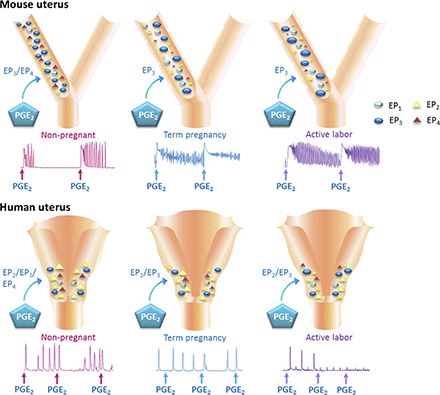
Visual Overview
Abstract
Prostaglandin (PG) E analogs are used clinically to ripen the cervix and induce labor. However, selective receptor agonists may have potential to improve induction response rates or manage unwanted uterine hypercontractility in conditions such as dysmenorrhea and preterm labor. To characterize their therapeutic value, PGE2 analogs were used to investigate the functional E‐type prostanoid (EP) receptor population in isolated human uterus. Responsiveness in mouse tissues was also examined to validate its use as a preclinical model. Uterine samples were obtained from mice at dioestrus (n = 12), term gestation (n = 14), and labor (n = 12) and from the lower uterus of women undergoing hysterectomy (n = 12) or Caesarean section (n = 18). Vehicle and agonist effects were assessed using superfusion and immersion techniques. PGE2 evoked predominant excitatory responses in mouse and relaxation in human tissues. Selective EP4 agonists inhibited tissue activity in both nonpregnant species, while the EP2 mimetic CP533536 also attenuated uterine contractions throughout gestation. The uterotonic effects of the EP3/1 agonist sulprostone were more pronounced than the EP1 agonist ONO-D1-004, corresponding to abundant EP3 receptor expression in all samples. The contractile phenotype in mouse compared with human uteri may relate to regional differences as well as high expression of EP3 receptor transcripts. Similarities in nonpregnant and gestational tissues across species suggest that EP3 may represent a valuable translational drug target for preventing uterine hypercontractility by employing a selective antagonist.
SIGNIFICANCE STATEMENT This research validates the use of nonpregnant mice for preclinical drug discovery of uterine EP receptor targets. To determine the utility of novel drugs and delivery systems at term pregnancy and labor, pharmacological agents interacting with EP3 receptors have clear translational value.
Introduction
Series two prostaglandins (PGs) consist of PGD2, PGE2, PGF2α, prostacyclin, and thromboxane A2. PGE2 exhibits a wide spectrum of physiological and pathological functions in various cells and tissues (Coleman et al., 1994; Sugimoto and Narumiya, 2007) and exerts these effects primarily via four EP receptor subtypes. EP1 and EP3 receptors produce smooth muscle contraction. The EP1 receptor mediates this response through an increase in intracellular calcium (Ca2+), while activation of the EP3 receptor predominantly inhibits smooth muscle relaxation through a decrease in adenylyl cyclase. In contrast, EP2 and EP4 receptors mediate uterine quiescence through the intracellular adenylyl cyclase and cAMP pathway (Sugimoto and Narumiya, 2007). EP1, EP2, EP3, and EP4 have all been isolated and cloned in the mouse (Sugimoto et al., 1992; Honda et al., 1993; Watabe et al., 1993; Nishigaki et al., 1995), revealing the presence of multiple splice variants for EP3 in mice (Sugimoto and Narumiya, 2007) and humans (Matsuoka and Narumiya, 2007) that are evolutionally conserved (Hla, 2005). Their pleiotropic effects are regulated by receptor expression, constitutive activity, signal transduction pathways, and agonist-induced desensitization (Sugimoto and Narumiya, 2007; Markovič et al., 2017) that differ in health and disease.
PGE2 mediates reproductive processes, including ovulation, fertilization, implantation, luteolysis, and uterine contractility (Challis et al., 2002; Valdes et al., 2009). In women, total PG concentrations increase at labor compared with term pregnancy (Durn et al., 2010), with a concurrent rise in intrauterine and amniotic fluid PGE2 release throughout parturition (Lee et al., 2008; Durn et al., 2010). A switch in the activator protein family of transcription factors regulates amniotic PGE2 synthesis and promotes uterine contraction (Lu et al., 2019). Cervical ripening and induction of labor with vaginal, intracervical, or oral PGE2 are recommended to safely expedite neonatal delivery (NICE, 2008; Vogel et al., 2017; Sheibani et al., 2018; Zhao et al., 2019) and reduce stillbirth rates (Hedegaard et al., 2014). However, aberrant PGE2 output and an imbalance in EP gene receptor expression are associated with in vitro fertilization failure (Ruan et al., 2012) preterm labor (Calder, 1990; Gouveia-Figueira et al., 2017) and a lack of response to labor induction (Konopka et al., 2015). Impaired ovulation, fertility, and decreased litter size were displayed in EP2-deficient mice (Hizaki et al., 1999; Kennedy et al., 1999; Tilley et al., 1999). However, the physiological role of each EP subtype in regulating uterine contraction is not well defined.
