of toxicity is closely correlated with striatal MPP+ concentrations (Giovanni et al., 1991). However, striatal MPP+ concentrations did not predict the toxicity to dopaminergic neurons in rats (Giovanni et al., 1994a). For example, striatal concentrations of MPP+ are greater in rats than in mice given an identical dose of MPTP (milligrams per kilogram basis), a dose that damages DA neurons in mice but not in rats. Furthermore, MPP+ concentrations are considerably greater (10- to 20-fold) in the striatum of rats than of mice when each species is given a dose of MPTP that produces a similar degree of damage. Therefore, inadequate exposure of DA neurons to MPP+ does not account for the lower vulnerability of the rat to this neurotoxin. In addition, the kinetics of MPP+ uptake into striatal synaptosomes from mice and rats are similar (Giovanni et al., 1994a). This latter finding implies that when exposed to the same extracellular content of MPP+, DA nerve terminals in both species achieve the same intracellular concentration of neurotoxin. Thus, it appears that DA neurons in rats have some mechanism or mechanisms that render them more resistant to the neurotoxic effects of MPP+ than those neurons in mice.
The discovery that MPP+ is accumulated into vesicles of chromaffin cells of the adrenal gland led Reinhard and colleagues (Reinhard et al., 1987, 1988, 1990; Daniel and Reinhard, 1988) to propose that this organ, which highly concentrates MPP+, is not damaged because of the removal of the neurotoxin from the cytosol of the cells. This prompted the hypothesis that vesicular sequestration of MPP+within neurons may provide a form of protection against this neurotoxin. Vesicles isolated from the brain of mice accumulate MPP+ (Del Zompo et al., 1993; Moriyama et al., 1993). Furthermore, cells transfected with cDNA for the vesicular monoamine transporter (VMAT1) become resistant to MPP+ compared with their wild-type phenotype, presumably because these cells have developed VMAT-containing organelles that can accumulate the MPP+ (Liu et al., 1992). Based on these observations, we hypothesized that vesicles within DA neurons of the rat may have a greater capacity to accumulate MPP+ than those neurons in mice.
In vivo studies have provided some indirect evidence that vesicular sequestration of MPP+ may differ between the two species. For example, in rats treated with the irreversible VMAT2 inhibitor reserpine plus MPTP, striatal concentrations of MPP+ are lower than those in nonreserpinized rats, findings that would be consistent with a loss of the vesicular storage sites (Russo et al., 1994). However, a similar treatment in mice did not significantly alter MPP+concentrations. These latter observations indicate that striatal vesicles in mice may have a lower propensity for accumulating MPP+ than those in rats.
The purpose of the present study was to determine directly whether striatal vesicles from rats have a greater ability to sequester the neurotoxin MPP+ than vesicles from mice. Specifically, the kinetics of uptake for [3H]MPP+ or [3H]DA, an endogenous substrate for VMAT2, into striatal vesicles were determined. In addition, binding of [3H]dihydrotetrabenazine ([3H]DTBZ), a selective ligand for VMAT2, in vesicle preparations was characterized. Electron microscopic (EM) studies were also conducted to compare the size of the vesicles in the striatal vesicle preparations.
Materials and Methods
Animals.
Male Swiss-Webster mice (30–35 g) and male Sprague-Dawley rats (250–300 g; Taconic Farms, Germantown, NY) approximately 2 months old were used in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and our local animal care committee. These strains of mice and rats were used based on our previous studies in which rat-versus-mouse differences were characterized (Giovanni et al., 1994a,b). Mice and rats were maintained at 20–22°C on a 12-h light/dark cycle with food and water available ad libitum. Mice were sacrificed by cervical dislocation, and rats were decapitated. Striata were rapidly dissected, weighed, homogenized, and assayed as described.
Drugs and Reagents.
