Abstract
The influence of the 5-hydroxytryptamine1A agonist 8-hydroxy-2-(di-n-propylamino)tetralin (DPAT) on locomotor hyperactivity induced by the acute and chronic administration of cocaine was assessed. Horizontal activity was measured in the periphery and center of an open field test enclosure equipped with photobeams; vertical activity was also recorded. Peripheral hyperactivity induced by an acute administration of cocaine (10 or 20 mg/kg) was significantly enhanced by 0.2 mg/kg DPAT. In contrast, central and vertical activities were reduced in a dose-related manner by DPAT (0.1 and 0.2 mg/kg); DPAT also suppressed central (0.2 mg/kg) and vertical (0.1 and 0.2 mg/kg) activities when administered alone. Similar observations were made on day 1 of chronic treatment with DPAT (0, 0.1, or 0.2 mg/kg) injected 15 min before an injection of cocaine (0, 10, or 15 mg/kg) administered twice daily for 7 days. By day 7 of repeated DPAT treatment, sensitization of DPAT-evoked peripheral activity developed, which contrasted with tolerance to the central and vertical hypoactivity evoked by DPAT. Sensitization developed to the repeated treatment with 15 mg/kg cocaine but not 10 mg/kg cocaine. Interestingly, enhancements of all activity measures were observed between days 1 and 7 in rats cotreated with DPAT plus either dose of cocaine. This sensitization to DPAT plus cocaine was expressed on challenge with DPAT and cocaine but not with cocaine alone. The present study implies that the stimulation of 5-hydroxytryptamine1A receptors is capable of modulating the hyperactivity evoked by cocaine, possibly via modulation of the mesoaccumbens dopamine circuit thought to mediate the behavioral effects of cocaine.
Stimulation of the dopamine (DA) pathway that arises from perikarya in the ventral tegmental area (VTA) to innervate the nucleus accumbens (NAc) appears to be an important mechanism underlying the behavioral effects of cocaine (COC; for a review, see Di Chiara, 1995). These circuits are also important in the enhancement of behaviors induced by repeated, intermittent administration of COC (“behavioral sensitization”;Kalivas et al., 1993). 5-Hydroxytryptamine (5-HT; serotonin) modulates mesoaccumbens pathways (Herve et al., 1979) and has been implicated as a candidate system that contributes to the behavioral effects of COC (for a review, see Walsh and Cunningham, 1997). Among the fourteen 5-HT receptor subtypes, the 5-HT1A receptor has been studied extensively as the presynaptic impulse-regulating autoreceptor found in the dorsal raphe nucleus (Sprouse and Aghajanian, 1986). A high density of postsynaptic 5-HT1Areceptors is also found in numerous limbic regions, particularly hippocampus, septum, and amygdala; lower densities are found in the VTA and NAc (Chalmers and Watson, 1991).
Pharmacological stimulation of 5-HT1A receptors after systemic administration of low doses of the selective 5-HT1A agonist 8-hydroxy-2-(di-n-propylamino)tetralin (DPAT) has been shown to modify the activity of both VTA neurons (Prisco et al., 1994) and the substantia nigra (Sinton and Fallon, 1988; Kelland et al., 1990;Arborelius et al., 1993), as well as neurons in the NAc (Nagaoka et al., 1998). The DPAT-evoked increase in spontaneous and burst activity of most DA neurons in the VTA is abolished by the selective destruction of 5-HT neurons, suggesting that the stimulation of DA neurons is a result of activation of 5-HT1A autoreceptors in raphe that results in reduced serotonergic input to DA neurons and a subsequent disinhibition of firing of DA neurons (Prisco et al., 1994). In keeping with this hypothesis, systemic DPAT administration has been shown to increase extracellular DA levels in the VTA (Chen and Reith, 1995) and NAc (Boulenguez et al., 1996). A role for a direct excitatory effect of 5-HT1A receptors in VTA is also suggested by the finding that the direct application of DPAT increased either firing rates of these cells (Arborelius et al., 1993) or burst firing (Zhang and Freeman, 1993); however, it is important to note that contrary evidence also exists (Guan and McBride, 1989; Prisco et al., 1994). NAc neurons also possess functional 5-HT1Areceptors (Nagaoka et al., 1998), and the local administration of DPAT via the microdialysis probe is reported to increase striatal DA release (Benloucif and Galloway, 1991).