The aim of this study was to characterize functional EP receptors as potential therapeutic targets in uterine tissues using highly selective agonists. Their temporal and regional expression was examined in the isolated mouse uterus during the oestrous cycle, at term gestation (day 18), and during labor. It was hypothesized that predominant excitatory EP1/3 receptors in the fundus and EP2/4 subtypes in the lower uterus would facilitate caudal contractions for menses and parturition. Efficacy was compared with uterine tissues from nonpregnant and term pregnant women to determine whether the mouse is a suitable system for investigating human uterine biology and pregnancy complications.
Materials and Methods
Experiments on Isolated Mouse Uterine Tissue.
Nonpregnant and pregnant female sexually mature Bankin Kingman White (BKW) mice (B & K Universal Ltd., Hull, UK) housed in groups were used throughout this study. All experimental procedures and animal care were carried out at the University of Bradford in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health, the amended Animals Scientific Procedures Act, UK 2012, and ARRIVE guidelines. The animals had free access to food and water and were exposed to a 12-hour light/12-hour dark cycle. Animals used in gestational studies were mated in a harem of three females to one male. The presence of a vaginal plug was observed as evidence of pregnancy; the day of plug detection was termed day 1 of gestation. The mice were weighed regularly to monitor the progression of pregnancy.
The mice were humanely killed by cervical dislocation when nonpregnant (n = 12), at day 18 of gestation (n = 14), or during labor (one to two pups delivered; n = 12). The uterus was dissected from the body, and uterine samples were taken from different anatomic segments along the length of each uterine horn (Griffiths et al., 2006). In nonpregnant animals, the stage of the oestrous cycle was determined by microscopic examination of the vaginal lavage (McLean et al., 2012). In the pregnant and laboring mice, the fetuses were carefully removed from the uterus and were sacrificed by carbon dioxide inhalation.
Uterine samples were immediately set up for superfusion as previously described (Griffiths et al., 2006). In brief, the strips were attached to isometric force transducers under a resting tension of 2 g, and aerated Krebs’ solution (95% O2/5% CO2) was driven through heating coils (37°C) at a rate of 2 ml/minute (Griffiths et al., 2006). The isolated uterine samples were equilibrated for 30 minutes before the start of drug treatments.
In uterine tissue samples from nonpregnant animals and those taken at late gestation, spontaneous activity diminished after 30 minutes. It was, therefore, difficult to examine the inhibitory effects of the agonists. The inclusion of 5-hydroxytryptamine (5-HT) at 10−6 M to the perfusate reservoir, which enhances acetylcholine and Ca2+ release (Griffiths, 2007), produced uniform, regular myogenicity, allowing the inhibitory properties of the compounds to be explored. Tissue taken during labor displayed a sustained level of activity, so 5-HT was not included in the reservoir for these strips.
PGE2 and the following selective EP agonists were used: ONO-D1-004, a selective EP1 agonist (Oka et al., 2003); the selective EP2 agonists butaprost (Gardiner, 1986) and CP533536 (Li et al., 2003; Paralkar et al., 2003); sulprostone, an EP1 and EP3 receptor agonist (Schaaf et al., 1981); and the EP4 agonists lactam derivative of 8-aza-11-deoxy-PGE1 (LAC-PGE) (Elworthy et al., 2004) and L-902688 (Billot et al., 2003). They were directly administered into the superfusate as 10 μl bolus doses (10−11–10−7 mol) at 10-minute intervals or until baseline activity had resumed. Agonists were administered immediately after a spontaneous contraction to avoid superimposing responses on background activity; this did not apply if there was no background activity present. Time-matched vehicle controls were also assigned, and only one dose-response curve was performed per tissue. At the end of each experiment, the Krebs’ superfusate was replaced with distilled water (Popat and Crankshaw, 2001); this induced a reference contraction (hypotonic shock) that was unique to each tissue strip.
Experiments on Isolated Human Uterine Tissue.