[3H]DTBZ (specific activity, 81 Ci/mmol) was a gift from Drs. K. Frey and M. Kilbourn (University of Michigan Medical Center, Ann Arbor, MI). The short-acting reversible VMAT inhibitors tetrabenazine (2-oxo-3-isobutyl-9,10-dimethoxy-1,2,3,4,6,7-hexahydro-benzo[α]chinolizin hydrochloride) and Ro 4-1284 (2-hydroxy-2-ethyl-3-isobutyl-9,10-dimethoxy-1,2,3,4,6,7-hexahydro-benzo[α]chinolizin hydrochloride) were gifts from Hoffman-La Roche (Nutley, NJ). GBR 12909 hydrochloride and MPP+ iodide were purchased from Research Biochemicals Inc. (Natick, MA). Sucrose, EDTA, MgSO4, and KCl were obtained from Fisher Scientific (Springfield, NJ). Potassium tartrate, EGTA, Mg2+-ATP, dopamine, ascorbic acid, HEPES, and polyethylenamine were obtained from Sigma Chemical Co. (St. Louis, MO). Whatman C and F 300 filters were purchased from Brandel (Gaithersburg, MD). The Protein Assay Kit was obtained from Bio-Rad Laboratories, Life Sciences Group (Melville, NY). [3H]DA (specific activity, 21.5 Ci/mmol), [3H]MPP+ acetate (specific activity, 79.9 Ci/mmol), and [3H]WIN 35,428 (specific activity, 84.5 Ci/mmol) were purchased from NEN Life Science Products (Boston, MA).
Vesicle Preparation.
Vesicles were prepared as described byDel Zompo et al. (1993) with slight modification. Striata from several mice or rats (for a total of 125–150 mg wet wt. tissue) were homogenized in 0.32 M sucrose (500 mg tissue/14 ml) in a glass homogenizer using 10 strokes of a Teflon pestle (clearance, 0.0229 cm). The homogenate was centrifuged at 2000g for 10 min. All centrifugations were performed at 4°C. The resulting supernatant was centrifuged at 10,000g for 30 min. The resulting pellet, which contains the synaptosomes, was resuspended by swirling in 2 ml of 0.32 M sucrose. The crude synaptosomal suspension was subjected to osmotic shock by the addition of 7 ml of distilled H2O and homogenized with five strokes of the Teflon pestle. The osmolarity was restored by the immediate addition of 900 μl of 0.25 M HEPES and 900 μl of 1.0 M potassium tartrate buffer (pH 7.5). The sample was centrifuged at 20,000g for 20 min. The resulting supernatant was centrifuged at 55,000gfor 60 min. The pellet was discarded, and MgSO4was added to the supernatant to bring the final magnesium concentration to 0.9 mM. The final centrifugation was performed at 100,000g for 45 min. The pellet was immediately resuspended in a minimal volume of vesicle assay buffer (VA Buffer) containing 25 mM HEPES, 100 mM potassium tartrate, 0.5 mM EDTA, 0.05 mM EGTA, 2 mM ATP-Mg2+, 1.7 mM ascorbic acid, and 4 mM KCl. Portions of the vesicle suspension were used for [3H]DA or [3H]MPP+ uptake, [3H]DTBZ binding, and protein determination.
Uptake of [3H]DA and [3H]MPP into Vesicles.
Vesicle suspensions (160 μl, 2–3 μg protein) were incubated with 40 μl of substrate (radiolabeled and unlabeled DA or radiolabeled and unlabeled MPP+) for 2 min at 30°C. Reactions were terminated by the addition of 2.5 ml of ice-cold VA Buffer (no ascorbate or ATP-Mg2+). Vesicles were collected onto a Whatman F glass fiber filter (soaked in 0.5% polyethylenimine for ∼2 h) using a Brandel cell harvester and rinsed three times with VA Buffer. Filter sections were immersed in 100% ethanol for 10 to 15 min to extract the radiolabeled compounds. Scintillation fluid (2.5 ml) was added, and radioactivity was determined by scintillation spectroscopy. Concentrations of compounds ranged from 50 to 1000 nM for DA, 10 to 4000 nM for MPP+, ∼37 nM (3–5 × 105 dpm) [3H]DA, and 30 to 35 nM (1.1–1.4 × 106 dpm) [3H]MPP+. Nonspecific uptake was defined as uptake that occurred in the presence of 10 μM Ro 4-1284 (reversible VMAT2 inhibitor). Kinetic experiments were carried out according to the method of Akera and Cheng (1977) in which the concentration of the nonradioactive substrate was varied and the concentration of tritiated substrate was held constant.