These data suggest that 5-HT1A interactions with DA systems could contribute to behaviors observed after the administration of psychostimulants such as COC that act predominantly via DA mesocorticolimbic circuits. The effects of 5-HT1A agonists and antagonists have been studied in the locomotor (Cunningham and Callahan, 1994; Przegalinski and Filip, 1997), discriminative stimulus (Callahan and Cunningham, 1995;Przegalinski and Filip, 1997), and reinforcing (Peltier and Schenk, 1993) effects of COC. For example, DPAT is reported to inhibit (Przegalinski and Filip, 1997), whereas the partial 5-HT1A agonist gepirone is reported to intensify, locomotor activity evoked by COC (Cunningham and Callahan, 1994). On the other hand, neither systemic (Callahan and Cunningham, 1995) nor intra-VTA (De La Garza et al., 1998) microinfusions of DPAT stimulated drug-lever responding in rats trained to discriminate COC from saline (SAL), effects that might have been predicted based on electrophysiological and neurochemical findings (Benloucif and Galloway, 1991; Arborelius et al., 1993). Yet, despite these inconsistent findings, acute administration of COC is known to be a potent and efficacious inhibitor of dorsal raphe neurons, at least in part via indirect activation of 5-HT1Aautoreceptors (Cunningham and Lakoski, 1990). In addition, COC sensitization is associated with specific modifications in 5-HT1A receptor sensitivity (Cunningham et al., 1992; King et al., 1993; Cunningham, 1995). Therefore, 5-HT1A receptor manipulations can modulate the behavioral effects produced by acute COC, and the mechanism via which COC elicits its chronic effects may be partially mediated by alterations in the sensitivity of the 5-HT1Areceptor.
Because DPAT may act to enhance DA neurotransmission in mesolimbic circuits important in the locomotor hyperactivity induced by COC and in light of conflicting evidence concerning the effects of DPAT on COC-elicited hyperactivity, we conducted a thorough analysis of hyperactivity elicited by COC in the presence versus the absence of DPAT. Furthermore, we sought to determine the effects of the selective 5-HT1A agonist DPAT on behavioral sensitization induced by COC.
Materials and Methods
Animals
Male Sprague-Dawley rats (200–250 g) were housed in groups of four in Plexiglas shoebox cages with bedding. The colony was maintained at constant temperature (21–23°C) and humidity (40–50%), and a 12-h light/dark cycle was used (lights on 7:00 AM to 7:00 PM). Animals were allowed free access to food and water, except during test sessions.
Apparatus
Behavioral activity was monitored and quantified using an open field activity system (San Diego Instruments, San Diego, CA) as described previously (McCreary et al., 1999; McMahon and Cunningham, 1999). Housed within sound attenuating enclosures, each chamber was a 40 × 40 × 40-cm clear Plexiglas enclosure containing a 4 × 4 photobeam matrix located 4 cm from the floor surface; breaks of these photobeams resulted in counts of activity in the peripheral and central fields of the chamber. Another horizontal row of 16 photobeams, located 12 cm from the floor surface, provided each chamber with a measurement of vertical (rearing) activity. Activity recorded in the inner 16 × 16 cm of the open field was counted as central activity, whereas the field bounded by the outer 16-cm band registered peripheral activity. Separate counts of peripheral and central horizontal and vertical beam interruptions were made with the control software (PAS; San Diego Instruments) and stored for subsequent statistical evaluation. Video cameras located above the chambers provided the ability to monitor activity continuously without disruption of behavior.
Design and Behavioral Procedures
Each rat was habituated to the locomotor chambers for 2 h per day for 3 days before the start of testing and for 30 min before every test session.
Experiment 1: Effects of Acute DPAT on Spontaneous and COC-Induced Activity.
Experimentally naive rats (n= 32) were injected with DPAT (0.1 or 0.2 mg/kg s.c.) or SAL (0.9% NaCl, 1 ml/kg s.c.) 15 min before an injection of COC (10 or 20 mg/kg i.p.) or SAL (1 ml/kg i.p.), which was immediately followed by a 45-min test session. All animals were tested under each condition (consisting of both doses of DPAT and COC) with drug tests spaced so that animals were tested twice weekly, with 2 to 3 days intervening between tests. Throughout the entire testing period of 8 weeks, each animal received only one injection of DPAT or COC during a 7-day period. The presentation of drugs was counterbalanced so the order of drug/dose testing was randomized for each individual animal.
Experiments 2 and 3: Effects of DPAT on Spontaneous and COC-Induced Activity and Behavioral Sensitization.
Experimentally naive rats (n = 8/group) were injected with DPAT (0.1 or 0.2 mg/kg s.c.) or SAL (1 ml/kg s.c.) 15 min before an injection of COC (10 or 15 mg/kg i.p.) or SAL (1 ml/kg i.p.) twice daily for 7 days; injections occurred at 8:00 AM and 4:00 PM. Animals were exposed to the locomotor chambers for 30 min after the injection of SAL or COC every morning and received the second series of injections in the home cage every afternoon. On days 1 and 7 of the chronic drug regimen, a 30-min habituation period was followed by an injection of either SAL or DPAT, followed 30 min later by an injection of SAL or COC. Monitoring of activity began immediately after the second injection.