Lower segment myometrial biopsies were obtained from premenopausal women undergoing hysterectomy for benign disorders, such as fibroids, pelvic pain, and heavy menstrual bleeding (aged 26–44; follicular stage; n = 12). At the time of surgery, none of the donors were using oral contraceptives or had received any hormone therapy. Segments of the upper margin of lower uterine muscle were also obtained at Caesarean section from healthy term pregnant women (aged 22–38; 37–40 weeks) not in labor (n = 10) or during active labor (regular contractions, cervical dilation 3–8.5 cm; n = 8). Indications included previous Caesarean section (n = 12), breech presentation (n = 2), fetal distress (n = 2), and prolonged labor (n = 2), none of which influenced in vitro phasic contractions (data not shown). Women who were taking regular prescription medication, who were with multiple fetuses, or who were associated with any major complication of pregnancy, such as hypertension, pre-eclampsia, and diabetes, were excluded from this study. Uterine specimens were obtained from the Yorkshire Clinic, Bradford, and Bradford Royal Infirmary; all women provided informed written consent prior to surgery. Ethical approval for all studies was obtained from the National Research Ethics Committee (LREC: 07/H1306/98).
Human uterine samples were prepared for immersion within an hour of collection as previously described (Fischer et al., 2008). In brief, myometrial strips (10 × 2 × 3 mm), trimmed of endometrial, serosal, fat, and fibrous tissue, were set up in organ baths containing oxygenated Krebs’ solution (95% O2, 5% CO2, 37°C) and attached to isometric force transducers under a resting tension of 2 g (Senior et al., 1991; Senior et al., 1993). Tissues from nonpregnant and term pregnant donors were equilibrated for at least 90 and 120 minutes, respectively, or until regular phasic contractions had developed. Vehicle and cumulative concentration-effect curves for PGE2 and EP agonists (10−10–10−5 M) were added at 30-minute intervals, and only one treatment was assigned per tissue. Responses were expressed as a percentage of hypotonic shock, induced by displacing the Krebs’ solution with distilled water (Popat and Crankshaw, 2001).
Measurement of Responses.
Uterine activity was measured via isometric force transducers (Grass Instruments Inc., RI, USA) linked to PowerLab hardware (AD Instruments Ltd., Dunedin, New Zealand). PowerLab was connected to a PC, and Chart 5 for Windows (AD Instruments Ltd., Dunedin, New Zealand) was used to display the changes in tissue tension. The integrated area under the curve was measured for 5 and 30 minutes after drug administration for mouse (Griffiths et al., 2006) and human (Fischer et al., 2008) tissues, respectively, and was expressed as a percentage of the hypotonic shock reference contraction. Superfusion was suitable for highly contractile mouse tissues (measured as moles within the Krebs’ superfusate), whereas the immersion technique better suited slow phasic human myometrial activity (measured as Molar in a defined 8 ml organ bath volume). Both are commonly used comparable methods for receptor characterization (Hillock and Crankshaw, 1999; Popat and Crankshaw, 2001; Duckworth et al., 2002; Griffiths et al., 2006; Fischer et al., 2008).
Real-Time Reverse-Transcription Polymerase Chain Reaction Analysis.
Total RNA was extracted from snap frozen mouse uterine tissues using TRIzol reagent (Invitrogen Life Technologies, Paisley, UK) followed by the mirVana miRNA isolation Kit (Thermo Fisher Scientific, Waltham, MA) according to the manufacturers’ instructions. RNA (1 μg) was converted into cDNA (QuantiTect Reverse Transcription kit; Qiagen, Manchester, UK), and gene expression of β-actin (NM_007393), glyceraldehyde-3-phosphate (GAPDH; NM_008084), EP1 (NM_013641), EP2 (NM_008964), EP3 (NM_011196), and EP4 receptors (NM_001136079) in uteri from nonpregnant, pregnant, and laboring mice were quantified by real-time reverse-transcription polymerase chain reaction (qRT-PCR). The probes for each gene (Qiagen, Manchester, UK) were used in combination with the QuantiTect SYBR Green Supermix (Qiagen, Manchester, UK) on a Quantstudio 12K flex real-time PCR system (Applied Biosystems, Paisley, UK). Absolute gene transcription was normalized to β-actin and GAPDH, and the relative values compared with internal controls shown.
Statistical Analysis.
Data were first tested for normality using a Shapiro-Wilk test. Changes in contractile activity and gene expression were determined using two-way ANOVA with Tukey’s post hoc test (GraphPad Prism 8.0 software; San Diego, CA). Where data were nonparametric, a Friedman’s two-way ANOVA with post hoc test was performed (SPSS version 22; NY). A probability of P < 0.05 was considered to be significant.
Compounds.