[3H]DTBZ Binding.
[3H]DTBZ binding to synaptic vesicles was performed according to the procedure described by Meshgin-Azarian et al. (1988) with slight modification. Striatal vesicles (3–5 μg of protein) were incubated with VA Buffer (without ascorbate or ATP-Mg2+) but containing 5 mM MgCl2, 10 mM NaCl, and [3H]DTBZ (concentrations of 0.5–4 nM; total assay volume of 1 ml) for 1 h. The vesicles were collected onto Whatman FP-300 glass fiber filters (soaked for ≥30 min in 0.5% polyethylenimine) using a Brandel cell harvester. The filter was washed three times with 3 ml of buffer. Radiolabeled compounds were extracted and counted as described earlier. Nonspecific binding was measured in the presence of 10 μM tetrabenazine.
[3H]WIN 35,428 Binding.
[3H]WIN 35,428 binding to membrane preparations was measured according to the procedure described by Madras et al. (1989) with slight modification. Two striata from mice or one striatum from rats (20 and 40 mg wet wt., respectively) were homogenized immediately in 1 ml of ice-cold VA Buffer (Polytron homogenizer, setting 3, 15 s; Brinkmann Instruments, Westbury, NY). The homogenates were centrifuged at 25,000g (18 min, 4°C). The supernatant was discarded, and the pellet was suspended in incubation buffer (5 mM HEPES, 30 mM NaCl, 0.32 mM sucrose, pH 7.5; 10 mg original wet wt./0.15 ml) using a Polytron homogenizer (setting 3, 15 s). Binding was performed using [3H]WIN 35,428 (concentrations ranging from 0.5 to 8 nM) in a total volume of 0.25 ml of incubation buffer and ∼7 mg tissue original wet wt. for 1 h in an ice bath. The tissue preparations were filtered and radioactivity was extracted and counted as described. Nonspecific binding was defined as disintegrations per minute bound in the presence of 0.2 μM GBR 12909 (reversible DAT inhibitor).
EM Studies.
Vesicles were obtained as described earlier but using ∼250 to 300 mg wet wt. of striata. The final pellet was fixed with 6% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, for 2 h. After a brief rinse in phosphate buffer, the pellets were dehydrated in graded ethanol and embedded in Embed-812 (EMS, Fort Washington, PA). Silver ultrathin sections were collected and photographed with a JEOL 1200 EM. One hundred small clear vesicles from each species were randomly chosen and measured on photographs (240 × 10 magnification).
Protein Determination.
The Bio-Rad Protein Assay Kit was used to measure protein concentrations in the vesicle and membrane preparations.
Data Analysis.
Results were compared by Student'st test (Instat; GraphPad Software, San Diego, CA). Differences were considered to be statistically significant atP < .05. Kinetic values for [3H]DA and [3H]MPP+ uptake studies were determined using Eadie-Hofstee plots (Prism, GraphPad Software). [3H]WIN 35,428 and [3H]DTBZ data were transformed and analyzed by Scatchard analysis using Inplot (GraphPad Software). The turnover rate was calculated as the uptake of substrate per transporter molecule per minute (i.e., Vmax of [3H]DA or [3H]MPP+uptake/Bmax of [3H]DTBZ binding). Results are expressed as mean ± S.E.