During the withdrawal period after the chronic drug regimen, animals were challenged to assess the expression of sensitization. In one challenge test (48 or 96 h after the last treatment injection), locomotor activity was assessed immediately after an injection of SAL. This test was included to assess conditioned activity in response to the test environment. In a second challenge test, activity levels were assessed immediately after an injection of COC (48 or 96 h after the last treatment injection). This test was designed to reveal differences in activity levels between animals with no previous exposure to COC versus animals subjected to the chronic COC regimen, thereby providing evidence of the expression of sensitization. In a final challenge test (144 h after the last treatment injection), animals received an injection of DPAT, followed 15 min later by an injection of COC. This test was designed to disclose whether DPAT altered COC-induced activity dependent on treatment.
Statistical Analysis
Experiment 1.
Total activity counts were summed for each individual animal for the 45-min test period; data are presented as mean total activity counts (±S.E.M.). A two-way repeated measures ANOVA was used to determine the main effects and interaction between DPAT (0, 0.1, and 0.2 mg/kg) and COC (0, 10, and 20 mg/kg) on total peripheral, central, and vertical activities observed during the 45-min test session. Planned group comparisons were assessed with a Student'st test with the experiment-wise error rate (α) set at .05.
Experiments 2 and 3.
Total activity counts were summed for each individual animal across the 45-min observation period; data are presented as mean total activity counts (±S.E.M.). A one-way ANOVA was used to assess the differences in total activity counts for the 45-min test session among the four treatment groups (SAL + SAL, DPAT + SAL, SAL + COC, and DPAT + COC) on days 1 and 7 and on challenges during withdrawal. A subsequent planned comparisons Student's ttest was performed among treatment groups. An ANOVA for repeated measures was used to analyze activity counts between days 1 and 7 within each treatment group. All statistical analyses were conducted with an experiment-wise error rate (α) set at .05.
Drugs
Doses of all drugs refer to the weight of the salt. Cocaine HCl (Sigma Chemical Co., St. Louis, MO) and DPAT (Research Biochemicals Inc., Natick, MA) were prepared in 0.9% SAL and injected i.p. and s.c., respectively, in a volume of 1 ml/kg.
Results
Experiment 1: Effects of DPAT on Spontaneous and COC-Induced Activity
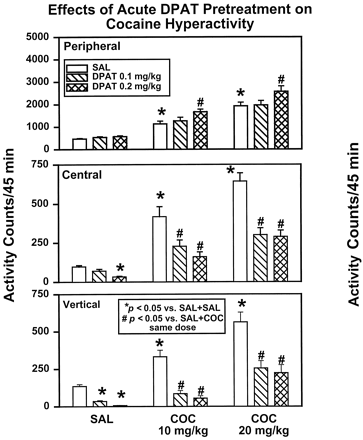
For all dependent measures, a significant main effect of DPAT (0, 0.1, and 0.2 mg/kg) and of COC (0, 10, or 20 mg/kg) and a significant interaction were observed (see Table 1for results of statistical analyses). Comparisons of group mean values indicated that pretreatment with DPAT (0.1 or 0.2 mg/kg) did not alter peripheral activity typically seen on SAL injection (Fig.1), but did reduce central (0.2 mg/kg,P < .05) and vertical (0.1 and 0.2 mg/kg,P < .05) activities. Clearly, 10 and 20 mg/kg COC (Fig. 1) produced dose-dependent increases in all activity measures (P < .05). Importantly, pretreatment with DPAT (0.1 and 0.2 mg/kg) led to significant decreases in COC-induced central and vertical activities, whereas peripheral activity was significantly increased at the 0.2 mg/kg dose of DPAT tested (Fig. 1;P < .05). A within-subjects design was used in this experiment to control interanimal variation and for economy of animal use; statistical analyses indicated that the observed results were not a consequence of the order of test (results of analyses not shown).
Acute DPAT modulation of cocaine hyperactivity: main effects of treatment
Effects of acute administration of DPAT on spontaneous and COC-induced locomotor activity. Rats received either SAL (open columns) or 0.1 mg/kg DPAT (hatched columns) or 0.2 mg/kg DPAT (cross-hatched columns) as a first injection, followed 15 min later by SAL or 10 mg/kg (COC 10) or 20 mg/kg (COC 20) COC (n = 32). Data are presented as mean activity counts (±S.E.M.) summed over the 45-min test session. Separate counts of peripheral (top), central (middle), and vertical (bottom) activities are represented independently. *Activity levels that differed significantly from SAL + SAL animals. #Activity levels that differed significantly from SAL + COC animals at the same dose of COC (P < .05).
