Abstract
Due to the ageing population, patients often present to the hospital with a high burden of comorbidities and polypharmacy. For patients admitted with decompensated heart failure (HF), the evidence on the effects of contraindicated drugs on long-term mortality is scarce. Therefore, we aimed to investigate the effect of contraindicated medications on outcomes of patients admitted with decompensated HF. We analyzed all consecutive patients from the National Heart Failure Audit admitted to two tertiary centers with acutely decompensated HF between April 2020 and October 2021. We included medication classes listed as contraindicated (class III) in the most recent European and American guidelines on the management of HF. The primary outcome measure was in-hospital mortality. The secondary outcome measure was overall mortality. Overall, 716 patients admitted with acute HF were included. One-fifth (n = 156, 21.8%) were on at least one contraindicated medication at admission. The prevalence of comorbidities was comparable between medication groups. During hospitalization, the use of nonsteroidal anti-inflammatory drugs (NSAIDs) was associated with increased in-hospital mortality (29% versus 9%, P = 0.013). On multivariable analyses, NSAID use was independently associated with worse in-hospital mortality (hazard ratio, 6.86; 95% confidence interval, 1.61–25.5; P = 0.005). However, other contraindicated medications were not associated with adverse outcomes. Postdischarge, the use of erythropoietin during admission was associated with increased mortality (54% versus 31%, P = 0.031). NSAID use is associated with increased in-hospital mortality for patients admitted with acute HF. However, inpatient use of other contraindicated medications was not associated with adverse in-hospital outcomes. Further studies are needed to confirm these results in larger and prospective cohorts.
SIGNIFICANCE STATEMENT Use of nonsteroidal anti-inflammatory drugs is associated with a worse in-hospital mortality in patients with decompensated heart failure. The prognostic role of other contraindicated medications remains still uncertain.
Introduction
Heart failure (HF) is one the leading causes of morbidity and mortality across the globe (Taylor et al., 2019). Its prevalence has increased significantly over the last 20 years, partly because of an aging population and patients surviving cardiovascular diseases earlier in life (Taylor et al., 2019; Groenewegen et al., 2020). Therefore, a large proportion of HF patients are elderly with a high comorbidity and medication burden (Wong et al., 2011; Pagell et al., 2016; Conrad et al., 2018; Unlu et al., 2020; Beezer et al., 2022). Thus, HF patients, especially the elderly, are at particularly high risk of being exposed to medications that are contraindicated in HF and may be harmful.
The European Society of Cardiology and the American Heart Association outline several medications that are contraindicated in HF due to their perceived harmful effects (McDonagh et al., 2021; Heidenreich et al., 2022). Guidelines relating to contraindicated medications in HF are based on a mixture of observational studies and clinical trials. Nonsteroidal anti-inflammatory drugs (NSAIDs), erythropoietin (EPO)-stimulating agents, α blockers, antidiabetic drugs such as thiazolidinediones and DPP4 inhibitors, and several antiarrhythmic medications have been linked to an increased risk of HF exacerbations and hospitalization (Mamdani et al., 2004; Pagell et al., 2016; McDonagh et al., 2021; Heidenreich et al., 2022).
For patients hospitalized with HF, there is a paucity of evidence relating to the effects of contraindicated drugs on subsequent long-term mortality. Also, most studies have investigated patients with chronic HF, whereas few have examined outcomes for patients with acutely decompensated HF. The aim of this study was to investigate the effect of contraindicated medications on outcomes of patients admitted to the hospital with decompensated HF.
Materials and Methods
Study Design and Population
All consecutive patients from the National Heart Failure Audit (NHFA) admitted to the King’s College Hospital or the Princess Royal University Hospital, London, UK, with acutely decompensated HF between April 2020 and October 2021 were included in the study. The NHFA holds data on acute HF hospitalizations of patients >18 years old in England and Wales. Patients were entered into the audit if they had a primary discharge diagnosis of HF. Data on each patient’s medication history prior to admission and medications administered during admission were extracted from the electronic patient record. We included relevant medication classes listed as contraindicated (class III) in the 2021 European Society of Cardiology heart failure guidelines or the 2022 American Heart Association heart failure guidelines (Table 1) (McDonagh et al., 2021; Heidenreich et al., 2022). Patients who were prescribed one or more contraindicated medication at the time of admission were compared with those who were not taking any contraindicated medications.