Results
Kinetics of [3H]DA Uptake into Striatal Vesicles.
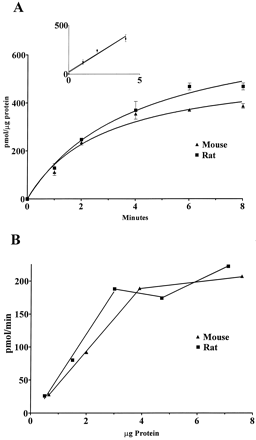
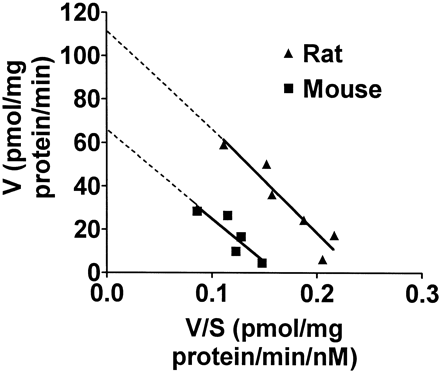
[3H]DA uptake into synaptic vesicles isolated from the striata of mice or rats was performed to determine whether there were any species differences in the functional capacity of these vesicles to accumulate the endogenous substrate for these vesicles. [3H]DA uptake was linear with protein concentration and time and was blocked by the reversible VMAT2 inhibitor Ro 4-1284 (Fig. 1). TheKm value for [3H]DA uptake into vesicle preparations from rats was slightly greater than that in mice, although this difference was not statistically significant (see Fig.2 for representative plot and Table1 for summary of results). However, theVmax value for [3H]DA uptake into striatal vesicles isolated from rats versus mice demonstrated a statistically significant 2-fold greater uptake. These kinetic values agree with those reported by Del Zompo et al. (1993) in mice and by Tanaka et al. (1976) and Erickson et al. (1990) in rats. The turnover number (minutes−1) for DA, calculated as molecules of DA transported per transporter per minute (Vmax of [3H]DA)/Bmaxof [3H]DTBZ), did not differ significantly between mice and rats (Table 1). These values approximate those reported by Floor et al. (1995).
Linearity of [3H]DA uptake into SSVs from mice and rats over time and tissue concentration. SSVs were prepared as described in Materials and Methods. Values are corrected for nonspecific uptake, which was defined as the uptake in the presence of 10 μM tetrabenazine. Uptake of [3H]DA into vesicles of mice and rats was linear up to 4 min (A) and 3 μg of protein (B).
Representative Eadie-Hofstee plot for [3H]DA uptake into SSVs from mice and rats. The assay was performed as described in Materials and Methods with unlabeled DA concentrations ranging from 50 to 1000 nM and 37 nM [3H]DA. The Km values from this representative experiment are 398 and 484 nM for mouse and rat, respectively. The Vmax values are 65 and 116 pmol/mg protein/min for mouse and rat, respectively. Mean ± S.E. values from three experiments are listed in Table 1.
Comparison of the kinetics for the uptake of [3H]DA and [3H]MPP+ into vesicles isolated from striata of mice and rats
Kinetics of [3H]MPP+ Uptake into Striatal Vesicles.
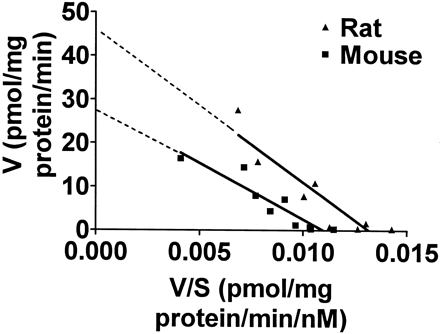
These studies were performed to directly investigate the accumulation of the neurotoxin into striatal vesicles from mice and rats. As was observed with [3H]DA uptake, theKm value for [3H]MPP+ uptake into vesicles from rats was also slightly, but not significantly, greater compared with that for mice, whereas theVmax value exhibited a significant 2.5-fold greater uptake into vesicles from rats than from mice (Fig.3, Table 1). These kinetic values for [3H]MPP+ uptake agree with those reported by Del Zompo et al. (1993) in mice. Furthermore, no significant difference was observed for turnover number (minutes−1) for [3H]MPP+ transport between the two species (Table 1).