Experiment 2: Effects of DPAT (0.2 mg/kg) on Spontaneous and COC-Induced Activity and Behavioral Sensitization
On the basis of the findings from the first experiment, in which DPAT potentiated COC-induced peripheral activity without observable effects on basal peripheral activity, we hypothesized that this 5-HT1A agonist would enhance the behavioral sensitization observed after repeated COC administration. The dose of 0.2 mg/kg for DPAT was chosen based on the positive interaction between DPAT and COC obtained in the first experiment. The regimen of COC administration (10 mg/kg twice daily for 7 days) was chosen as a threshold regimen unlikely to elicit sensitization (K.A.C., unpublished data) to assess a potential enhancement induced by DPAT.
Effects of Pretreatment: Day 1.
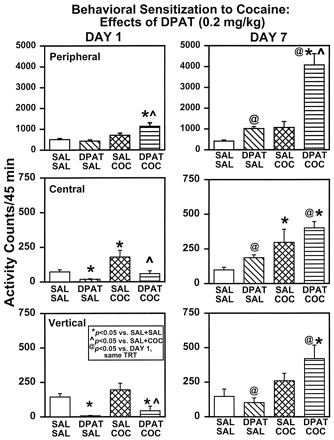
The first day of testing provided the opportunity to reanalyze the acute effects of DPAT on spontaneous and COC-induced locomotor activity (Fig.2, left). There was a main effect of treatment for peripheral (F3,28 = 9.27, P = .0002), central (F3,28 = 6.18, P = .0023), and vertical (F3,28 = 7.68,P = .0007) activities. Animals administered DPAT (DPAT + SAL) exhibited significant decreases in central and vertical activities (P < .05) compared with SAL + SAL controls, whereas peripheral activity was unaffected. Acute COC exposure (SAL + COC) resulted in modest increases in activity levels that reached significance for central activity only (P < .05) compared with SAL + SAL controls. Treatment with DPAT (DPAT + COC) resulted in significantly increased peripheral and decreased central and vertical activities evoked by COC compared with SAL + COC rats (P < .05).
Effects of DPAT (0.2 mg/kg) on day 1 and day 7 of chronic treatment. Rats received either SAL or DPAT (0.2 mg/kg), followed 30 min later by an injection of SAL or COC (10 mg/kg). The four treatment groups (n = 6/group) included SAL + SAL (open columns), DPAT + SAL (left-hatched columns), SAL + COC (cross-hatched columns), and DPAT + COC (horizontally lined columns). Data are presented as mean activity counts (±S.E.M.) summed over the 45-min period beginning immediately after the second injection (SAL or COC) on day 1 (left) and day 7 (right) of treatment. *Activity levels that differed significantly from SAL + SAL animals on the same treatment day (P < .05). ∧Activity levels in DPAT + COC animals that differed from SAL + COC animals on the same treatment day (P < .05).@Activity levels on day 7 that differed significantly from day 1 for a given treatment (P < .05).
Effects of Pretreatment: Day 7.
On day 7, locomotor activity was assessed again in each of the four treatment groups (Fig. 2, right). There was a main effect of treatment for peripheral (F3,28 = 28.05, P = .0001), central (F3,28 = 5.88,P = .0030), and vertical (F3,28 = 5.33, P = .0050) activities. In direct contrast to day 1, DPAT + SAL rats exhibited levels of peripheral, central, and vertical activities not significantly different from that of SAL + SAL rats on day 7 (P < .05). After a COC injection on day 7, SAL + COC rats exhibited significantly higher central, but not peripheral or vertical, activity compared with SAL + SAL rats (P < .05). Importantly, DPAT + COC rats exhibited significantly greater peripheral, central, and vertical activities compared with SAL + SAL controls (P < .05). Significant differences between SAL + COC and DPAT + COC rats existed on day 7 for peripheral activity only, although there was a clear trend toward a significant enhancement of vertical activity in the DPAT + COC rats as well (P< .05).
Effects of Pretreatment: Day 1 to Day 7.
