Abstract
This study was undertaken to evaluate the role of glutamate receptors at spinal synapses on the ascending limb of the micturition reflex. In urethane-anesthetized female rats, a tungsten electrode was inserted stereotaxically into the dorsal part of the rostral pons to record field potentials which were evoked by electrical stimulation of the pelvic nerve (PLN) (1–15 V, 0.05 ms pulse duration at 100–300 Hz, 5–30 ms train duration). The effects of glutamate receptor antagonists administered intrathecally (i.t.) on the PLN-evoked field potentials in the dorsal part of the rostral brainstem were examined. PLN stimulation evoked short latency (10–22 ms) negative field potentials (85 ± 4 μV) in a limited area of the dorsal part of the rostral pons (bregma −9.0 to −8.4, L 0.5 to 1.5, H 4.2 to 5.4). The i.t. administration of LY215490 (0.1–30 μg), a competitive α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor antagonist, reduced the amplitude of the evoked potentials in a dose-dependent manner; 84 ± 6%, 59 ± 11% (P < .001), 31 ± 10% (P < .001), 17 ± 9% (P < .001) of control after 0.1, 1, 10, 30 μg of LY215490, respectively. The i.t. administration of MK-801 (1–100 μg), a noncompetitive N-methyl-d-aspartate (NMDA) receptor antagonist, also reduced the amplitude of the evoked potentials in a dose-dependent manner; 93 ± 21%, 76 ± 14%, 52 ± 9% (P < .001), 39 ± 9% (P < .001) of control after 1, 10, 30, 100 μg of MK-801, respectively. Combined administration of LY215490 (0.1 μg) and MK-801 (1 μg), in doses which individually did not elicit a significant effect, markedly reduced the amplitude of the evoked potentials (27 ± 9% of control, P = .0002). These results suggest that AMPA and NMDA glutamatergic synaptic mechanisms play a key role in the spinal processing of afferent input from the bladder and that these mechanisms function synergistically in the ascending limb of the spinobulbospinal micturition reflex pathway.
Micturition is mediated by a spinobulbospinal reflex pathway consisting of: (1) an ascending limb from the lumbosacral spinal cord, (2) an integration center in the rostral brainstem known as the PMC and (3) a descending limb from the PMC back to the parasympathetic nucleus in the lumbosacral spinal cord (Kuru, 1965; Mallory et al., 1989;Noto et al., 1991; de Groat et al., 1993). Previous pharmacological studies revealed that glutamic acid plays an important role in the reflex pathways controlling the lower urinary tract. Administration of NMDA receptor antagonists (MK-801 or LY274614) or AMPA/kainate receptor antagonists (GYKI 52466 or LY215490) to urethane-anesthetized rats depressed the amplitude of reflex bladder contractions evoked by bladder distension (Maggi et al., 1990; Yoshiyama et al, 1991, 1993a, b, 1995a; Kakizakiet al, 1996) or by electrical stimulation of the PMC (Matsumoto et al., 1995a, b). Further, microinjection of glutamate or related excitatory amino acids into the PMC or adjacent areas facilitated micturition in the rat and cat (Willette et al, 1988; Mallory et al., 1991). These data indicate that glutamatergic mechanisms play a pivotal role at synapses in the PMC and in the descending limb of the micturition reflex pathway. However, a possible role of glutamate in the ascending limb of the micturition reflex pathway has not been examined.
In the present study electrophysiological techniques were used to evaluate the contribution of spinal NMDA and AMPA glutamatergic mechanisms to the ascending limb of the micturition reflex pathway in the rat. Previous studies revealed that glutamatergic mechanisms are involved in spinal nociceptive and non-nociceptive transmission (Dougherty et al., 1992; Coderre, 1993; Cumberbatch et al., 1994; Birder and de Groat, 1992; Kakizaki et al., 1996). In addition, synergistic interactions between NMDA and AMPA glutamate receptors have been detected in the spinal processing of nociceptive input from the lower urinary tract (Kakizaki et al., 1996). Possible interactions between these two types of glutamate receptors in the ascending limb of the micturition reflex pathway were also examined in this study.
Preliminary accounts of these findings have appeared in an abstract (Kakizaki et al., 1997).
Materials and Methods
Animal preparation.