Included contraindicated medications
Baseline Characteristics
For each patient, data on demographics, HF parameters [including left ventricular systolic dysfunction, New York Heart Association HF class], comorbidities, and cardiovascular risk factors were recorded in the NHFA. The standard NHFA dataset proforma is available from the National Institute for Cardiovascular Outcomes Research (https://www.nicor.org.uk/national-cardiac-audit-programme/datasets/). Contraindicated medications for HF with reduced ejection fraction (HFrEF) were grouped into four classes: α blockers, NSAIDs, EPO, and antiarrhythmic medications.
Outcome Measures
The primary outcome of the study was in-hospital mortality in patients taking contraindicated medications during admission, which was collected from hospital records. The secondary endpoint was overall mortality. Follow-up mortality status was identified from National Health Service Digital using a National Health Service number, a unique identifier allocated at first use of the National Health Service in the UK. Subgroup analyses examined the effect of medications taken prior to admission on both endpoints (in-hospital and overall mortality).
Statistical Analysis
Variables are reported as median and interquartile range, mean and standard deviation, or numbers and percentages as appropriate. Baseline characteristics were analyzed using Pearson χ2 for categorical variables, Student’s t test for continuous variables, and one-way ANOVA for multiple comparisons. Patients were grouped according to class of contraindicated medication. Kaplan-Meier curves for in-hospital and overall mortality were compared using the log-rank test. Uni- and multivariable Cox proportional hazards models were used to adjust for confounders. Clinically relevant and demographic variables known to impact prognosis for patients with HF were selected and included in the univariable model. For the multivariable model, only variables with a P < 0.10 at the univariable were included in the model. A sensitivity analysis including only patients with HFrEF was conducted. All data analyses were performed using SPSS statistics software and R (R-Project).
Results
Study Population
Seven hundred sixteen patients admitted to the hospital with acute HF during the study period were included. Of those admitted, approximately one-fifth (n = 156, 21.8%) were taking at least one contraindicated medication at the time of their admission. Baseline characteristics of the study population are reported in Table 2. The prevalence of comorbidities, including asthma, diabetes, hypertension, valve disease, atrial fibrillation/flutter, chronic obstructive pulmonary disease, and ischemic heart disease, was comparable between medication groups. However, patients on α blockers were more frequently male compared with other classes of drugs (50% for those not taking contraindicated drugs, 78% for α blockers, 59% for EPO, and 50% for NSAIDs, P < 0.001), and those taking α blockers were generally older than those taking other medications (84 years for α blockers versus 76 years for NSAIDs versus 72.5 years for EPO, P < 0.001). There was a higher prevalence of patients with a history of malignancy taking EPO compared with those taking either no contraindicated medications or another class of contraindicated medication [29% for EPO versus 4.7% for α blockers versus 14% for NSAIDs versus 12% for no contraindicated drugs (P = 0.007)]. Patients with cardiomyopathy were less likely to be taking contraindicated medications than to be taking a contraindicated medication [28% for no contraindicated drugs versus 13% for α blockers versus 11% for EPO versus 15% for NSAIDs (P = 0.003)]. There were no patients who remained on antiarrhythmic medications following hospital admission in our cohort.