To determine whether significant changes occurred during the course of the chronic regimen, comparisons of activity levels were made between days 1 and 7 within treatment groups (Fig. 2). In the SAL + SAL treatment group, neither peripheral (F1,14 = 1.57,P = .2303), central (F1,14 = 1.03, P = .3281), nor vertical (F1,14<.0001, P = .9488) activity differed between day 1 and day 7. This was also the case for SAL + COC rats, in which peripheral (F1,14 = 1.38,P = .2600), central (F1,14 = 1.26, P = .2801), and vertical (F1,14 = .78,P = .3915) activities did not differ from day 1 to day 7. In contrast, in DPAT + SAL rats, peripheral (F1,14 = 31.40, P = .0001), central (F1,14 = 62.32,P = .0001), and vertical (F1,14 = 7.82, P = .0143) activities increased from day 1 to day 7. Similarly, in DPAT + COC rats, peripheral (F1,14 = 27.18,P = .0001), central (F1,14 = 1.03, P = .0001), and vertical (F1,14 = 13.10,P = .0028) activities were significantly enhanced from day 1 to day 7.
Expression of Sensitization: Challenges during Withdrawal.
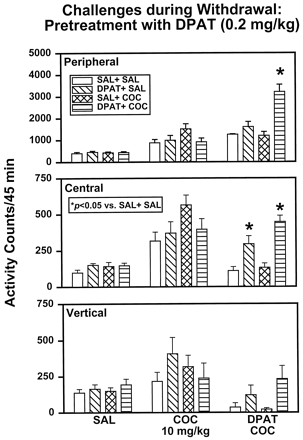
Locomotor activity was assessed after an injection of SAL 48 h after the last treatment injection (Fig.3, left). An ANOVA indicated no main effect of treatment on peripheral (F3,28 = .20, P = .8951), central (F3,28 = 1.33,P = .2831), or vertical (F3,28 = .62, P = .6064) activity. In a second challenge test (Fig. 3, middle), activity levels were assessed after an injection of COC (10 mg/kg) 96 h after the last treatment injection. An ANOVA indicated no main effect of treatment on peripheral (F3,28 = 2.26, P = .1033), central (F3,28 = 2.29, P = .1006), or vertical (F3,28 = .89,P = .4595) activity. In a final challenge test 144 h after the last treatment injection (Fig. 3, right), activity was reassessed in response to the administration of COC (10 mg/kg) preceded by DPAT (0.2 mg/kg). An ANOVA indicated a main effect of treatment on peripheral (F3,28 = 14.67,P = .0001) and central (F3,28 = 14.84, P = .0001) activities, but only a trend toward significance was observed for vertical activity (F3,28 = 2.71,P = .0639). Planned comparisons among groups showed that rats previously treated with DPAT + SAL exhibited significantly greater central activity (P < .05) compared with SAL + SAL controls when exposed to the combined challenge with DPAT + COC. Previous exposure to DPAT + COC was also associated with significantly increased peripheral and central activities (P < .05) compared with SAL + SAL rats, whereas activity levels in SAL + COC rats were not different than those in SAL + SAL rats on challenge with DPAT + COC.
DPAT (0.2 mg/kg) treatment: challenge tests during withdrawal. At 48, 96, and 144 h after the last treatment injection, SAL + SAL, DPAT + SAL, SAL + COC, and DPAT + COC rats were challenged with SAL (left), COC (10 mg/kg, middle), or DPAT (0.2 mg/kg, right) plus COC (10 mg/kg), respectively. *Activity levels that differed significantly from SAL + SAL animals on a given challenge test (P < .05).
Experiment 3. Effects of DPAT (0.1 mg/kg) on Spontaneous and COC-Induced Activity and Behavioral Sensitization
In this experiment, we tested the hypothesis that stimulation of 5-HT1A receptors may enhance COC sensitization with a dose of DPAT (0.1 mg/kg s.c.) that was less efficacious in inducing acute hypomotive effects (see Fig. 1) and a COC treatment regimen (15 mg/kg twice daily for 7 days) that had previously been shown to induce more robust COC sensitization (Cunningham et al., 1992).
Effects of Pretreatment: Day 1.
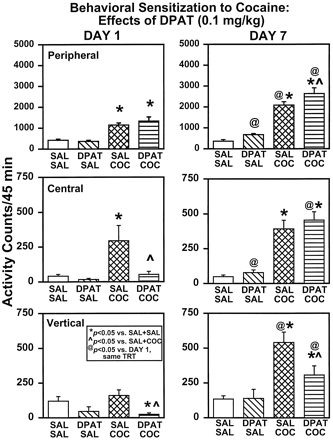
An ANOVA indicated a main effect of treatment for peripheral (F3,28 = 18.75, P = .0001), central (F3,28 = 5.32,P = .0050), and vertical (F3,28 = 4.11, P = .0155) activities on day 1. Treatment with 0.1 mg/kg DPAT (DPAT + SAL) did not significantly alter basal activity compared with SAL + SAL controls on day 1 (Fig. 4, left). SAL + COC rats exhibited significant increases in peripheral and central (P < .05), but not vertical, activities compared with SAL + SAL rats. A significant decrease in central and vertical activities was observed in DPAT + COC rats compared with SAL + COC rats (P < .05).