5-HT was obtained from Sigma Aldrich Chemical Co. (Poole, Dorset, UK). Butaprost, PGE2, and sulprostone were obtained from Cayman Chemicals (distributed by Alexis Corporation (UK) Ltd., Bigham, Notts, UK). LAC-PGE (7-2-[(E)-3-hydroxy-4-((3-trifluoromethyl-phenyl)-phenyl)-but-1-enyl]-5-oxo-pyrrolidin-1-yl-heptanoic acid), CP533536, ONO-D1-004, and L-902688 were received from Allergan Inc. (Irvine, CA, USA) as gifts. Stock solutions of 10−2 M were prepared by dissolving the compounds in ethanol. Further dilutions were made with 0.9% (w/v) normal saline. The Krebs’ solution (pH 7.4) was freshly prepared at the following composition (mM): NaCl 118.9; KCl 4.7; KH2PO4 1.2; MgSO4 1.2; and CaCl2 2.5, NaHCO3 25.0, and glucose 10.0, oxygenated with 95% O2 and 5% CO2.
Results
Effect of PGE2 on Myometrial Activity.
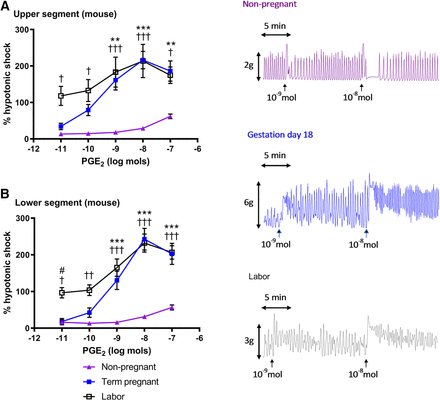
In all murine tissues, PGE2 evoked a dose-dependent excitation curve. In both upper (Fig. 1A) and lower (Fig. 1B) uterine segments, a greater excitatory response to PGE2 was observed in tissues taken during gestation compared with dioestrus (P < 0.05 to P < 0.001). Preliminary studies showed that this PGE2 effect was similar in tissues taken throughout the oestrous cycle (Supplemental Fig. 1). With PGE2 at 10−11 mol, lower segment tissue taken at labor exhibited a greater contractile response than tissue taken on day 18 of gestation (P < 0.05). When responses to PGE2 in lower and upper tissue segments were compared at each gestational stage, no significant regional variation was observed along the length of the uterine horns.
Dose-response curves (10−11–10−7 mol) and typical traces for PGE2 in (A) upper and (B) lower segments of mouse uterine horn. Matching tissues were obtained from nonpregnant (n = 6), pregnant (gestation day 18; n = 7), and laboring mice (n = 8). Tissue strips were superfused with Krebs’ solution. Activity was determined over 5-minute periods (area under the curve) and expressed as percentage of a reference contraction induced by hypotonic shock. Data are expressed as arithmetic means ± S.E.M., and statistical significance was determined by two-way ANOVA with Tukey’s post hoc test. **P < 0.01; ***P < 0.001 for nonpregnant compared with gestation day 18; †P < 0.05; †† P < 0.01; †††P < 0.001 for nonpregnant compared with labor; #P < 0.05 for gestation day 18 compared with labor.
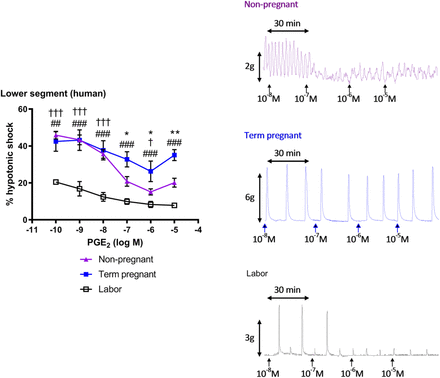
Human lower segment myometrial strips exhibited strong spontaneous contractions when taken during the follicular phase and at term pregnancy before labor. In these tissues, PGE2 caused predominant utero-relaxant effects (10−10–10−6 M), with tissue activity partially restored at 10−5 M (Fig. 2). Spontaneous contractions approximately halved during labor. PGE2 fully inhibited this myogenicity in a monophasic concentration-related manner by suppressing contractile amplitude (P < 0.001).
Concentration-effect curves (10−10–10−5 M) and typical traces for PGE2 in isolated lower segment myometrium obtained from nonpregnant (n = 11), term pregnant, not in labor (n = 6), and laboring women (n = 4). Tissue strips were immersed in Krebs’ solution. Activity was determined over 30-minute periods (area under the curve) and expressed as percentage of a reference contraction induced by hypotonic shock. Data are shown as arithmetic means ± S.E.M., and statistical significance was determined by two-way ANOVA with Tukey’s post hoc test. *P < 0.05; **P < 0.01 for nonpregnant compared with term pregnant; †P < 0.05; †††P < 0.001 for nonpregnant compared with labor; ##P < 0.01; ###P < 0.001 for term pregnant compared with labor.
Effect of EP2 Agonists on 5-HT–Induced and Spontaneous Myometrial Activity.