Demographic characteristics of study population
Outcomes
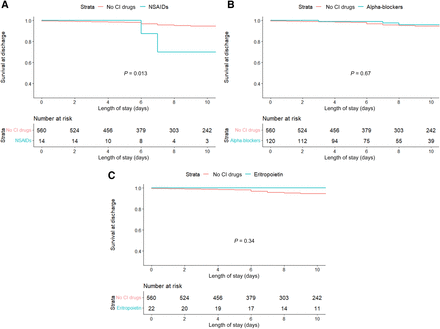
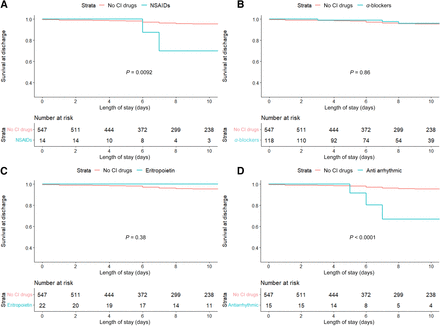
During hospitalization, the use of NSAIDs was associated with an increased in-hospital mortality (29% versus 9%, P = 0.013 by log-rank) (Fig. 1A). Other contraindicated medications, including α blockers and EPO, were not associated with an increased in-hospital mortality (Fig. 1, B and C). Having a history of NSAID use (P = 0.0092) or antiarrhythmic use (P < 0.0001) prior to admission was associated with increased in-hospital mortality (Fig. 3, A and B). Use of α blockers and erythropoietin in the medication history was not associated with an increased in-hospital mortality (Fig. 3, C and D). Multivariable analyses, adjusted for age, sex, New York Heart Association class, and left ventricular ejection fraction, demonstrated that NSAID use during admission was independently associated with worse in-hospital mortality (hazard ratio, 6.86; 95% confidence interval, 1.61–25.5; P = 0.005).
Kaplan-Meier curves for in-hospital mortality for patients taking contraindicated (CI) medications during admission (grouped according to drug class) versus no contraindicated medications during admission. (A) NSAIDs. (B) α blockers. (C) Erythropoietin.
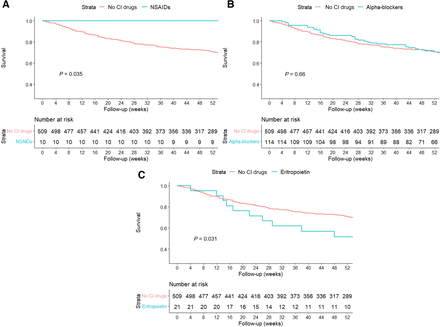
Regarding the secondary outcome of mortality following discharge, over a median follow-up of 55 weeks (interquartile range, 25–76 weeks), only the use of EPO during admission was associated with increased mortality (54% versus 31%, P = 0.031) (Fig. 2A). Conversely, the use of NSAIDs during admission was associated with a reduced postdischarge mortality (P = 0.035) (Fig. 2B). α blockers were not associated with a difference in postdischarge mortality (P = 0.66) (Fig. 3C).
Kaplan-Meier curves for out-of-hospital mortality according to contraindicated (CI) medication class during admission. (A) Erythropoietin. (B) NSAIDs. (C) α blockers.
Kaplan-Meier curves for in-hospital mortality according to contraindicated (CI) medication class prior to admission (medication history). (A) NSAIDs. (B) Antiarrhythmic medication. (C) α blockers. (D) Erythropoietin.
Sensitivity analyses for patients with HF with reduced ejection fraction (HFrEF) showed a trend toward higher in-hospital mortality for patients taking NSAIDs during admission (P = 0.07) but not for α blockers (P = 0.62) or erythropoietin (P = 0.30). A similar trend was also observed in the postdischarge period for the use of NSAIDs, whereas no differences were observed for α blockers (P = 0.49). Compared with patients on no contraindicated drugs, HFrEF patients receiving EPO were more likely to experience an adverse outcome following discharge (P = 0.021).
Discussion
Several treatments have been shown to improve the prognosis of patients with HF, especially for those with reduced ejection fraction (McDonagh et al., 2021). However, some medications have been associated with an increased risk of adverse events (McDonagh et al., 2021). Although the impact of several contraindicated medications on incident HF risk and hospitalization has been investigated in previous studies (Mamdani et al., 2004; Pagell et al., 2016), little is known about their effect on in-hospital and follow-up mortality in acute decompensated HF. We report an association between NSAID use prior to and during admission for acute HF and increased in-hospital mortality. Interestingly, there was no association with increased in-hospital mortality for inpatient use of α blockers or erythropoietin-stimulating agents.