Effects of DPAT (0.1 mg/kg) on day 1 and day 7 of chronic treatment. See Fig. 2 for explanation of symbols.
Effects of Pretreatment: Day 7.
On day 7, locomotor activity was reassessed (Fig. 4, right) and a main effect of treatment was observed for peripheral (F3,27 = 44.64, P = .0001), central (F3,27 = 32.25, P = .0001), and vertical (F3,27 = 9.86,P = .0001) activities. Comparisons among groups revealed similar levels of peripheral, central, and vertical activities in SAL + SAL and DPAT + SAL rats on day 7. In addition, both SAL + COC and DPAT + COC rats exhibited significantly higher peripheral, central, and vertical activities versus SAL + SAL rats (P < .05). Peripheral activity was significantly increased in DPAT + COC over SAL + COC rats (P < .05), whereas vertical activity was decreased in DPAT + COC versus SAL + COC rats (P < .05).
Effects of Pretreatment: Day 1 to Day 7.
To determine whether significant changes occurred during the course of the chronic regimen from day 1 to day 7, comparisons of activity levels were made within treatment groups (Fig. 4). In SAL + SAL rats, neither peripheral (F1,14 = .42, P = .5267), central (F1,14 = .20,P = .6583), nor vertical (F1,14 = .13, P = .7227) activity differed from day 1 to day 7. In contrast, peripheral (F1,13 = 27.71, P = .0002) and vertical (F1,13 = 21.56,P = .0005) activities, but not central activity (F1,13 = 1.34, P = .2682), differed from day 1 to day 7 in SAL + COC rats, indicating that sensitization developed in SAL + COC rats. In DPAT + SAL rats, peripheral (F1,14 = 18.29,P = .0008) and central (F1,14 = 9.09, P = .0093) activities, but not vertical activity (F1,14 = 1.67, P = .2166), were significantly different from day 1 to day 7. In DPAT + COC rats, peripheral (F1,14 = 15.22,P = .0016), central (F1,14 = 39.83, P = .0001), and vertical (F1,14 = 18.44,P = .0007) activities were significantly different from day 1 to day 7.
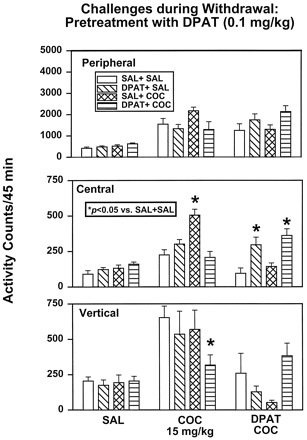
Expression of Sensitization: Challenges during Withdrawal.
All rats were challenged with SAL 96 h after the last treatment injection (Fig. 5, left). No main effect of treatment was observed for peripheral (F3,28 = 2.61, P = .0712), central (F3,28 = 2.07,P = .1270), or vertical (F3,28 = 3.29, P = .0552) activity. Activity was also assessed after challenge with COC (15 mg/kg i.p.) 48 h after the last treatment injection (Fig. 5, middle). A main effect of treatment was observed for central (F3,28 = 12.53, P = .0001) and vertical (F3,28 = 4.89,P = .0074) activities but not for peripheral activity (F3,28 = 2.38, P = .0905). Group comparisons indicated that SAL + COC rats differed significantly from SAL + SAL rats for central activity (P < .05) but not for peripheral or vertical activities induced by the challenge with COC. On challenge with COC, DPAT + COC rats exhibited significantly decreased vertical activity compared with SAL + SAL rats. In a final challenge with COC (15 mg/kg) preceded by injection of DPAT (0.1 mg/kg) 144 h after the last treatment injection (Fig. 5, right), a main effect of pretreatment was present for central activity (F3,28 = 8.79, P = .0003) but not peripheral (F3,28 = 2.35, P = .0942) or vertical (F3,28 = 2.89,P = .0532) activity. Group comparisons indicated that DPAT + SAL rats differed significantly from SAL + SAL rats on central activity (P < .05). In addition, central activity in DPAT + COC rats was significantly higher than that in SAL + SAL rats.
DPAT (0.1 mg/kg) treatment: challenge tests during withdrawal. At 96, 48, and 144 h, SAL + SAL, DPAT + SAL, SAL + COC, and DPAT + COC rats were challenged with SAL (left), COC (15 mg/kg, middle), or DPAT (0.1 mg/kg, right) plus COC (15 mg/kg), respectively. *Activity levels that differed significantly from SAL + SAL animals on a given challenge test (P < .05).