Experiments were performed on 20 female Wistar rats weighing 265 to 320 g (mean-286 g). The animals were anesthetized with a subcutaneous injection of urethane (1.2 g/kg), and cannulae (PE-50) were placed in the carotid artery for monitoring blood pressure and in the external jugular vein for intravenous drug administration. A tracheostomy tube (PE-240) was inserted to facilitate respiration and permit artificial ventilation after neuromuscular blockade.
An i.t. catheter was inserted with a modification of the technique described by Yaksh and Rudy (1976). The atlanto-occipital membrane was incised at the midline with the tip of an 18-gauge needle. A catheter (PE-10), which was filled with artificial CSF (Feldberg and Fleischhauer, 1960), was inserted through the slit into the subarachnoid space and advanced caudally to the L6-S1 level of the spinal cord. The volume of fluid within the catheter was kept constant at 10 μl for all experiments by adjusting the length of the tubing precisely (16 cm). At the end of experiment, a laminectomy was performed to verify the position of the catheter tip.
Through a mid-line abdominal incision, the PLN was isolated and prepared for stimulation. To prevent the bladder from filling with urine during the experiment, both ureters were tied distally, cut and the proximal ends cannulated (PE-10) and drained externally. The urinary bladder was cannulated by passing a catheter (PE-90) through the external urethral orifice. The catheter was secured in place by a ligature around the urethral orifice and connected to a pressure transducer to monitor intravesical pressure.
The rat was placed in a stereotaxic apparatus and a small craniotomy was performed to insert a recording electrode into the dorsal pontine tegmentum. After completion of the surgical procedures, the animal’s body was rotated 120° to expose the urinary bladder and PLN. Abdominal skin flaps were tied to a metal frame to form a cavity that was filled with warm mineral oil. The PLN was placed on bipolar silver electrodes for stimulation. A fine monopolar tungsten electrode (diameter, 10–20 μm), insulated with Teflon except for the tip, was used for recording in the brainstem. Animals were paralyzed with α-bungarotoxin (0.4 mg/kg i.v.), a noncompetitive blocker of striated muscle nicotinic receptors, and artificially ventilated. This agent has a long duration of action which allowed one dose to produce complete neuromuscular blockade throughout the experiment (3–6 hr) (Chiappinelli, 1985).
Initially, the bladder was filled slowly with saline (0.04 ml/min) to confirm the presence of reflex bladder contractions. Then the bladder was emptied through the urethral catheter. A recording electrode was introduced stereotaxically into the medial part of the dorsal pontine tegmentum in 0.25- or 0.5-mm steps. Sites in the pons were identified where pelvic afferent stimulation (1–15 V, 0.05 ms pulse duration at 100–300 Hz, 5–30 ms train duration) evoked field potentials. These stimulus parameters were based on a previous report (Noto et al., 1991). Neural activity was displayed on an oscilloscope, and 10 successive evoked potentials were averaged with a digital computer and plotted on a plotter. The latency measurements were made from computer-averaged evoked potentials. The latencies were measured from the stimulus artifact to the peak amplitude of the evoked potentials. Stimulation parameters were varied to evoke a response of maximal amplitude and shortest measurable latency. Recording sites were identified by stereotaxic coordinates. Histological confirmation of the location of the tip of the recording electrode was not performed in this study.
Glutamate antagonists were administered i.t.. Before drug administration, control injections (10 μl) of artificial CSF were tested to evaluate possible injection artifacts. Drug solution was injected slowly for a period of 30 sec to 1 min to minimize volume effects. Cumulative drug dose-response curves were obtained by giving i.t. injections of LY215490, a selective, competitive AMPA receptor antagonist (Ornstein et al., 1993a, b; Gill and Lodge, 1994;Schoepp et al., 1995) or MK-801, a noncompetitive NMDA receptor antagonist, every 15 min. In this study, cumulative doses of LY215490 and MK-801 injected i.t. were 0.1, 1, 10 and 30 μg and 1, 10, 30 and 100 μg, respectively. To accomplish sequential administrations of several concentrations of drugs, the intrathecal catheter, which was initially filled with 10 μl of artificial CSF (vehicle), was flushed sequentially with the following volumes of drugs and vehicle; LY215490: (1) 10 μl of 0.01 μg/μl solution; (2) 9 μl of 0.1 μg/μl solution and 1 μl of vehicle; (3) 9 μl of 1 μg/μl solution and 1 μl of vehicle; (4) 10 μl of 10 μg/μl solution; and (5) 2 μl of 10 μg/μl solution; MK-801: (1) 10 μl of 0.1 μg/μl solution, (2) 9 μl of 1 μg/μl solution and 1 μl of vehicle, (3) 10 μl of 10 μg/μl solution, (4) 2 μl of 10 μg/μl solution, and (5) 7 μl of 10 μg/μl solution. These doses were chosen based on the results of pilot experiments (n = 5) as well as previous experiments (Yoshiyamaet al., 1997).