Representative Eadie-Hofstee plot of [3H]MPP+ uptake into SSVs from mice and rats. The assay was performed as described in Materials and Methods with unlabeled MPP+ concentrations ranging from 10 to 4000 nM and 30 nM [3H]MPP+. TheKm values from this representative experiment are 2589 and 3474 nM for mouse and rat, respectively. TheVmax values are 28 and 46 pmol/mg protein/min for mouse and rat, respectively. Mean ± S.E. values from three experiments are listed in Table 1.
Kinetics of [3H]DTBZ Binding to Striatal Vesicles.
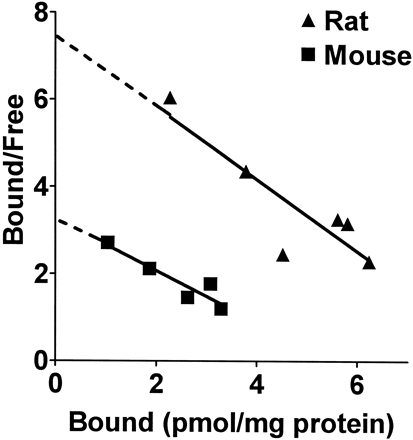
The VMAT2 ligand [3H]DTBZ was used to determine the maximal number of VMAT2 binding sites in the vesicle preparations. Kd values for [3H]DTBZ binding to striatal synaptic vesicles from mouse and rat were similar (Fig. 4, Table 1). However, Bmax values were 2-fold greater in vesicles from rats than from mice. This species difference in Bmax values for [3H]DTBZ likely accounts for the differences inVmax values for [3H]DA and [3H]MPP+ uptake between rats and mice. The Kd andBmax values obtained in our preparations approximate those observed for [3H]DTBZ binding in mouse (Sherman, 1986) and bovine vesicles (Meshgin-Azarian et al., 1988).
Scatchard plot analysis of [3H]DTBZ binding to VMAT2 in SSVs from mice and rats. The assay was performed as described in Materials and Methods with [3H]DTBZ concentrations ranging from 0.5 to 4 nM. TheKd values from this representative experiment are 1.7 and 1.2 nM for mouse and rat, respectively. TheBmax values are 5.5 and 9.1 pmol/mg protein for mouse and rat, respectively. Mean ± S.E. values from three experiments are listed in Table 1.
EM Studies of Striatal Vesicle Pellets from Mice and Rats.
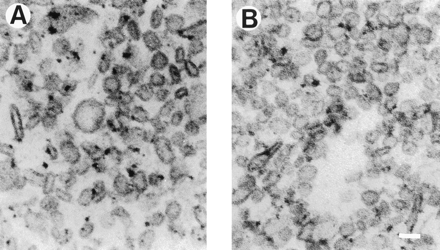
Vesicles from mouse and rat striata were examined and compared using an EM. One hundred randomly chosen vesicles from each species were analyzed. Vesicle preparations from both species showed predominantly small synaptic vesicles (SSVs) with little membrane contamination (Fig.5, A and B, shows representative electron micrographs from mouse and rat, respectively). No significant species difference in the physical appearance or size of the vesicles was observed in the electron micrographs. The diameters of the vesicles were 32.3 ± 5.5 and 35.5 ± 4.5 nm (mean ± S.D.) in mice and rats, respectively. The size of the vesicles corresponds with that reported in the literature for mice (Del Zompo et al., 1993) and rats (Teng et al., 1997).