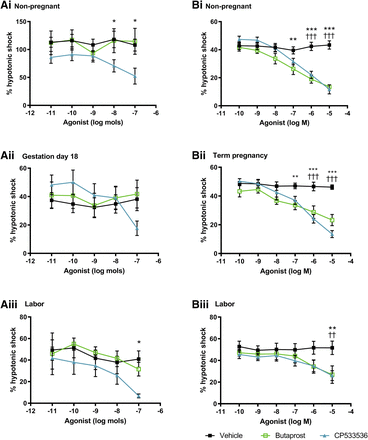
The EP2 agonist butaprost did not inhibit 5-HT–induced myogenicity in nongestational and gestational mouse tissues or spontaneous contractions in uterine strips taken during labor (Fig. 3A). Similarly, the nonprostanoid EP2 agonist CP533536 had no effect on 5-HT–driven activity in tissues taken on day 18 of gestation. In tissues taken during dioestrus and labor, CP533536 attenuated both 5-HT–induced and spontaneous myometrial contractions by 52% and 83%, respectively (P < 0.05).
Vehicle and dose-response curves for EP2 receptor agonists butaprost and CP533536 in uterine samples taken from (A) mice at (i) dioestrus (n = 6), (ii) gestation day 18 (n = 6–8), and (iii) during labor (n = 3–5) as well as from (B) human donors in (i) the follicular phase (n = 6–9), (ii) term pregnancy (n = 6), and (iii) during labor (n = 4–6). Upper segment uteri from mice were superfused, and lower myometrial biopsies from women were immersed in Krebs’ solution. Responses are shown as percentage of a reference contraction induced by hypotonic shock. Data are expressed as arithmetic mean ± S.E.M., and statistical significance was determined by two-way ANOVA with Tukey’s post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001 for vehicle compared with butaprost; ††P < 0.01; †††P < 0.001 for vehicle compared with CP533536.
In immersed lower segment myometrium from nonpregnant and pregnant women, both butaprost and CP533536 caused a gradual decline in contractility compared with vehicle (P < 0.01 to P < 0.001; Fig. 3B). Potency values were similar for both EP2 mimetics (pEC50: 6.7 ± 0.4 and 6.5 ± 0.5 M, respectively). Complete cessation of activity was achieved in many tissue strips at 10−5 M, especially when taken during labor.
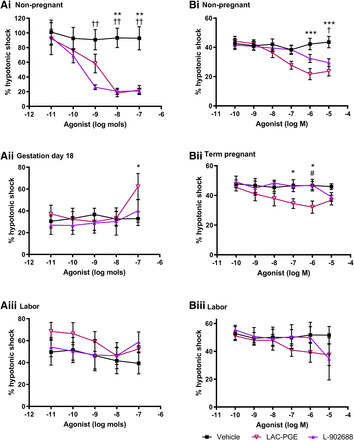
Effect of EP4 Agonists on 5-HT–Induced and Spontaneous Myometrial Activity.
The EP4 agonists evoked dose-dependent inhibition of 5-HT–induced myogenicity in mouse tissues obtained in the nongravid state (P < 0.01; Fig. 4A), with L-902688 more potent than LAC-PGE. In gestational uteri, L-902688 had no effect on 5-HT or spontaneously driven uterine contractions. However, bolus doses of LAC-PGE produced a biphasic excitatory response followed by a period of inhibition. When compared with vehicle, LAC-PGE enhanced contractility in tissues taken on day 18 of gestation (P < 0.05).
Vehicle and dose-response curves for EP4 receptor agonists LAC-PGE and L-902688 in uterine samples taken from (A) mice at (i) dioestrus (n = 6), (ii) gestation day 18 (n = 6–7), and (iii) during labor (n = 3–4) as well as from (B) human donors in (i) the follicular phase (n = 3–7), (ii) term pregnancy (n = 3–6), and (iii) during labor (n = 3–5). Upper segment uteri from mice were superfused, and lower myometrial biopsies from women were immersed in Krebs’ solution. Responses are shown as percentage of a reference contraction induced by hypotonic shock. Data are expressed as arithmetic mean ± S.E.M., and statistical significance was determined by two-way ANOVA with Tukey’s post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001 for vehicle compared with LAC-PGE; †P < 0.05; ††P < 0.01 for vehicle compared with L-902688; #P < 0.05 for LAC-PGE compared with L-902688.
The concentration-effect curves for LAC-PGE and L-902688 exhibited different responses in isolated human myometrium. When taken during the follicular phase, both EP4 agonists gradually reduced myogenic activity compared with time-matched vehicles (P < 0.05 to P < 0.001); at 10−6 M, responses to LAC-PGE plateaued (Fig. 4B). LAC-PGE induced a similar biphasic response at term pregnancy (P < 0.05) but not during labor. Despite a decrease at 10−5 M, L-902688 had no significant effect on myometrial tonus or contractility.