Evaluation and statistical analysis.
The effects of drugs on the latency and amplitude at the peak of the evoked potentials were evaluated 10 min after each dose. Latency to peak rather than to the onset of the evoked potentials was used because it exhibited greater stability during the experiment. All values in the text are expressed as mean ± S.E.M. The changes in the peak amplitude of the evoked responses after drug treatments were evaluated statistically with repeated measures analysis of variance (ANOVA) followed by Tukey-Kramer test as a post hoc multiple comparison procedure. For all statistical tests, P < .05 was considered significant.
Drugs.
Drugs used in this study include: α-bungarotoxin (Sigma Chemical Co., St. Louis, MO), LY215490 [(3SR,4aRS,6RS,8aRS)-6-[2-(1H-tetrazol-5-yl)ethyl]decahydroisoquinoline-3-carboxylic acid, Lilly Research Laboratories, Indianapolis, IN], MK-801 (dizocilpine, Merck, Sharp & Dohme Research Laboratories, West Point, PA). LY215490 and MK-801 were dissolved in artificial CSF and these solutions were then adjusted to pH 7.2 to 7.4. α-Bungarotoxin was dissolved in saline.
Results
Evoked responses in the dorsal part of the rostral pons after PLN stimulation.
PLN stimulation evoked short latency (10–22 ms) negative field potentials (85 ± 4 μV) in a relatively limited area of the PAG (bregma −9.0 to −8.4, L 0.5 to 1.5, H 4.2 to 5.4). The threshold intensity of PLN stimulation ranged from 1 to 4 V in different animals. The latency from the onset of stimulation to the peak of the evoked potentials was 37.4 ± 1.8 ms. In preliminary experiments (n = 3), repeated injections of 10 μl of artificial CSF (up to five or six times, every 15 min) did not change the latency and amplitude of the evoked potentials.
Effects of AMPA receptor antagonist (LY215490) on the evoked response elicited by PLN stimulation.
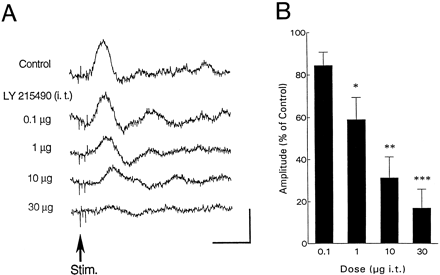
Intrathecal injection of vehicle (artificial CSF) did not have any effect on the evoked potentials. Cumulative doses of LY215490 (0.1, 1, 10 and 30 μg) were injected i.t. every 15 min (n = 5). Intrathecal injection of LY215490 reduced the amplitude of the evoked potentials in a dose-dependent manner (fig. 1): 84 ± 6%, 59 ± 11% (P < .001), 31 ± 10% (P < .001) and 17 ± 9% (P < .001) of control after 0.1, 1, 10 and 30 μg of LY215490, respectively. The evoked responses did not recover during the 4-hr period after the highest dose of LY215490. The drug did not change the latency of the evoked potentials (table1).
(A) Effect of i.t. administration of AMPA antagonist (LY215490) on the potentials in the PAG evoked by PLN stimulation (upward trace indicates negative potential). Each record is made by averaging 10 successive evoked responses. Cumulative doses of LY215490 are indicated. Vertical and horizontal calibrations correspond to 100 μV and 50 ms, respectively. (B) Dose-response relationship after i.t. administration of LY215490 (n = 5). Abscissa, the dose of LY215490; ordinate, amplitude of the evoked potentials. * P < .001 vs. control, P < .05vs. 0.1 μg. ** P < .001 vs. control and 0.1 μg, P < .01 vs. 1 μg. *** P < .001 vs. control, 0.1 and 1 μg.
Effect of i.t. administration of AMPA (LY215490) and NMDA (MK-801) glutamate antagonists on the latency (ms) of potentials in the PAG evoked by PLN stimulation
Effects of NMDA receptor antagonist (MK-801) on the evoked responses elicited by PLN stimulation.