Representative electron micrographs of SSVs. Vesicles were prepared as described in Materials and Methods. Shown are electron micrographs of SSVs from mouse (A) and rat (B). Scale bar, 50 nm. Both preparations contain predominantly small, round, clear vesicles of a similar size with little membrane contamination.
Kinetics of [3H]WIN 35,428 Binding to Striatal Membrane Preparations.
To determine whether any differences existed in the maximal number of DAT binding sites within the striatum of the two species, [3H]WIN binding was performed in synaptosomal preparations. [3H]WIN binding to striatal membrane preparations was saturable, linear with protein content, and blocked by the DAT inhibitor GBR 12909 (data not shown). In comparing results from rats and mice, no significant species differences in Kd (7.5 ± 1.9 and 9.7 ± 2.5 nM, respectively; mean ± S.E., n= 3 experiments) or Bmax (3.4 ± 0.9 and 2.1 ± 0.3 pmol/mg protein; respectively; mean ± S.E., n = 3 experiments) values for [3H]WIN 35,428 binding to striatal membrane preparations were observed. These values are comparable to those previously reported for [3H]GBR 12935 binding in rat striatal membranes (Izenwasser et al., 1990; Richfield, 1991) and [3H]mazindol binding in mouse striatal membranes (Zimanyi et al., 1989). These data also support previous studies that demonstrated no differences between the two species in either the affinity or maximal rate of uptake of [3H]MPP+ into striatal synaptosomes (Giovanni et al., 1994a).
Discussion
Previous studies have been unable to resolve reasons for the differential sensitivity of mice and rats to MPP+-induced toxicity. Although DA neurons in mice are highly sensitive to MPP+, those in rats exhibit considerably less vulnerability (Giovanni et al., 1994a). Diminished sensitivity of rat DA neurons to MPP+does not appear to be due to species differences in the uptake of the neurotoxin into the neurons (Giovanni et al., 1994a). The present results, using [3H]WIN binding, confirm that there is no significant species difference in striatal plasmalemmal DAT numbers. These findings suggest that MPP+accumulation within the DA neurons of both species is similar. Consequently, the lower vulnerability of rat versus mouse DA neurons to MPP+ toxicity cannot be explained by lower intracellular levels of MPP+ in rat DA neurons. A better explanation is that the intracellular compartmentalization of MPP+, and thus the cytosolic content of free toxin within the DA neurons, differs between the two species. MPP+ is actively accumulated into vesicles by VMAT2 (Del Zompo et al., 1993; Moriyama et al., 1993). The possibility that there might be a greater accumulation of MPP+ into vesicles of DA-containing neurons in rats than in mice could provide an explanation for the reduced vulnerability of these neurons in rats to MPP+toxicity. The present findings, in which the sequestration of MPP+ into striatal vesicles of rats was found to significantly exceed that of mice, provide direct evidence in support of this hypothesis.
The greater accumulation of MPP+ into striatal vesicles from rats versus mice can be attributed to more VMAT2 in rat striatal vesicles. Not only was the maximal transport rate (Vmax) for both [3H]MPP+ and [3H]DA 2-fold greater in rat than in mouse vesicles, but also [3H]DTBZ binding to the vesicles was 2-fold greater in striatal preparations from rats than from mice. A higher Km value was observed for VMAT2-mediated uptake of [3H]DA and [3H]MPP+ in rat versus mouse vesicles. However, this difference did not achieve statistical significance and would not contribute to the species difference observed in vivo because a greater affinity in the mouse would, if anything, enhance MPP+ accumulation into vesicles and thus reduce the sensitivity of mice to MPP+. No species differences were observed in turnover number (Vmax/Bmax) for DA or MPP+, although DA turnover in both species was greater than MPP+ turnover. These data indicate that VMAT2 function is similar in the vesicles of the two species and confirm that the largerVmax value for MPP+ uptake into vesicles from rats versus mice is due to higher levels of VMAT2 rather than to other variables that might influence Vmax values. Also noteworthy is that although we found differences in VMAT2 number in striatal vesicles from mice and rats, Kilbourn and Frey (1996) did not find significant differences in VMAT2 number between two strains of mice that exhibit substantial differences in their sensitivity to MPTP toxicity. This implies that vesicular storage of MPP+ within DA neurons of these mouse strains cannot explain the differences in sensitivity. Instead, Giovanni et al. (1991) demonstrated that more extensive damage is observed in strains of mice that exhibit higher MPP+ levels. These findings imply that the greater the striatal content of MPP+, the more MPP+ is available in the cytosol of the neuron to poison mitochondria.