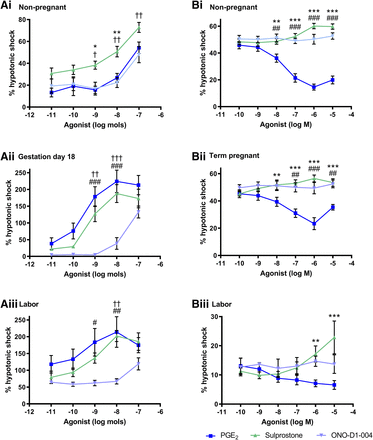
Effect of EP1/3 Agonists on Myometrial Activity.
The EP3/1 agonist sulprostone and EP1 agonist ONO-D1-004 evoked excitation in all murine tissues. Sulprostone produced greater contractile effects than both ONO-D1-004 (P < 0.05 to P < 0.01) and PGE2 (P < 0.05 to P < 0.01) in nonpregnant tissues (Fig. 5A). In uterine tissue taken on day 18 of gestation and during labor, PGE2 and sulprostone were more potent than ONO-D1-004 (P < 0.05 to P < 0.001). The excitatory effects of sulprostone and ONO-D1-004 were higher in gestational over nongestational tissues.
Dose-response curves for PGE2, the EP3/1 receptor agonist sulprostone and the EP1 receptor agonist ONO-D1-004 in uterine samples taken from (A) mice at (i) dioestrus (n = 11–12), (ii) gestation day 18 (n = 5–7), and (iii) during labor (n = 5–8) as well as from (B) human donors in (i) the follicular phase (n = 3–8), (ii) term pregnancy (n = 4–10), and (iii) during labor (n = 3–7). Upper segment uteri from mice were superfused, and lower myometrial biopsies from women were immersed in Krebs’ solution. Responses are shown as percentage of a reference contraction induced by hypotonic shock. Data are expressed as arithmetic mean ± S.E.M., and statistical significance was determined by two-way ANOVA with Bonferroni’s post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001 for PGE2 compared with sulprostone; †P < 0.05; ††P < 0.01; †††P < 0.001 for sulprostone compared with ONO-D1-004; #P < 0.05; ##P < 0.01; ###P < 0.001 for PGE2 compared with ONO-D1-004.
In myometrium from nonpregnant, term pregnant, and laboring women, ONO-D1-004 had no effect on phasic contractions (Fig. 5B). Sulprostone evoked excitatory responses compared with ONO-D1-004 and PGE2 (P < 0.01 to P < 0.001). The induced rise in activity compared with vehicle (12.5%, 9.4%, and 12.6%, respectively) corresponded to intensified frequency and amplitude of contractions.
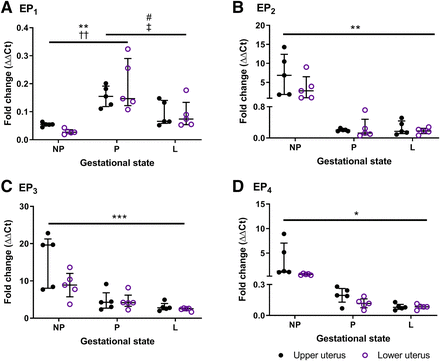
Abundance and Distribution of EP Receptors in Mouse Uterus.
Gene expression of each EP receptor transcript was equivalent in upper and lower regions of the isolated nonpregnant, term pregnant, and laboring mouse uterus (Fig. 6). Detection of EP1 mRNA was relatively low but peaked at term pregnancy compared with tissues taken at dioestrus (P < 0.01) and during labor (P < 0.05). In contrast, abundant EP3 compliments were notable in all uterine samples, especially in the nongravid uterus. Relative EP3 expression decreased with advancing gestation (P < 0.001); interestingly, this was not consistent with its contractile effects (Fig. 5A). A reduction in EP2 (P < 0.01) and EP4 (P < 0.05) receptors was also observed in gestational tissues, better reflecting their agonist-induced effects (Fig. 3A; Fig. 4A).
Expression of (A) EP1, (B) EP2, (C) EP3, and (D) EP4 transcripts in upper and lower mouse uterus taken at dioestrus (NP; n = 5), term gestation day 18 (P; n = 5), and during labor (L; n = 5). Total RNA was extracted using the TRIzol method and quantified using real-time reverse-transcription PCR, and gene expression was normalized against β-actin and GAPDH relative to an internal control. Data are shown as the median and interquartile range and analyzed using a Friedman’s two-way ANOVA with Dunn’s multiple comparisons post hoc test; *P < 0.05; **P < 0.01; ***P < 0.001 for upper and ††P < 0.01 for lower uterus from nonpregnant mice compared with gestational tissues; and #P < 0.05 for upper and ‡P < 0.05 for lower uterus from gestation day 18 mice compared with laboring tissues.