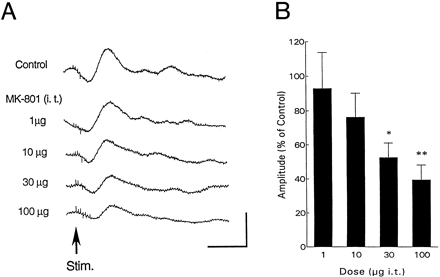
Cumulative doses of MK-801 injected i.t. every 15 min (n = 5) reduced the amplitude of the evoked potentials in a dose-dependent manner (fig.2). The potentials were reduced to 93 ± 21%, 76 ± 14%, 52 ± 9% (P < .001) and 39 ± 9% (P < .001) of control after 1, 10, 30 and 100 μg of MK-801, respectively. The evoked responses did not recover during the 4-hr period after the highest dose of MK-801. The latency of the evoked potentials was not altered by MK-801 (table 1).
(A) Effect of i.t. administration of NMDA antagonist (MK-801) on the potentials in the PAG evoked by PLN stimulation. Each record is made by averaging 10 successive evoked responses. Cumulative doses of MK-801 are indicated. Vertical and horizontal calibrations correspond to 100 μV and 50 ms, respectively. (B) Dose-response relationship after i.t. administration of MK-801 (n = 5). Abscissa, the dose of MK-801; ordinate, amplitude of the evoked potentials. * P < .001 vs. control, P < .01 vs. 1 μg. ** P < .001vs. control and 1 μg, P < .01 vs. 10 μg.
Effects of combined administration of MK-801 (1 μg) and LY215490 (0.1 μg) on the potentials evoked by PLN stimulation.
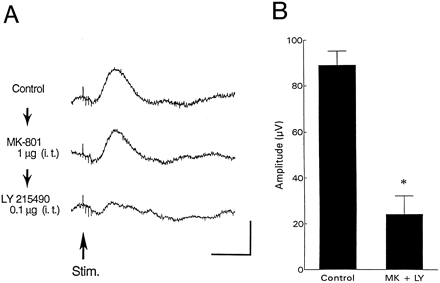
Combined i.t. administration of MK-801 and LY215490 was examined in five rats by injecting one of the drugs and then 15 min later injecting the second drug. Combined administration of 1 μg of MK-801 and 0.1 μg of LY215490, which individually did not have a significant effect on the amplitude of the evoked potentials (figs.1 and 2), significantly reduced (to 27% of control, P = .0002) the amplitude of the evoked potentials (fig. 3). Combined administration of MK-801 and LY215490 did not change the latency of the evoked potentials (table 1).
(A) Effect of combined i.t. administration of MK-801 (1 μg) and LY215490 (0.1 μg) on the potentials in the PAG evoked by PLN stimulation. Vertical and horizontal calibrations correspond to 100 μV and 50 ms, respectively. (B) Changes of the amplitude of the evoked potentials after combined administrations of MK-801 (1 μg) and LY215490 (0.1 μg) (n = 5). * P = .0002 vs. control.
Discussion
This study revealed that both AMPA and NMDA glutamatergic transmission plays a key role in the spinal processing of afferent input from the bladder and that spinal NMDA and AMPA glutamatergic synaptic mechanisms interact synergistically in the ascending limb of the spinobulbospinal micturition reflex pathway.
In the present study, short latency (10–22 ms) field potentials were evoked by PLN stimulation in a relatively limited area of the PAG. These findings confirm the results of a previous study which showed that short latency (13 ± 3 ms) field potentials were elicited by PLN stimulation in the PAG (Noto et al., 1991). Because the latency for PLN to the PAG potentials was shorter than the latency for PLN to the PMC potentials (Noto et al, 1991), it was suggested that the PAG might function as the receiving area for the ascending limb of the micturition reflex pathway and that neurons in the PAG might relay information to neurons in the PMC which then provide an input back to the sacral parasympathetic nucleus. Further support for this concept has been obtained in axonal tracing studies in the cat (Blok and Holstege, 1994; Blok et al, 1995; VanderHorst et al, 1996).
Intrathecal injection of an AMPA or NMDA antagonist reduced the amplitude of the evoked potentials in a dose-dependent fashion (figs. 1and 2). Glutamate is present in both primary afferent pathways as well as in interneurons of the dorsal horn (Cotman et al., 1987;Wanaka et al., 1987; Westlund et al., 1990; Araki and de Groat, 1996). Thus glutamate receptor antagonists injected i.t. might act at several sites. For example, they might reduce the synaptic transmission from primary afferent fibers to interneurons or spinal tract neurons, or depress interneuronal transmission.