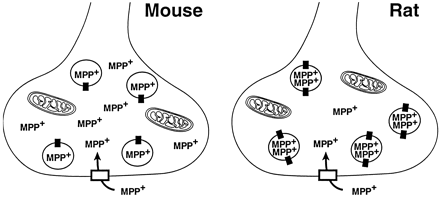
The model that best explains our data suggests that rat striatal vesicles contain more VMAT2 molecules per vesicle than those from mice (Fig. 6). This is supported by data that demonstrate the Vmax value of MPP+ uptake is greater in rats than in mice, as is the Bmax value for [3H]DTBZ binding. It is of interest to note that in this model, under conditions in which the mouse and rat DA terminals contain the same number of MPP+molecules, the ratio of vesicular to cytosolic MPP+ is 0.7:1 in the mouse and 4:1 in the rat. This model demonstrates the potential power of the vesicles to sequester MPP+ in rats compared with mice. Thus, it is possible that vesicular sequestration could be an important factor in the species difference in sensitivity to MPP+. Additional studies with EM immunohistochemical techniques will be needed to determine the validity of the model.
Hypothetical models to explain mouse-versus-rat differences in susceptibility to MPP+ neurotoxicity. DA nerve terminals in mice and rats have a similar number of plasmalemmal DAT molecules, allowing similar amounts of MPP+ to be taken up into the nerve terminal of these two species. However, the synaptic vesicles in the rat have twice as many VMAT2 molecules as those from mice, resulting in a greater sequestration of MPP+. As a consequence, there is more MPP+ available to act on mitochondria in nerve terminals of mice versus rats. Therefore, degeneration of DA neurons in mice occurs at lower MPP+concentrations than in rats.
The hypothesis that there are species differences in the number of VMAT2 molecules per synaptic vesicles is based on several assumptions. One is that the percentage of recovery of VMAT2-containing vesicles in the preparations from both species is similar. This seems likely because the initial amount of striatal tissue used for making vesicle preparations from rats and mice was similar (i.e., 120–150 mg tissue) and yielded similar protein content in the final vesicle preparations (60–90 μg protein). It is probable that any substantial differences in recovery of vesicles from rat versus mouse preparations would have yielded differences in final protein content of vesicle preparations. A second assumption is that the VMAT2-containing vesicles in the preparations were predominantly SSVs from DA nerve terminals and not of larger dense-core vesicles from other monoaminergic neurons. Recent EM studies reveal that SSVs are the major VMAT2-containing organelles that store DA in the rat striatum, whereas other monoaminergic neurons contain both SSVs and large dense-core vesicles (Nirenberg et al., 1997). A disproportionate number of large compared with small VMAT2-containing vesicles in the striatal preparations of mice versus rats could differentially modify the kinetics of vesicular uptake in the two species. However, our EM studies demonstrate that the vesicles in the preparations from both species are of similar size (35 nm) and correspond to SSVs. The size of the vesicles in our preparations also corresponds with that reported in the literature for vesicle preparations from mice (Del Zompo et al., 1993) and rats (Teng et al., 1997). They are also consistent with the size of vesicles within the DA neurons of rats as demonstrated by in situ techniques (Nirenberg et al., 1997). However, further in situ studies in mice and rats are needed to more closely examine vesicle size within DA neurons of the two species. In addition, the ratio of DA to serotonin in the striatum of mice and rats is similar in both species (approximately 20:1; our unpublished observations). Thus, it would be predicted that the ratio of SSVs to large vesicles would also be similar in both species, although confirmation of this proposition remains to be determined. Overall, given the high density of DA nerve terminals in the striatum, it is likely that the majority of the VMAT2-containing vesicles are derived from DA neurons.