Discussion
These studies confirm the presence of a functionally heterogeneous EP receptor population in mouse and human uteri, which was characterized for the first time across gestational states. It was discovered that EP3-mediated excitation predominates with uterotonic effects enhanced in the late gestational and laboring uterus, indicating the EP3 receptor as a therapeutic target in women’s reproductive health.
PGE2 has previously been shown to exhibit diverse effects on smooth muscle contractility via EP1-4 subtypes (Coleman et al., 1994). In this study, EP receptor agonist effects varied according to hormonal milieu, most likely because of the differential regulation of EP3 receptors that play an important role in the labor process. Limited EP2 receptor agonist function in mouse but not human tissues substantiate previous findings that murine EP2 mRNA was localized to luminal epithelial cells rather than uterine myocytes (Lim and Dey, 1997; Yang et al., 1997). This suggests that changes in the spatial and temporal expression of EP receptors may mediate uterine events during the oestrous and menstrual cycle, pregnancy, and parturition.
Maintained excitatory response to PGE2 along the length of the mouse uterine horn reflects the relatively uniform EP receptor complements in nongestational and gestational tissues. However, in human myometrium, the expression and ratio of EP subtypes were shown to differ. While the expression of EP1 and EP4 transcripts remain constant throughout the uterus, EP2 receptors increased toward the cervix, whereas EP3 receptors were reported to be the same (Arulkumaran et al., 2012) or more abundant in the upper uterine segment (Astle et al., 2005; Grigsby et al., 2006). Predominant PGE2 tissue excitation in the mouse uterus is likely to represent upper segment function in women. In litter-bearing mice, this may indicate its role to deliver multiple fetuses. It is possible that the uterine body distal to the uterine horns is more translational to the lower segment in women. The clinical implications of sampling upper uterine tissue and ethical restrictions limiting sampling to the lower human uterus prevented the investigation of regional differences.
Because of the decline in spontaneous activity in myometrial strips obtained from nonpregnant and pregnant (gestation day 18) mice (Griffiths, 2007), 5-HT was used to drive in vitro phasic contractions. The biphasic response to PGE2 observed in the 5-HT–induced nongravid tissues indicated the presence of both inhibitory and excitatory EP receptors. These findings were supported by functional studies using the isolated porcine (Cao et al., 2005) and human uterus (Senior et al., 1991).
To identify the functional population of inhibitory EP receptors, selective EP2 and EP4 agonists were used in this study. The EP2 agonists butaprost (Gardiner, 1986) and CP533536 (Li et al., 2003; Paralkar et al., 2003) are well documented for high selectivity at the EP2 receptor with respective Ki values of 110 and 50 nM (Kiriyama et al., 1997; Li et al., 2003; Paralkar et al., 2003). Even so, butaprost showed no significant effects in mice, while CP533536 only attenuated myometrial activity at the highest dose. This suggests a paucity of functional uterine EP2 receptors, which corresponded to findings in the guinea pig (Lebel et al., 2004). However, EP2 mRNA expression was shown to increase during pregnancy and decline at term labor in the rat uterus (Brodt-Eppley and Myatt, 1998; Dong and Yallampalli, 2000). Furthermore, in isolated human myometrium, responsiveness to PGE2 is thought to be primarily mediated via the EP2 receptor (Senior et al., 1991; Senior et al., 1993; Duckworth et al., 2002; Fischer et al., 2008) and cAMP signaling (Sooranna et al., 2005), with its expression altered in a temporal and regional manner. Myometrial EP2 receptors decrease toward term gestation (Matsumoto et al., 1997; Brodt-Eppley and Myatt, 1999; Leonhardt et al., 2003) and are reported to not change (Dong and Yallampalli, 2000; Lebel et al., 2004; Kandola et al., 2014) or increase during parturition (Grigsby et al., 2006). The expression of EP2 receptors is also more intense in the lower rather than upper human uterine segments (Astle et al., 2005; Grigsby et al., 2006). This may explain similar EP2-mediated effects in this study, contributing to the relaxant phenotype of the lower uterus during parturition for successful neonatal delivery.