Discussion
The present results demonstrate that DPAT selectively, but differentially, modulates behaviors evoked by administration of COC in the open field, such that this 5-HT1A agonist enhanced peripheral activity but suppressed central and vertical activities induced by COC. Behavioral competition may account for the diminished COC-induced hyperactivity evoked by the highest dose of DPAT, but the suppression of COC-induced central and vertical activities was not predicted at the low dose of DPAT (0.1 mg/kg), which did not uniformly inhibit basal activity. Furthermore, DPAT (0.2 mg/kg) enhanced COC-induced peripheral activity, without any overt effects on basal peripheral activity. In a sense, these data may clarify reported inconsistencies concerning the effects of DPAT on COC-induced locomotor activity. Although an enhancement of COC-evoked horizontal activity was observed after treatment with the partial 5-HT1Aagonist gepirone (Cunningham and Callahan, 1994), a dose-dependent suppression of COC-evoked locomotor activity was reported after cotreatment with DPAT (Przegalinski and Filip, 1997). Based on the current findings, this outcome may be related to an DPAT-evoked diminution of COC-induced activity in the center of the field. Thus, the use of a global horizontal activity measurement would have obscured the influence of DPAT on the spatial profile of the hyperactivity evoked by COC. These data reiterate the importance of a detailed microanalysis of behavior (Paulus et al., 1993) when studying the interaction of COC with drugs that have prominent behavioral effects alone.
Changes in the spatial distribution of activity in an open field arena may reflect the influence of a drug on “emotionality” or exploration (Denenberg, 1969). Rodents placed in a novel, brightly lit open field will normally avoid the central portion of the field and gravitate toward the periphery, which is suggestive of “anxiogenic,” or stress, reactions. In the present study of general motor activity, care was taken to habituate rats to the environment and to use low light illumination to minimize the novelty of the test situation. Under these habituated conditions, COC uniformly increased activity in peripheral, central, and vertical planes, effects that are inconsistent with an interpretation that COC evoked an anxiogenic-like response. However, the pattern of activity seen after DPAT administration, alone or in combination with COC, does suggest an anxiogenic-like response. An analysis of the spatial distribution of activity under the conditions that are classically considered to tap emotionality in the open field (Denenberg 1969) will be required to clarify the possibility that DPAT and COC combinations result in a synergistic enhancement of anxiogenic-like behaviors in the rat.
Repeated, intermittent COC administration is known to induce a progressive augmentation of locomotion and stereotypical behaviors (“sensitization”; Kalivas et al., 1993). In the present study, the development and expression of sensitization were observed in rats treated twice daily for 7 days with 15 mg/kg (but not 10 mg/kg) COC. Treatment with DPAT intensified COC-induced peripheral hyperactivity on days 1 and 7 of both chronic treatment regimens. Rats cotreated with DPAT and 10 mg/kg COC showed the progressive increase in activity typically associated with sensitization. Because this regimen of COC treatment alone did not result in a robust sensitization, the presence of DPAT appeared to enhance the development of sensitization. Curiously, despite this observation during treatment, rats previously exposed to DPAT plus COC did not express sensitization on COC challenge during withdrawal. However, when challenged with the combination of DPAT plus COC, enhanced activity levels were again observed in rats previously treated with DPAT plus COC. These data suggest that the expression of sensitization in the DPAT + COC rats is dependent on coadministration of the two drugs and may also suggest that the expression of sensitization is a state-dependent phenomenon (Wise et al., 1996).
Challenges with COC in the presence or absence of DPAT were conducted at early withdrawal time points (<1 week) and the times of challenges varied between 48 and 144 h of withdrawal. Rats exposed to intermittent injections of psychostimulants have actually been reported to express tolerance on challenge with the psychostimulant at withdrawal periods of less than 1 week, whereas longer withdrawal periods seem to be optimal to observe a sensitized response to COC (Pierce et al., 1995). Clearly, the withdrawal period is a time of dynamic fluctuations in monoamine neurotransmission (Kalivas et al., 1993), and therefore we cannot rule out the possibility that the expression of sensitization in various treatment groups or on specific challenge tests might be distinct at longer withdrawal time points.