Previous studies have shown that c-fos expression, a functional marker for nociceptive pathways in CNS, was increased in the spinal cord after chemical irritation of the lower urinary tract and that AMPA and NMDA antagonists (LY215490 and MK-801, respectively) reduced the c-fos expression in all of the relevant areas of the spinal cord, including dorsal horn, dorsal commissure and lateral laminae V–VII (Birder and de Groat, 1992; Kakizaki et al., 1996). These observations are consistent with the present findings; together they provide strong support for the view that glutamatergic synapses are essential for the spinal processing of nociceptive and non-nociceptive afferent input from the bladder.
In the present study, LY215490 was used as an AMPA receptor antagonist. Previous in vivo and in vitro studies indicate that this drug is a potent, competitive antagonist at AMPA receptors.In vitro binding studies indicate that this agent acts as a competitive antagonist at AMPA receptors with an IC50 of 1.8 to 4.8, but at a 10-fold higher concentration it can also act as an antagonist at NMDA receptors (Schoepp et al., 1996). In vivo at doses between 25 and 75 mg/kg i.p. LY215490 elicited a selective antagonism of the neurotoxic effect of AMPA in the rat striatum, without affecting the neurotoxicity to NMDA (Schoepp et al., 1996). In the mouse, even much larger doses (up to 320 mg/kg i.p.) did not alter NMDA toxicity (Ornstein et al., 1993a, b). However, a concern in interpreting the present data is whether the doses of LY215490 administered i.t. act selectively on AMPA receptors or whether they also have some effect on NMDA receptors. Indirect evidence suggests that the effects of at least the low doses of the drug (0.1 and 1 μg) on the ascending limb of the micturition reflex are caused by block of AMPA receptors. For example, it is clear from other studies in vivo and in vitro that the micturition reflex depends on AMPA glutamatergic mechanisms as evidenced by block of AMPA receptors with various drugs (CNQX, GYKI52466) (Matsumoto et al., 1991; Sugaya and de Groat, 1994; Araki and de Groat, 1996;Yoshiyama et al., 1995a) and that NMDA mechanisms also play an important role (Maggi et al., 1990; Yoshiyama et al., 1991, 1993a, b). However, under certain conditions such as the decerebrate unanesthetized rat, AMPA mechanisms play an essential role and NMDA mechanisms are not required. In these animals low doses of LY215490 administered i.v. (1–10 mg/kg) or i.t. (0.1–10 μg) depressed the micturition reflex (Yoshiyama et al, 1997); whereas even large doses of MK-801 (up to 30 mg/kg i.v.) did not induce a detectable block (Yoshiyama et al., 1994). These data indicate that the lower doses (0.1–1 μg) of LY215490 used in the present experiments acted selectively on AMPA receptors in the spinal cord. However, it is impossible to determine whether the higher doses of the drug (10–30 μg i.t.) acted selectively or whether the effects of these doses were caused by a combined block of both types of glutamatergic receptors.
Combined administration of low doses of AMPA and NMDA antagonists, which individually did not have a significant effect on PLN-evoked potentials, dramatically reduced the amplitude of the evoked potentials (mean decrease-73%). This result indicates that fast (AMPA) and slow (NMDA) types of glutamatergic transmission interact synergistically in the spinal processing of afferent input from the bladder. Synergistic interactions between AMPA and NMDA glutamate receptor antagonists to depress spinal c-fos expression after lower urinary tract irritation also have been demonstrated previously (Kakizaki et al., 1996). This synergism is attributable to the action of AMPA/kainate receptors to induce synaptic depolarization, which facilitates the opening of NMDA receptor channels (Collingridge and Lester, 1989). Membrane depolarization removes the voltage-dependent blockade of the NMDA receptor by Mg++ (MacDonald and Nowak, 1990) and thereby enhances NMDA receptor-mediated transmission. This unblocking of NMDA receptor channels is likely to occur during high levels of afferent input induced by bladder distension or electrical stimulation of afferent fibers in PLN that would release glutamate as well as other transmitters at spinal synapses. Thus synergistic interactions between AMPA and NMDA synaptic mechanisms seem to be necessary for optimal transmission of afferent information from the bladder to the PMC. Consequently, combinations of AMPA and NMDA receptor antagonists produce a more dramatic depressant effect than administration of individual antagonists.