In contrast, EP4 receptors in mouse uterus showed gestational but not regional-related changes. The pharmacological effects of LAC-PGE and L-902688 diminished during pregnancy and labor, following the EP4 expression profile. At term gestation, tissue excitation evoked by LAC-PGE may be attributed to off-target activation of EP3 receptors (Maruyama et al., 2001). L-902688 produced the greatest inhibition and was reported to be 3 to 5 times more potent than PGE2 (Billot et al., 2003). EP4 receptors were also consistently expressed in human uterus (Astle et al., 2005; Grigsby et al., 2006) and were reported to have a negligible function (Hillock and Crankshaw, 1999). The LAC-PGE analog used in this study displayed utero-relaxant effects, possibly because of its high affinity and 1000-fold subtype selectivity for EP4 (Ki: 4.5 nM) (Elworthy et al., 2004) compared with the previously studied EP4 receptor antagonist AH23848B (Ki: 2690 nM) (Davis and Sharif, 2000). Even so, efficacy was low, especially at term gestation and labor, substantiating that inhibitory receptors are predominantly of the EP2 subtype. Because of its involvement in cervical ripening (Schmitz et al., 2001), EP4 receptors may have a distinct role from modulating uterine contractions in mouse and human uteri at term.
Despite predominant human uterine quiescence, responses evoked by PGE2 suggest the presence of contractile EP receptor subtypes in all tissue types. EP3 receptor mRNA is highly expressed in the mouse uterus (Sugimoto et al., 1992) and has been detected on uterine smooth muscle cells (Katsuyama et al., 1997; Yang et al., 1997). However, there are conflicting reports concerning the EP1 subtype with either low (Yang et al., 1997) or undetectable levels of mRNA identified (Katsuyama et al., 1997), perhaps relating to the nuclear sequestration of EP1 during labor (Arulkumaran et al., 2012). ONO-D1-004, which is a specific EP1 agonist, has been shown to be eightfold less potent than PGE2 (Oka et al., 2003). Nevertheless, in this study, PGE2 and ONO-D1-004 evoked analogous uterotonic responses in isolated tissue from nonpregnant animals. Sulprostone, an EP3/1 agonist, augmented uterine contractility, indicating EP1 and EP3 receptor function. Given that PGE2 binds to its receptor subtypes with a rank order of affinity of EP3 > EP4 > EP2 > EP1 (Kiriyama et al., 1997), its contractile effects may have been moderated by its actions on inhibitory EP receptors. Even so, unlike the hypothesis, EP3 receptors appear to have a greater role.
In mice, tissue excitation was upregulated in late gestational compared with nongestational tissues. While overall expression was lower, proportionally EP3 receptors were threefold higher than EP2 and EP4 subtypes at term pregnancy and labor than in the nonpregnant uterus. Because of the excitatory profile maintained during labor, it is likely that EP3 receptors are involved in the contractile force associated with parturition. In human, baboon, and guinea-pig myometrium at term pregnancy and labor, EP3 receptor expression was similarly reported to be greater than that of EP2 and EP4 receptors (Matsumoto et al., 1997; Smith et al., 2001; Terry et al., 2008). This provides support to exploit EP3 receptors as a potential target for tocolysis or improved labor induction. Antagonizing EP3 receptors also shows pharmacological promise through their apparent antithrombotic and antiulcer effects, which are currently under clinical evaluation (Markovič et al., 2017; Xiang et al., 2019).
In conclusion, a heterogeneous EP receptor population characterized in mouse and human female reproductive tracts showed some similarities. In lower segment nongestational tissues, both EP4 and EP2 receptor agonists suppressed uterine activity without affecting resting tone. Their contrasting tocolytic potential at term pregnancy perhaps reflected uterine topography. In all tissues, EP3-mediated excitation seemed to predominate with uterotonic effects enhanced in the late gestational and laboring uterus. These findings support development of new selective EP3 receptor modalities, which are currently being investigated for local drug delivery. Examining their effects in mice offers valuable preclinical insight that could be used to improve response rates for labor induction or as a therapy for menstrual cramps, pelvic pain, or preterm labor caused by uterine hypercontractility.
Acknowledgments
The authors would like to thank Allergan Inc. for their generous supply of compounds and for supporting D.P.F., A.L.G., and U.J.S.
Authorship Contributions
Participated in research design: Fischer, Griffiths, Lui, Woodward, Marshall.
Conducted experiments: Fischer, Griffiths, Lui.
Contributed new reagents or analytical tools: Woodward, Marshall.
Performed data analysis: Fischer, Griffiths.
Wrote or contributed to the writing of the manuscript: Fischer, Griffiths, Sabar, Farrar, O’Donovan, Woodward, Marshall.
Footnotes
- Received October 30, 2019.
- Accepted March 10, 2020.
↵1 D.P.F. and A.L.G. contributed equally.
↵
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
Abbreviations
- 5-HT
- 5-hydroxytryptamine
- Ca2+
- calcium EP prostaglandin E2 receptor
- GAPDH
- glyceraldehyde-3-phosphate
- LAC-PGE
- lactam derivative of 8-aza-11-deoxy-PGE1
- PCR
- polymerase chain reaction
- PG
- prostaglandin
- Copyright © 2020 by The Author(s)
This is an open access article distributed under the CC BY-NC Attribution 4.0 International license.