In the early 2000s, data began to emerge about the cardiotoxic effects of cyclooxygenase-2 (COX-2) NSAIDs (Arfè et al., 2016), followed by safety concerns relating to traditional nonselective NSAIDs (Trelle et al., 2011; Bhala et al., 2013). In HF specifically, a few studies have suggested that NSAID use in chronic HF is associated with increased mortality (Hudson et al., 2005; Gislason et al., 2009), an increased risk of incident HF, decompensation, and hospitalization for HF (Feenstra et al., 2002; Mamdani et al., 2004; Scott et al., 2008; Arfè et al., 2016). Acutely, the pathophysiological mechanism may be related to NSAID-mediated COX and prostaglandin inhibition leading to increased peripheral systemic resistance and reduced renal perfusion (Arfè et al., 2016). This, in turn, affects fluid balance, causing increased fluid retention and blood pressure fluctuation (Gislason et al., 2009).
Our results suggest that the association with increased mortality can be observed in the acute setting as well. Our study corroborates the evidence previously reported for elderly patients, where NSAID use was associated with worse long-term all-cause mortality (Sunaga et al., 2020). Furthermore, adverse events with NSAIDs have been reported to occur close to the time of administration and even with short-term use (Gislason et al., 2006, 2009). Our data on inpatient use of NSAIDs and in-hospital mortality are consistent with this finding that NSAIDs are harmful in the short term as well as the medium term during hospitalization. Although a different trend may be seen in the postdischarge period (Fig. 3), this association may be impacted by a selection of patients at a lower risk of events who survived the index admission. Given the relatively low number of patients, larger prospective studies are needed to address the relationship between NSAIDs and postdischarge survival in patients with HF.
NSAIDS are common medications that are frequently bought over the counter and used for several chronic inflammatory or painful conditions. Although they have important impacts on quality of life (Gislason et al., 2009; Scarpignato et al., 2015), it may be reasonable to forego this for short periods given the association with in-hospital mortality. Given the lack of information on renal parameters, the association between NSAID use and worsening of renal function cannot be ascertained in this analysis, and it is only speculative. Our finding of an association between NSAID use and increased in-hospital mortality in decompensated HF supports recommendations that NSAID use in acute HF settings should be restricted.
Conversely, use of erythropoietin during admission was not associated with an increased in-hospital mortality but was associated with an increased overall mortality. EPO use in HF remains controversial, and the evidence is contradictory (Volpe et al., 2015). The benefits to symptoms, hospitalization, and mortality that were reported in earlier epidemiologic studies have been contradicted by high-quality randomized trials (Mastromarino et al., 2013). These demonstrated no benefit of EPO in reducing HF hospitalizations or mortality and reported increased thromboembolic events (Swedberg et al., 2013; Bello et al., 2015; Kang et al., 2016). One randomized trial showed significantly greater incidence of HF hospitalization, death, myocardial infarction, and stroke in patients with chronic kidney disease (CKD) receiving a high dose of EPO (Singh et al., 2006). Furthermore, the mechanism of potential toxicity of EPO in patients with HF may be multifactorial and synergistic with other comorbidities (such as CKD). Therefore, the detrimental effect may be present in the medium or long term rather than acutely. Nevertheless, a complete separate evaluation of the prognostic role of CKD requiring EPO administration was not possible in this cohort. Overall, our results are consistent with these findings that EPO does not provide mortality benefits in HF and may have harmful effects, especially in the long term, postdischarge.