An apparent behavioral tolerance developed to the suppressive effects of DPAT on central and vertical activities, whereas a significant enhancement of peripheral activity suggestive of sensitization was observed on repeated, intermittent exposure to DPAT. We propose that forward locomotion in the periphery typically associated with acute DPAT exposure (Alhenius et al., 1993), but which were below the limits of detection on day 1, became sensitized after repeated exposure to drug. Sensitization of activity in the periphery that develops after chronic administration of DPAT is a novel finding that contrasts with the tolerance observed to other DPAT-induced behaviors reported previously (Johansson et al., 1990; Renyi and Jimenez, 1993), but parallels the sensitization that developed during cotreatment with DPAT plus COC. At 18 h after the termination of treatment with 15 mg/kg COC (twice daily for 7 days), a heightened pharmacological supersensitivity of 5-HT1A receptors in the absence of changes in the density of [3H]DPAT binding has been demonstrated (Cunningham et al., 1992). The enhanced sensitivity to DPAT and DPAT plus COC in the present study may be related to altered sensitivity of 5-HT1Areceptors, although these modifications are probably not sufficient to generate behavioral sensitization because increased COC-evoked hyperactivity was not observed in rats treated with DPAT alone.
The actions of DPAT at 5-HT1A receptors are most likely to mediate its effects in the present study, although we did not analyze the ability of a selective 5-HT1Aantagonist to reverse the effects of DPAT on hyperactivity evoked by either acute or chronic administration of COC. This outcome would most likely be the case because DPAT induction of the 5-HT syndrome, including increases in forward locomotion (Przegalinski et al., 1994;Forster et al., 1995), its discriminative stimulus properties (Cunningham et al., 1987), and, for that matter, most of its neurochemical (Davidson and Stamford, 1995) and electrophysiological (Forster et al., 1995) effects, are reversed by 5-HT1A antagonists. Furthermore, the suppression of COC-evoked activity by DPAT was reversed by WAY 100135, although the hyperactivity evoked by COC was unaffected (Przegalinski and Filip, 1997). However, despite the relative selectivity of DPAT for 5-HT1A receptors, DPAT exhibits high affinity for the 5-HT7 receptor (e.g., McLean and Coupar, 1996); thus, the role of 5-HT7 receptor in behaviors evoked by DPAT cannot yet be ruled out.
The ability of DPAT to alter the activity profile observed after acute and chronic COC exposure might be related to its ability to modulate DA function by virtue of its actions as a 5-HT1Aagonist. The fact that activation of 5-HT1Areceptors after systemic administration of DPAT at low doses can increase DA cell firing in the VTA and substantia nigra (Sinton and Fallon, 1988; Kelland et al., 1990; Arborelius et al., 1993; Prisco et al., 1994) and increase extracellular DA levels in NAc (Boulenguez et al., 1996) and VTA (Chen and Reith, 1995) suggests that actions of DPAT at the level of the mesoaccumbens circuit may contribute to the observed behavioral effects. However, multiple sites of action for DPAT at 5-HT1A receptors to affect COC-induced behaviors may exist, and the contribution of specific 5-HT1A receptor populations to the observed effects of DPAT can be assessed by using microinjection techniques to isolate the location and nature of the interaction between 5-HT1A receptors and DA function.
In conclusion, we have shown that acute administration of the 5-HT1A agonist DPAT produced specific changes in locomotor activity patterns induced by COC, which are likely to be mediated by 5-HT1A receptors. We have also corroborated the importance of detailed microanalyses in the complicated data sets that arise from experiments in which locomotor activity results from cotreatment with drugs. Finally, our findings suggest that stimulation of 5-HT1Areceptors is capable of altering the patterns of activity evoked by COC. The manner in which this occurs, possibly via 5-HT1A interactions with DA mesolimbic systems that mediate the in vivo effects of COC, remains to be established.
Acknowledgments
We thank Drs. Malzorgata Filip and Mary Thomas for their helpful editorial insights into this research.
Footnotes
-
Send reprint requests to: Dr. Kathryn A. Cunningham, Department of Pharmacology and Toxicology, University of Texas Medical Branch, Galveston, TX 77555-1031.
-
↵1 This work was supported by National Institute on Drug Abuse Grants DA05708, DA06511 (K.A.C.), and DA05638 (R.D.). Portions of this research were presented at the annual meeting of the Society for Neuroscience, Miami, FL, November, 1994, and at the annual meeting of the College on the Problems of Drug Dependence, Scottsdale, AZ, June, 1995. This research was presented to the Graduate School of the Biomedical Sciences at the University of Texas Medical Branch in partial fulfillment of the requirements for the degree of Doctor of Philosophy.
-
↵2 Present address: Department of Psychiatry, Yale University School of Medicine, New Haven, CT 06520.
- Abbreviations:
- DA
- dopamine
- 5-HT
- 5-hydroxytryptamine (serotonin)
- COC
- cocaine
- SAL
- saline
- DPAT
- 8-hydroxy-2-(di-n-propylamino)tetralin
- NAc
- nucleus accumbens
- VTA
- ventral tegmental area
- Received February 3, 1999.
- Accepted September 13, 1999.
- The American Society for Pharmacology and Experimental Therapeutics