Vesicular sequestration of MPP+, however, cannot totally explain the species differences in susceptibility to MPP+. In microdialysis studies, in which toxicity produced by intrastriatal MPP+ infusions was compared between mice and rats, a 10- to 25-fold higher concentration of MPP+ was needed in rats than in mice to produce striatal lesions of similar magnitude (Giovanni et al., 1994b;Staal and Sonsalla, 2000). This contrasts with the 2-fold higherVmax value observed in the present study. It is possible that the in vitro vesicular preparation does not entirely mimic vesicular function in vivo and may underestimate the actual accumulation of MPP+ that occurs in vivo. Even so, it seems unlikely that the relatively small species differences in vesicular function identified in vitro in the present study can adequately explain the much larger differences in MPP+ sensitivity observed in vivo. Consistent with this, we have found that blockade of VMAT2 in rats does enhance the toxicity of intrastriatally infused MPP+, although this pharmacological treatment does not shift the dose-response curve for toxicity all the way to that seen in mice (Staal and Sonsalla, 2000). These observations imply that there are mechanisms other than vesicular sequestration that are also involved in providing protection to rat DA neurons from the neurotoxicity of MPP+. Attempts to identify such mechanism or mechanisms are under way. Nevertheless, the data support the hypothesis originally proposed by Reinhard and colleagues (Reinhard et al., 1987,1988, 1990; Russo et al., 1994) and subsequently by others (Liu et al., 1992; Takahashi et al., 1997; Gainetdinov et al., 1998; Speciale et al., 1998) that MPP+ sequestration into vesicles can protect against this neurotoxin.
In summary, the lower vulnerability of DA neurons in rats to neurotoxicity produced by MPTP or MPP+ can be attributed, at least in part, to the greater sequestration of MPP+ into synaptic vesicles of this species compared with mice. This greater sequestration into vesicles of rats can remove MPP+ from the cytosol, thus preventing it from exerting its deleterious effects on mitochondria. These observations suggest that a secondary role of VMAT2, in addition to concentrating monoamines in vesicles for neurotransmission, may be to remove toxic substances from the cytosol and to prevent them from reaching their site of action.
Acknowledgments
We thank Drs. Kirk A. Frey and Michael Kilbourn for the generous gift of [3H]DTBZ. We also acknowledge L. Manzino for expert technical assistance.
Footnotes
-
Send reprint requests to: Dr. Patricia K. Sonsalla, Department of Neurology, University of Medicine and Dentistry of New Jersey, Robert Wood Johnson Medical School, 675 Hoes Lane, Piscataway, NJ 08854-4535. E-mail: sonsalla{at}umdnj.edu
-
↵1 This work was supported by National Institutes of Health Grant AG08479 and National Institute of Environmental Health Sciences Grant ES07148. The synthesis and characterization of [3H]DTBZ binding were supported by National Institutes of Health Grants AG08671 and MH47611 to Drs. Michael Kilbourn and Kirk Frey (University of Michigan).
- Abbreviations:
- MPTP
- 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- VMAT2
- vesicular monoamine transporter 2
- DAT
- dopamine transporter
- DA
- dopamine
- DTBZ
- dihydrotetrabenazine
- MPP+
- 1-methyl-4-phenylpyridinium
- SSV
- small synaptic vesicle
- EM
- electron microscopic (microscope)
- Received April 6, 1999.
- Accepted February 14, 2000.
- The American Society for Pharmacology and Experimental Therapeutics