α blockers are a widely prescribed class of medication, especially in elderly males with benign prostate hypertrophy. Use of α blockers prior to or during admission was not associated with increased in-hospital or overall mortality. Previous studies have suggested an increased risk of incident HF with some α blockers such as doxazosin (Alderman, 2003; Bryson et al., 2004). Very few studies have examined the effect of α blockers in pre-existing HF. One study linked increased HF hospitalization only in patients taking α blockers without concurrent β blockade, although this study was underpowered (Dhaliwal et al., 2009). A possible mechanism for this is that unopposed α blockade leads to overstimulation of β adrenergic receptors, increasing plasma renin, aldosterone activity, and fluid retention (Pagell et al., 2016). Our study supports other epidemiologic studies that reported no or minimal association with HF-related hospitalization or mortality, and one study has even reported reduced hospitalization and mortality rates in α blocker users (Spoladore et al., 2009; Jackevicius et al., 2018). However, these epidemiologic studies require further confirmation in prospective or randomized trials. The potential mechanism of action may relate to the vasodilatory properties of this class of drugs. However, use of vasodilators in HF remains controversial following the results of the VICTORIA Trial using vericiguat (Armstrong et al., 2020). Furthermore, there is little evidence that α blockers, particularly if used concurrently with β blockade, are harmful in pre-existing HF. Randomized trials are needed to confirm this.
In this study, there were no patients taking antiarrhythmic medications during admission. However, analyses on patients taking antiarrhythmic medications prior to admission showed a significant association with higher in-hospital mortality. This corroborates several previous studies that have suggested they may depress left ventricular function and worsen HF (Pagell et al., 2016).
Overall, the findings of this study support the recommendation from the major HF guidelines on contraindicated medications. Some medications, especially NSAIDs, may be harmful and are associated with worse in-hospital mortality. However, further studies are required to confirm these results in larger populations.
Strengths and Limitations
The NHFA is an established audit that collects data from hospitals across England and Wales.
In our analysis, the main results are obtained using electronically prescribed inpatient medications and pharmacist-reconciled medication histories, which minimizes unrecorded medication use. This mitigates the limitation of previous studies investigating the impact of medications in HF as some of these medications are available over the counter (such as NSAIDs), and as such, their use may not be recorded. However, the effect of medications available over the counter cannot be entirely ascertained and requires dedicated studies monitoring the administration of specific drugs in the community setting.
There is a possibility of bias from unmeasured confounders, and causative links cannot be established. Similarly, investigating the differences between the prescription of contraindicated drugs, the severity of the clinical picture, and the causative prognostic effect is not possible in this type of analysis. Given the nature of this study, the results may not be generalizable to other populations and represent association rather than causation. The sample size of the study was relatively small with a low number of patients who were taking contraindicated medications during admission. Due to small sample sizes, comments about specific medications within medication classes are not possible. Furthermore, given the heterogeneity of indications for specific classes of drugs and the difficulties of ascertaining the correct indication, it was not possible to adjust for these confounders. Investigating the synergistic effect of different classes of medications and the specific indications may require a larger sample size and was out of the scope of the present analysis. The daily dose of medications was not available for each patient. Further studies may be warranted to investigate this aspect. Cause-specific death was not available. Therefore, we used all-cause mortality to capture the largest number of adverse events across the spectrum of comorbidities. Lastly, data on renal profile, such as eGFR (estimated Glomerular Filtration Rate) or creatinine, were not available in this cohort of patients, limiting the possibility to correlate the effect of specific drugs on renal profiles.
Conclusion
NSAID use during and prior to admission for acute HF is associated with increased in-hospital mortality. Inpatient use of other contraindicated medications listed in HF guidelines, including α blockers and erythropoietin, was not associated with in-hospital mortality. Further studies are needed to confirm these results in larger cohorts and to investigate whether this association extends to long-term mortality, as well as the interaction of different classes of medications on mortality during admission for acute HF.
Authorship Contributions
Participated in research design: Parish, Cannata, Bromage.
Performed data analysis: Cannata.
Wrote or contributed to writing of the manuscript: Parish, Cannata, Shamsi, Jordan-Rios, Albarjas, Piper, Scott, Bromage, McDonagh.
Footnotes
- Received August 14, 2022.
- Accepted April 5, 2023.
This work received no external funding.
No author has actual or perceived conflict of interest with the contents of this article.
Abbreviations
- CKD
- chronic kidney disease
- EPO
- erythropoietin
- HF
- heart failure
- HFrEF
- heart failure with reduced ejection fraction
- NHFA
- National Heart Failure Audit
- NSAID
- nonsteroidal anti-inflammatory drug
- Copyright © 2023 by The American Society for Pharmacology and Experimental Therapeutics