Previous studies of somatic sensory pathways have indicated that non-NMDA receptors mediate monosynaptic activation of dorsal horn neurons by primary afferent fibers, whereas NMDA receptors mediate polysynaptic inputs to the dorsal horn (Davies and Watkins, 1983;Schouenborg and Sjolund, 1986; Evans and Long, 1989; Morris, 1989;Dickenson and Sullivan, 1990). A similar organization of glutamatergic mechanisms has been described in the afferent pathways to primate spinothalamic tract neurons (Dougherty et al., 1992) and may be important in processing afferent input from the bladder.
In this study, i.t. injection was used to deliver drugs exclusively to the spinal cord. A previous study showed that 15 min after i.t. injection of 10 μl of dye (1% methylene blue) at the caudal lumbar level, the dye was distributed to the T9-S4 level of the spinal cord (Igawa et al., 1993). Thus in the present experiments there was sufficient time for drugs administered at the L6-S1 level to have acted at other levels of the neuraxis. However, because afferent pathways in the PLN project almost exclusively to L6-S1 (de Groat et al., 1993) and because these segments are important for spinal processing of afferent input from the bladder (Birder and de Groat, 1993), it is unlikely that the spread of drugs beyond the L6-S1 level of the spinal cord would have prominent qualitative influence on the results of this study. This conclusion is supported further by the finding that injections of LY215490 at rostral levels of the spinal cord (C2) elicited a very delayed (75 min) and weak depression of bladder reflexes (Yoshiyama et al., 1997), whereas injections at L6-S1produced a rapid onset and complete block of reflexes.
Previous pharmacological studies have revealed that administration of NMDA receptor antagonists (MK-801 or LY274614) or AMPA/kainate receptor antagonists (GYKI 52466 or LY215490) to the urethane-anesthetized rats depressed the amplitude of reflex bladder contractions evoked by bladder distension (Maggi et al., 1990; Yoshiyama et al, 1991, 1993a, b, 1995a) or by electrical stimulation of the PMC (Matsumoto et al., 1995a, b). Synergistic depressant effects of AMPA and NMDA glutamate receptor antagonists on the amplitude of reflex bladder contractions also have been detected in unanesthetized decerebrate rats (Yoshiyama et al, 1995b,1997). Patch-clamp studies in the neonatal rat spinal slice preparation indicate that AMPA and NMDA glutamate receptors are important in the mediation of fast and slow transmission, respectively, between excitatory interneurons and preganglionic neurons in the lumbosacral parasympathetic nucleus (Araki and de Groat, 1996) These data indicate that glutamatergic transmission plays a crucial role in the function of the descending or efferent component of the micturition reflex pathway. This study provides evidence that spinal glutamatergic transmission also plays a pivotal role in the ascending limb of the micturition reflex pathway. Thus, glutamate receptors at spinal synapses on both ascending and descending limbs of the micturition reflex pathway are important for the control of micturition.
In summary, the results of this and previous studies indicate that glutamate is an important neurotransmitter in the micturition reflex pathway in the rat. The spinal processing of afferent input from the urinary bladder depends on transmission mediated by both AMPA and NMDA receptors. In addition, a synergistic interaction between AMPA and NMDA transmission appears to be important in the ascending limb of the spinobulbospinal micturition reflex pathway.
Acknowledgments
The authors are grateful to Eli Lilly and company, and Merck, Sharp & Dohme Research Laboratories for gifts of LY215490 and MK-801, respectively.
Footnotes
-
Send reprint requests to: William C. de Groat, Ph.D., Department of Pharmacology, University of Pittsburgh School of Medicine, W1357 Biomedical Science Tower, Pittsburgh, PA 15261.
-
↵1 This work was supported by National Institutes of Health Grants DK-49430 (W.D.) and DK-51402 (W.D.).
-
↵2 Present address: Department of Urology, Hokkaido University School of Medicine, Sapporo, 060 Japan.
- Abbreviations:
- AMPA
- α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- NMDA
- N-methyl-d-aspartate
- PMC
- pontine micturition center
- CSF
- cerebrospinal fluid
- PLN
- pelvic nerve
- PAG
- periaqueductal gray
- CNS
- central nervous system
- i.t.
- intrathecal
- Received April 24, 1997.
- Accepted November 28, 1997.
- The American Society for Pharmacology and Experimental Therapeutics